Is There a Role for Exercise When Treating Patients with Cancer with Immune Checkpoint Inhibitors? A Scoping Review
Abstract
Simple Summary
Abstract
1. Introduction
- Is there any evidence that suggests that exercise has a demonstratable effect on improving the oncological outcomes of patients with cancer receiving ICIs?
- Is there any evidence that suggests that exercise (including which type, timing and dosage of exercise) has a demonstratable effect on improving the QoL of patients with cancer receiving ICIs?
- What are the biological mechanisms, if any, that could be responsible for the effects exerted by exercise on improving the oncological outcomes and QoL of patients with cancer receiving ICIs?
2. Materials and Methods
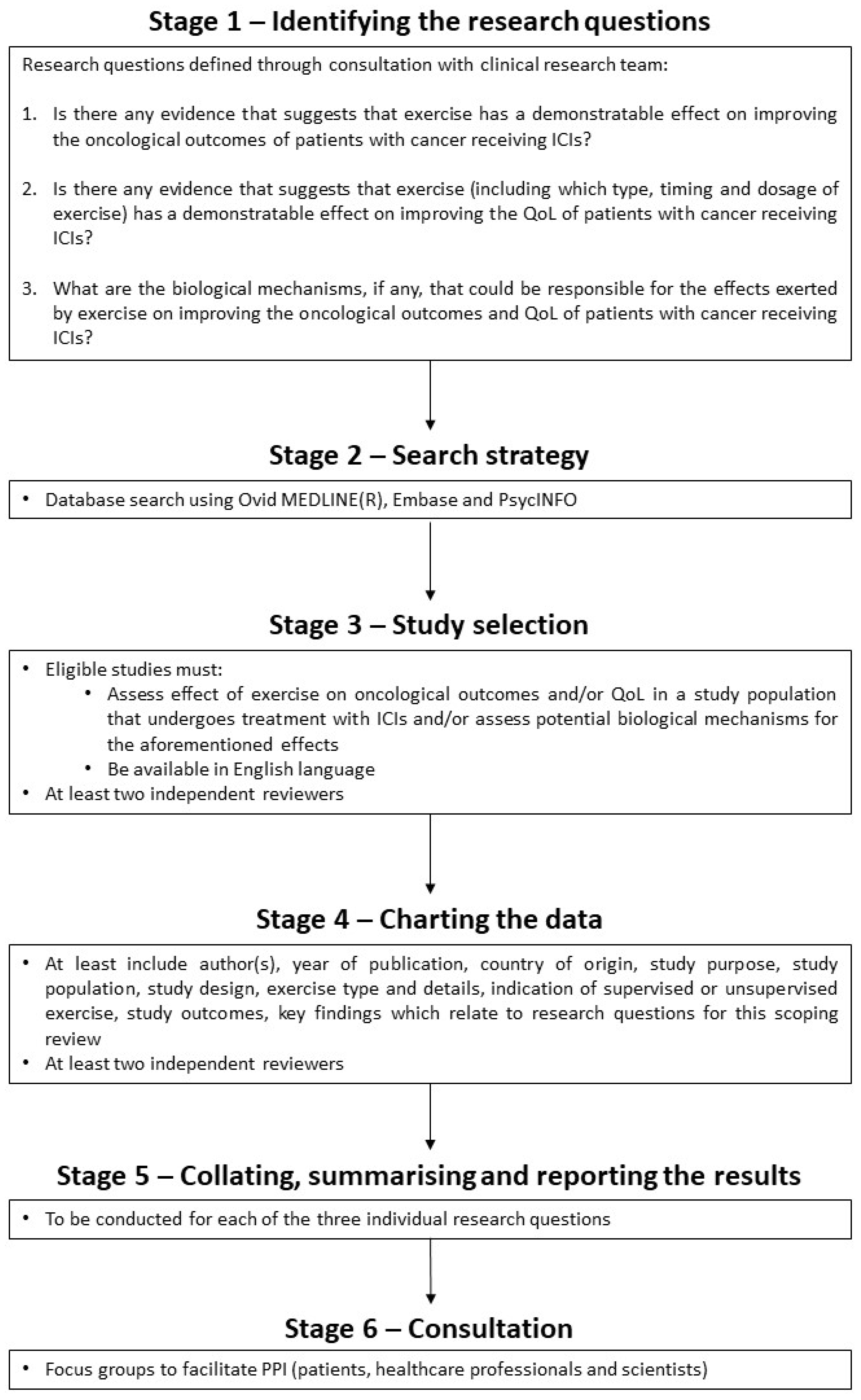
2.1. Protocol and Reporting
2.2. Database Search
2.3. Identifying Eligible Studies
2.4. Charting and Summarising the Data
2.5. Consultation Phase
3. Results
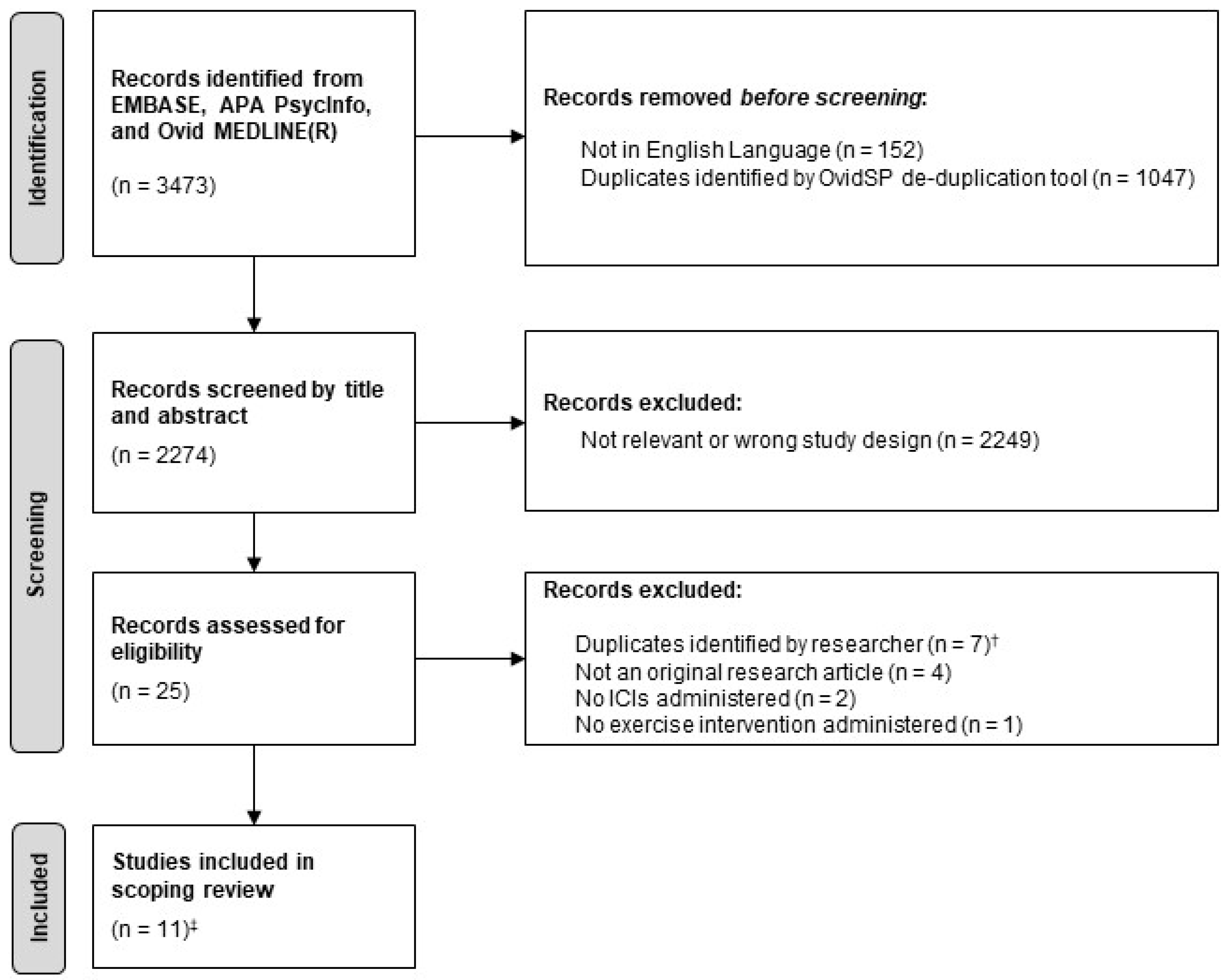
3.1. Study Characteristics
3.2. The Effect of Exercise on Oncological Outcomes
3.3. The Effect of Exercise on QoL
3.4. Biological Mechanisms
3.5. Consultation Phase
4. Discussion
4.1. Oncological Outcomes
4.2. Biological Mechanisms
4.3. QoL
4.4. Clinical Research
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Eno, J. Immunotherapy Through the Years. J. Adv. Pract. Oncol. 2017, 8, 747–753. [Google Scholar] [PubMed]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of Resistance to Immune Checkpoint Inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef]
- Gide, T.N.; Wilmott, J.S.; Scolyer, R.A.; Long, G.V. Primary and Acquired Resistance to Immune Checkpoint Inhibitors in Metastatic Melanoma. Clin. Cancer Res. 2018, 24, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Arnaud-Coffin, P.; Maillet, D.; Gan, H.K.; Stelmes, J.-J.; You, B.; Dalle, S.; Péron, J. A Systematic Review of Adverse Events in Randomized Trials Assessing Immune Checkpoint Inhibitors. Int. J. Cancer 2019, 145, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Physical Activity. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 4 April 2022).
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical Activity, Exercise, and Physical Fitness: Definitions and Distinctions for Health-Related Research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Campbell, K.L.; Winters-Stone, K.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.; Matthews, C.; Ligibel, J.; Gerber, L.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Katzmarzyk, P.T.; Rhodes, R.E.; Shephard, R.J. Evidence-Informed Physical Activity Guidelines for Canadian Adults. Can. J. Public Health Rev. Can. Sante Publique 2007, 98 (Suppl. S2), S16–S68. [Google Scholar]
- Mishra, S.I.; Scherer, R.W.; Snyder, C.; Geigle, P.M.; Berlanstein, D.R.; Topaloglu, O. Exercise Interventions on Health-related Quality of Life for People with Cancer during Active Treatment. Cochrane Database Syst. Rev. 2012, 2012, CD008465. [Google Scholar] [CrossRef]
- Gerritsen, J.K.W.; Vincent, A.J.P.E. Exercise Improves Quality of Life in Patients with Cancer: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Br. J. Sports Med. 2016, 50, 796–803. [Google Scholar] [CrossRef]
- Ferioli, M.; Zauli, G.; Martelli, A.M.; Vitale, M.; McCubrey, J.A.; Ultimo, S.; Capitani, S.; Neri, L.M. Impact of Physical Exercise in Cancer Survivors during and after Antineoplastic Treatments. Oncotarget 2018, 9, 14005–14034. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Bohlke, K.; May, A.M.; Clinton, S.K.; Demark-Wahnefried, W.; Gilchrist, S.C.; Irwin, M.L.; Late, M.; Mansfield, S.; Marshall, T.F.; et al. Exercise, Diet, and Weight Management during Cancer Treatment: ASCO Guideline. J. Clin. Oncol. 2022, 40, 2491–2507. [Google Scholar] [CrossRef]
- Zheng, X.; Cui, X.-X.; Huang, M.-T.; Liu, Y.; Shih, W.J.; Lin, Y.; Lu, Y.P.; Wagner, G.C.; Conney, A.H. Inhibitory Effect of Voluntary Running Wheel Exercise on the Growth of Human Pancreas Panc-1 and Prostate PC-3 Xenograft Tumors in Immunodeficient Mice. Oncol. Rep. 2008, 19, 1583–1588. [Google Scholar]
- Higgins, K.A.; Park, D.; Lee, G.Y.; Curran, W.J.; Deng, X. Exercise-Induced Lung Cancer Regression: Mechanistic Findings from a Mouse Model. Cancer 2014, 120, 3302–3310. [Google Scholar] [CrossRef]
- Piguet, A.-C.; Saran, U.; Simillion, C.; Keller, I.; Terracciano, L.; Reeves, H.L.; Dufour, J.-F. Regular Exercise Decreases Liver Tumors Development in Hepatocyte-Specific PTEN-Deficient Mice Independently of Steatosis. J. Hepatol. 2015, 62, 1296–1303. [Google Scholar] [CrossRef]
- Hagar, A.; Wang, Z.; Koyama, S.; Serrano, J.A.; Melo, L.; Vargas, S.; Carpenter, R.; Foley, J. Endurance Training Slows Breast Tumor Growth in Mice by Suppressing Treg Cells Recruitment to Tumors. BMC Cancer 2019, 19, 536. [Google Scholar] [CrossRef]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef]
- Van Waart, H.; Stuiver, M.M.; van Harten, W.H.; Geleijn, E.; Kieffer, J.M.; Buffart, L.M.; de Maaker-Berkhof, M.; Boven, E.; Schrama, J.; Geenen, M.M.; et al. Effect of Low-Intensity Physical Activity and Moderate- to High-Intensity Physical Exercise During Adjuvant Chemotherapy on Physical Fitness, Fatigue, and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial. J. Clin. Oncol. 2015, 33, 1918–1927. [Google Scholar] [CrossRef]
- Dimeo, F.C.; Stieglitz, R.-D.; Novelli-Fischer, U.; Fetscher, S.; Keul, J. Effects of Physical Activity on the Fatigue and Psychologic Status of Cancer Patients during Chemotherapy. Cancer 1999, 85, 2273–2277. [Google Scholar] [CrossRef]
- Sturgeon, K.; Schadler, K.; Muthukumaran, G.; Ding, D.; Bajulaiye, A.; Thomas, N.J.; Ferrari, V.; Ryeom, S.; Libonati, J.R. Concomitant Low-Dose Doxorubicin Treatment and Exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R685–R692. [Google Scholar] [CrossRef]
- Courneya, K.S.; Segal, R.J.; McKenzie, D.C.; Dong, H.; Gelmon, K.; Friedenreich, C.M.; Yasui, Y.; Reid, R.D.; Crawford, J.J.; Mackey, J.R. Effects of Exercise during Adjuvant Chemotherapy on Breast Cancer Outcomes. Med. Sci. Sports Exerc. 2014, 46, 1744–1751. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the Immune System: Regulation, Integration, and Adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef]
- Gustafson, M.P.; Wheatley-Guy, C.M.; Rosenthal, A.C.; Gastineau, D.A.; Katsanis, E.; Johnson, B.D.; Simpson, R.J. Exercise and the Immune System: Taking Steps to Improve Responses to Cancer Immunotherapy. J. Immunother. Cancer 2021, 9, e001872. [Google Scholar] [CrossRef] [PubMed]
- Sellami, M.; Gasmi, M.; Denham, J.; Hayes, L.D.; Stratton, D.; Padulo, J.; Bragazzi, N. Effects of Acute and Chronic Exercise on Immunological Parameters in the Elderly Aged: Can Physical Activity Counteract the Effects of Aging? Front. Immunol. 2018, 9, 2187. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Chen, M.; Rai, R.; Fox, L.; Moss, C.L.; George, G.; Karagiannis, S.N.; Enting, D.; Joseph, M.; Peat, N.; Russell, B.; et al. Is There a Role for Physical Activity When Treating Patients with Cancer with Immune Checkpoint Inhibitors? Protocol for a Scoping Review. BMJ Open 2021, 11, e046052. [Google Scholar] [CrossRef]
- Aromataris, E.; Munn, Z. JBI Manual for Evidence Synthesis. Available online: https://synthesismanual.jbi.global (accessed on 4 April 2022).
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping Studies: Advancing the Methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- González-Blanch, C.; Hernández-de-Hita, F.; Muñoz-Navarro, R.; Ruíz-Rodríguez, P.; Medrano, L.A.; Cano-Vindel, A. The Association between Different Domains of Quality of Life and Symptoms in Primary Care Patients with Emotional Disorders. Sci. Rep. 2018, 8, 11180. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Serrano, J.A.; Hagar, A. Run for Your Life: An Integrated Virtual Tissue Platform for Incorporating Exercise Oncology into Immunotherapy. Cancer Immunol. Immunother. 2021, 70, 1951–1964. [Google Scholar] [CrossRef] [PubMed]
- Martín-Ruiz, A.; Fiuza-Luces, C.; Rincón-Castanedo, C.; Fernández-Moreno, D.; Gálvez, B.G.; Martínez-Martínez, E.; Martín-Acosta, P.; Coronado, M.J.; Franco-Luzón, L.; González-Murillo, Á.; et al. Benefits of Exercise and Immunotherapy in a Murine Model of Human Non-Small-Cell Lung Carcinoma. Exerc. Immunol. Rev. 2020, 26, 100–115. [Google Scholar] [PubMed]
- Wennerberg, E.; Lhuillier, C.; Rybstein, M.D.; Dannenberg, K.; Rudqvist, N.-P.; Koelwyn, G.J.; Jones, L.W.; Demaria, S. Exercise Reduces Immune Suppression and Breast Cancer Progression in a Preclinical Model. Oncotarget 2020, 11, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Santos, I.L.; Amoozgar, Z.; Kumar, A.S.; Ho, W.W.; Roh, K.; Talele, N.P.; Curtis, H.; Kawaguchi, K.; Jain, R.K.; Fukumura, D. Exercise Training Improves Tumor Control by Increasing CD8+ T-Cell Infiltration via CXCR3 Signaling and Sensitizes Breast Cancer to Immune Checkpoint Blockade. Cancer Immunol. Res. 2021, 9, 765–778. [Google Scholar] [CrossRef]
- Turbitt, W.J.; Xu, Y.; Mastro, A.M.; Rogers, C.J. Abstract 1260: Diet and Exercise-Induced Weight Maintenance May Be Preventing Mammary Tumor Growth and Metastatic Burden by Enhancing Antitumor Immunity and/or Reducing Protumorigenic Factors. Cancer Res. 2017, 77, 1260. [Google Scholar] [CrossRef]
- Unterrainer, N.; Pedersen, K.S.; Bay, M.L.; Pedersen, B.K.; Gehl, K.J.; Hojman, P. PO-364 Effect of Exercise and Immunotherapy on Tumour Immunogenicity and Growth. ESMO Open 2018, 3, A371. [Google Scholar] [CrossRef]
- Hyatt, A.; Drosdowsky, A.; Williams, N.; Paton, E.; Bennett, F.; Andersen, H.; Mathai, J.; Milne, D. Exercise Behaviors and Fatigue in Patients Receiving Immunotherapy for Advanced Melanoma: A Cross-Sectional Survey via Social Media. Integr. Cancer Ther. 2019, 18, 1534735419864431. [Google Scholar] [CrossRef]
- Lacey, J.; Lomax, A.J.; McNeil, C.; Marthick, M.; Levy, D.; Kao, S.; Nielsen, T.; Dhillon, H.M. A Supportive Care Intervention for People with Metastatic Melanoma Being Treated with Immunotherapy: A Pilot Study Assessing Feasibility, Perceived Benefit, and Acceptability. Support. Care Cancer 2019, 27, 1497–1507. [Google Scholar] [CrossRef]
- Bay, M.L.; Unterrainer, N.; Stagaard, R.; Pedersen, K.S.; Schauer, T.; Staffeldt, M.M.; Christensen, J.F.; Hojman, P.; Pedersen, B.K.; Gehl, J. Voluntary Wheel Running Can Lead to Modulation of Immune Checkpoint Molecule Expression. Acta Oncol. 2020, 59, 1447–1454. [Google Scholar] [CrossRef]
- Buss, L.A.; Williams, T.; Hock, B.; Ang, A.D.; Robinson, B.A.; Currie, M.J.; Dachs, G.U. Effects of Exercise and Anti-PD-1 on the Tumour Microenvironment. Immunol. Lett. 2021, 239, 60–71. [Google Scholar] [CrossRef]
- Charles, C.; Bardet, A.; Ibrahimi, N.; Aromatario, O.; Cambon, L.; Imbert, A.; Pons, M.; Raynard, B.; Sauveplane, D.; Pouchepadass, C.; et al. Delivering Adapted Physical Activity by Videoconference to Patients with Fatigue under Immune Checkpoint Inhibitors: Lessons Learned from the PACTIMe-FEAS Feasibility Study. J. Telemed. Telecare, 2021; online ahead of print. [Google Scholar] [CrossRef]
- Shaver, A.L.; Sharma, S.; Nikita, N.; Lefler, D.S.; Basu-Mallick, A.; Johnson, J.M.; Butryn, M.; Lu-Yao, G. The Effects of Physical Activity on Cancer Patients Undergoing Treatment with Immune Checkpoint Inhibitors: A Scoping Review. Cancers 2021, 13, 6364. [Google Scholar] [CrossRef]
- Thomas, R.; Al-Khadairi, G.; Decock, J. Immune Checkpoint Inhibitors in Triple Negative Breast Cancer Treatment: Promising Future Prospects. Front. Oncol. 2021, 10, 600573. [Google Scholar] [CrossRef]
- Donlon, N.E.; Power, R.; Hayes, C.; Reynolds, J.V.; Lysaght, J. Radiotherapy, Immunotherapy, and the Tumour Microenvironment: Turning an Immunosuppressive Milieu into a Therapeutic Opportunity. Cancer Lett. 2021, 502, 84–96. [Google Scholar] [CrossRef]
- Wennerberg, E.; Vanpouille-Box, C.; Bornstein, S.; Yamazaki, T.; Demaria, S.; Galluzzi, L. Immune Recognition of Irradiated Cancer Cells. Immunol. Rev. 2017, 280, 220–230. [Google Scholar] [CrossRef]
- Chen, I.X.; Chauhan, V.P.; Posada, J.; Ng, M.R.; Wu, M.W.; Adstamongkonkul, P.; Huang, P.; Lindeman, N.; Langer, R.; Jain, R.K. Blocking CXCR4 Alleviates Desmoplasia, Increases T-Lymphocyte Infiltration, and Improves Immunotherapy in Metastatic Breast Cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 4558–4566. [Google Scholar] [CrossRef]
- Kurz, E.; Hirsch, C.A.; Dalton, T.; Shadaloey, S.A.; Khodadadi-Jamayran, A.; Miller, G.; Pareek, S.; Rajaei, H.; Mohindroo, C.; Baydogan, S.; et al. Exercise-Induced Engagement of the IL-15/IL-15Rα Axis Promotes Anti-Tumor Immunity in Pancreatic Cancer. Cancer Cell 2022, 40, 720–737.e5. [Google Scholar] [CrossRef]
- Li, H.-B.; Yang, Z.-H.; Guo, Q.-Q. Immune Checkpoint Inhibition for Pancreatic Ductal Adenocarcinoma: Limitations and Prospects: A Systematic Review. Cell Commun. Signal. 2021, 19, 117. [Google Scholar] [CrossRef]
- Hanson, E.D.; Sakkal, S.; Que, S.; Cho, E.; Spielmann, G.; Kadife, E.; Violet, J.A.; Battaglini, C.L.; Stoner, L.; Bartlett, D.B.; et al. Natural Killer Cell Mobilization and Egress Following Acute Exercise in Men with Prostate Cancer. Exp. Physiol. 2020, 105, 1524–1539. [Google Scholar] [CrossRef]
- Gebhardt, K.; Krüger, K. Supporting Tumor Therapy by Exercise: Boosting T Cell Immunity by Myokines. Signal Transduct. Target. Ther. 2022, 7, 292. [Google Scholar] [CrossRef]
- Goulart, K.N.d.O.; Resende, N.M.; Oliveira, L.M.; Drummond, M.D.M.; Lima, F.V.; Fujiwara, R.T.; Couto, B.P. Cytokine Response to Resistance Training Sessions Performed after Different Recovery Intervals. Sport Sci. Health 2022, 18, 743–749. [Google Scholar] [CrossRef]
- Riechman, S.E.; Balasekaran, G.; Roth, S.M.; Ferrell, R.E. Association of Interleukin-15 Protein and Interleukin-15 Receptor Genetic Variation with Resistance Exercise Training Responses. J. Appl. Physiol. 2004, 97, 2214–2219. [Google Scholar] [CrossRef] [PubMed]
- Calle, M.C.; Fernandez, M.L. Effects of Resistance Training on the Inflammatory Response. Nutr. Res. Pract. 2010, 4, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Ibañez, J.; Calbet, J.A.L.; Navarro-Amezqueta, I.; González-Izal, M.; Idoate, F.; Häkkinen, K.; Kraemer, W.J.; Palacios-Sarrasqueta, M.; Almar, M.; et al. Cytokine and Hormone Responses to Resistance Training. Eur. J. Appl. Physiol. 2009, 107, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Pudkasam, S.; Tangalakis, K.; Chinlumprasert, N.; Apostolopoulos, V.; Stojanovska, L. Breast Cancer and Exercise: The Role of Adiposity and Immune Markers. Maturitas 2017, 105, 16–22. [Google Scholar] [CrossRef]
- Mustian, K.M.; Alfano, C.M.; Heckler, C.; Kleckner, A.S.; Kleckner, I.R.; Leach, C.R.; Mohr, D.; Palesh, O.G.; Peppone, L.J.; Piper, B.F.; et al. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-Analysis. JAMA Oncol. 2017, 3, 961–968. [Google Scholar] [CrossRef]
- Naidoo, J.; Page, D.B.; Li, B.T.; Connell, L.C.; Schindler, K.; Lacouture, M.E.; Postow, M.A.; Wolchok, J.D. Toxicities of the Anti-PD-1 and Anti-PD-L1 Immune Checkpoint Antibodies. Ann. Oncol. 2015, 26, 2375–2391. [Google Scholar] [CrossRef]
- Zaleta, A.K.; Miller, M.F.; Olson, J.S.; Yuen, E.Y.N.; LeBlanc, T.W.; Cole, C.E.; McManus, S.; Buzaglo, J.S. Symptom Burden, Perceived Control, and Quality of Life Among Patients Living With Multiple Myeloma. J. Natl. Compr. Cancer Netw. 2020, 18, 1087–1095. [Google Scholar] [CrossRef]
- Piraux, E.; Caty, G.; Aboubakar Nana, F.; Reychler, G. Effects of Exercise Therapy in Cancer Patients Undergoing Radiotherapy Treatment: A Narrative Review. SAGE Open Med. 2020, 8, 2050312120922657. [Google Scholar] [CrossRef]
- Hall, E.T.; Singhal, S.; Dickerson, J.; Gabster, B.; Wong, H.; Aslakson, R.A.; Schapira, L.; Aslakson, R.; Ast, K.; Carroll, T.; et al. Patient-Reported Outcomes for Cancer Patients Receiving Checkpoint Inhibitors: Opportunities for Palliative Care—A Systematic Review. J. Pain Symptom Manag. 2019, 58, 137–156.e1. [Google Scholar] [CrossRef]
- Wiskemann, J. Sportivumab—Feasibility of Exercise as a Supportive Measure for Patients Undergoing Checkpoint-Inhibitor Treatment. 2022. Available online: https://clinicaltrials.gov/ (accessed on 22 July 2022).
- Straten, P. High Intensity Aerobic Exercise Training and Immune Cell Mobilization in Patients With Lung Cancer (HI AIM). 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04263467 (accessed on 22 July 2022).
| Variable | Definition |
|---|---|
| Authors | Name(s) of the author(s) of the study |
| Country | Country in which study was conducted |
| Year | Year of publication |
| Study design | Broad study type (e.g., preclinical/observational/quasi-experimental/randomised controlled trial) |
| Population | Total number and characteristics of study participants |
| Purpose | Aim(s) of the study |
| Cancer | Tumour type(s) represented amongst the study population |
| ICI | Method of immune checkpoint blockade, including any other oncological intervention delivered concurrently |
| Exercise intervention | Details of the exercise intervention, including type (e.g., aerobic/resistance/mixed), intensity, frequency and any other supportive care intervention delivered concurrently |
| Exercise intervention oversight | Level of supervision of the exercise intervention (e.g., supervised/unsupervised/mixed) |
| Concurrent exercise and ICI | Indication of whether the study included any concurrent delivery of the exercise and ICI interventions or not (e.g., yes/no) |
| Scoping objective(s) | The research question(s) of the present scoping review addressed by the study |
| Outcome measure(s) | The endpoints used in the study which relate to the research question(s) of the present scoping review |
| Relevant findings | Results of the study which relate to the research questions(s) of the present scoping review |
| Authors, Country, Year | Study Design, Population | Purpose | Cancer | ICI | Exercise Intervention | Exercise Intervention Oversight | Concurrent Exercise and ICI | Scoping Objectives 1 | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Serrano & Hagar, USA, 2021 | Preclinical (in silico computer model) Virtual cohort of patients with early-stage solid tumour cancer (n = 200) | To describe an in-silico model simulating early-stage solid tumour growth and anti-tumour immune response, and demonstrate its utility through two virtual experiments. | Solid tumour cancer | Treatment with ICIs was modelled by reducing the inhibitory parameter of Tregs, resulting in increased efficacy of cytotoxic T lymphocytes. | Aerobic fitness was a pre-existing parameter in the virtual model. The model used clinical and epidemiological data on aerobic fitness to divide the virtual cohort into aerobically fit or sedentary groups. | N/A | N/A | 2 | [32] |
| Martin-Ruiz et al., Spain, 2020 | Preclinical Mouse tumour model (n = 22) | To determine the effects of exercise on tumour growth and its potential adjuvant effects when combined with αPD-1 immunotherapy (nivolumab). | NSCLC | αPD-1 | Mixed (combination of aerobic and resistance). Moderate intensity. 40–60 min per session. 5 days per week for 8 weeks. | Supervised | Yes (Post-implant exercise initiated when tumour reached 100 mm3. ICI initiated 15 days later. ICI and exercise were then delivered concurrently for 6 weeks) | 1, 3 | [33] |
| Wennerberg et al., USA, 2020 | Preclinical Mouse tumour model (n = 42) | To determine whether exercise has anti-tumour effects in a model of established triple-negative breast cancer. | Breast cancer (triple negative) | αPD-1 (+RT) | Aerobic (treadmill running). 30 min per session. 5 days/week for ~3 weeks. | Supervised | Yes (Post-implant exercise initiated 8 days after tumour inoculation and continued until end of experiment. αPD-1 delivered on day 15, 19 and 23) | 1, 3 | [34] |
| Gomes-Santos et al., USA, 2021 | Preclinical Mouse tumour model (total n not explicity specified) | To determine whether CD8+ T-cells mediate effect of exercise on tumour control, and to examine effect of exercise on response to immunotherapy. | Breast cancer | αPD-1 ± αCTLA-4 | Aerobic (treadmill running). Moderate-vigorous intensity. 30–45 min per session. 5 days per week for 1–2 weeks. | Supervised | Yes (Exercise and ICI interventions initiated concomitantly when tumours reached prespecified volume) | 1, 2, 3 | [35] |
| Turbitt et al., USA, 2017 | Preclinical Mouse tumour model (n = 64) | To determine whether preventing weight gain (through diet and exercise interventions) improves responses to whole tumour cell vaccine and PD-1 checkpoint blockade. | Breast cancer | αPD-1 (±whole tumour cell vaccine) | Aerobic (voluntary wheel running). Range of distance run not reported. Continued for 8 weeks + 35 days. Delivered alongside a dietary intervention (10% reduction in calorie intake). | Unsupervised | Yes (8 weeks of pre-implant exercise plus 35 days of post-implant exercise. Whole tumour cell vaccine initiated 7 days post-implant and αPD-1 initiated 9–12 days post-implant) | 1, 3 | [36] 2 |
| Unterrainer et al., Denmark, 2018 | Preclinical Mouse tumour model (n not provided) | To determine whether exercise enhances responses to ICIs given an exercise-dependent increase in intratumoural immune cell infiltration. | Melanoma | αPD-L1 | Aerobic (voluntary wheel running). Ranged from 1.8–5.4 km per day for 4 weeks. | Unsupervised | No (4 weeks of pre-implant exercise followed by αPD-L1, which was initiated 4 days post-implant) | 1, 3 | [37] 2 |
| Hyatt et al., Australia, 2019 | Observational (survey) Patients with unresectable stage 3/4 melanoma receiving immunotherapy (n = 55) | To describe the levels of fatigue, exercise behaviours, and barriers/facilitators to engaging with exercise in patients receiving immunotherapy for melanoma. | Melanoma | Mixed (n = 8 known to have received αPD-1 or αCTLA-4, however no data provided on remainder of cohort) | Mixed (exercise regimes were individual to each patient and were aerobic or a combination of aerobic/resistance). Of those who exercised during immunotherapy treatment (n = 31), most spent <60 min per session and exercised <5 times per week. | Mixed | Yes (Participants were asked to recall the impact of exercise, if undertaken, whilst they were undergoing immunotherapy) | 2 | [38] |
| Lacey et al., Australia, 2019 | Quasi-experimental (pre-post test) Patients with metastatic melanoma receiving pembrolizumab (n = 28) | To assess the feasibility of a multimodal supportive care intervention in patients with metastatic melanoma receiving pembrolizumab. | Melanoma | αPD-1 | Mixed (exercise regimes were individual to each patient and were aerobic, resistance, or a combination of modes). 16 exercise sessions over 8 weeks. Delivered as part of a wider holistic supportive care program (including dietary advice, non-invasive complementary therapies and psychological consultation). | Mixed | Yes (Exercise intervention prospectively delivered in participants who were also undergoing ICI treatment) | 2 | [39] |
| Bay et al., Denmark, 2020 | Preclinical Mouse tumour model (n = 112) | To determine whether voluntary wheel running leads to increased expression of checkpoint molecules and achieves an additive effect when combined with ICIs. | Mixed (Melanoma, breast cancer, LLC, HNSCC) | αPD-1 or αPD-L1 | Aerobic (voluntary wheel running). Ranged from 4–8 km per day (in αPD-1 experiment) or 0.9–5.8 km per day (in αPD-L1 experiment) for 5 weeks. | Unsupervised | No (5 weeks of pre-implant exercise followed by αPD-1 or αPD-L1, which was initiated 4 days post-implant) | 1, 3 | [40] |
| Buss et al., New Zealand, 2021 | Preclinical Mouse tumour model (n = 104) | To determine whether exercise after introduction of cancer cells enhances efficacy of concurrent αPD-1 treatment and increases infiltration of cytotoxic immune cells, improves perfusion, and reduces hypoxia. | Mixed (Melanoma, breast cancer) | αPD-1 | Aerobic (voluntary wheel running). Range of distance run not reported. Continued until tumours reached maximum ethical size (2–5 weeks, median 19 days) | Unsupervised | Yes (Exercise and αPD-1 interventions initiated post-implant and continued until tumours reached maximum ethical size) | 1, 3 | [41] |
| Charles et al., France, 2021 | Quasi-experimental (pre-post test) Patients with cancer undergoing treatment with ICIs, and presenting with moderate to severe fatigue (n = 16) | Firstly, to determine whether a 6-month videoconference programme promoting exercise is feasible in patients with cancer undergoing immunotherapy and, secondly, to assess whether exercise reduces patients’ fatigue. | Mixed (melanoma, lung cancer, other) | αPD-1 | Mixed (combination of aerobic, resistance, other). Aimed for at least 150 min of exercise per week for 6 months. | Mixed | Yes (Exercise intervention prospectively delivered in participants who were also undergoing ICI treatment) | 2 | [42] |
| Authors | Outcome Measure(s) | Relevant Findings | Ref. |
|---|---|---|---|
| Martin-Ruiz et al. | Final tumour volume, percentage change in tumour volume, necrotic index, cell proliferation index, apoptotic index | Exercise, but not ICI treatment (αPD-1), alone significantly suppressed tumour growth. Combining ICI treatment with exercise did not result in a significant difference in tumour growth, necrosis, apoptosis, or cell proliferation compared to ICI intervention alone. | [33] |
| Wennerberg et al. | Tumour volume over time, final tumour volume, metastatic burden in lungs | Exercise alone and ICI treatment (αPD-1+RT) alone significantly suppressed tumour growth. Moreover, combining ICI treatment with exercise further enhanced the response, with significantly slowed tumour growth compared to ICI treatment alone. | [34] |
| Gomes-Santos et al. | Tumour volume over time, final tumour volume | Exercise alone significantly suppressed tumour growth (according to final tumour volume), however ICI treatment (αPD-1 ± αCTLA-4) did not. Combining ICI treatment with exercise sensitised established breast cancer tumours to ICIs, with significantly greater tumour suppression compared to ICI treatment alone. | [35] |
| Turbitt et al. | Final tumour volume, final tumour weight, metastatic burden in lungs | The weight management intervention (exercise + calorie restriction) alone suppressed tumour growth. Compared to the weight management intervention alone, no statistically significant benefit on tumour control or metastatic burden was observed when combined with αPD-1 or αPD-1 + whole tumour cell vaccine, respectively. | [36] 1 |
| Unterrainer et al. | Tumour growth (not otherwise specified) | Exercise alone had an overall suppressive effect. Combining ICI treatment (αPD-L1) and exercise appeared to suppress tumour growth more than exercise alone, but this was not a statistically significant effect. | [37] 1 |
| Bay et al. | Tumour volume over time, final tumour weight | Exercise, but not ICI treatment (αPD-1 or αPD-L1), alone significantly suppressed tumour growth. Combining ICI treatment and exercise did not result in a statistically significant additive effect on tumour control compared to exercise alone. A non-significant trend towards greater tumour control with αPD-1+exercise compared to αPD-1 alone was observed. | [40] |
| Buss et al. | Tumour growth (not otherwise specified) | Exercise alone, ICI treatment (αPD-1) alone, nor their combination significantly altered tumour growth rate compared to sedentary, isotype control treated mice. | [41] |
| Authors | Outcome Measure(s) | Relevant Findings | Ref. |
|---|---|---|---|
| Serrano & Hagar 1 | Cytotoxicity parameter in model (surrogate for treatment related adverse effects) | Virtual patients who were aerobically fit were more likely to experience adverse effects when receiving the same dose of ICI as compared to sedentary patients. | [32] |
| Gomes-Santos et al. 1 | Exercise capacity (surrogate for fatigue) | Mice with established cancer undergoing ICI treatment alone (αPD-1 ± αCTLA-4) had significantly reduced exercise capacity (i.e., increased fatigue) compared to normal controls. However, combining ICI treatment with an exercise intervention restored exercise capacity (i.e., prevented the increase in fatigue). | [35] |
| Hyatt et al. | Patient-reported free-text responses to survey questions | In patients who exercised during immunotherapy (n = 31), many reported benefits of exercise with the most common being reduced treatment-related fatigue. Other benefits included increased energy, improved overall wellbeing, improved sleep, and improved mental health. | [38] |
| Lacey et al. | PROM questionnaires | In patients who carried out exercise (as part of a wider holistic supportive care intervention) alongside ICI treatment (αPD-1) (n = 13), a clinically meaningful improvement in memory but worsening in dry mouth was observed where comparing post- vs. pre- supportive care intervention. There was no clinically meaningful change in anxiety/depression or general HRQoL. | [39] |
| Charles et al. | PROM questionnaires | For patients receiving ICIs (αPD-1) who completed the pre- and post- exercise intervention assessments (n = 13), a significant improvement in fatigue was observed as well as improvement in overall perception of physical and mental health. | [42] |
| Authors | Outcome Measure(s) | Relevant Findings | Ref. |
|---|---|---|---|
| Martin-Ruiz et al. | TIME composition (all leukocytes, neutrophils, monocytes/eosinophils), expression of murine genes relating to the immune system | Combining ICI treatment (αPD-1) with exercise did not result in a significant difference in intratumoural leukocyte, neutrophil, or monocyte/eosinophil infiltration when compared to ICI treatment alone. ICI treatment alone, or in combination with exercise, resulted in significantly increased intratumoural vegf-a expression compared to exercise alone. | [33] |
| Wennerberg et al. | TIME composition (MDSCs), splenic composition (CD8+ T-cells and NK cells), expression of Ki67, CD69, PD-1 | Combining ICI treatment (αPD-1+RT) with exercise significantly reduced intratumoural MDSC infiltration, increased splenic NK cell infiltration, and reduced PD-1 expression on splenic NK and T cells compared to ICI treatment alone. | [34] |
| Gomes-Santos et al. | TIME composition (CD8+ T-cells, activated CD8+ T-cells) | Combining ICI treatment (αPD-1 ± αCTLA-4) with exercise synergistically increased the proportion of activated CD8+ T-cells compared to ICI treatment alone. | [35] |
| Turbitt et al. | Splenic composition (MDSCs) | Combining ICI treatment (αPD-1 + whole tumour cell vaccine) with a weight management intervention (exercise + calorie restriction) did not significantly affect splenic MDSC infiltration compared to the weight management intervention alone. | [36] 1 |
| Unterrainer et al. | TIME composition (cytotoxic NK cells and T-cells), expression of immune checkpoint molecules | Exercise alone increased intratumoural expression of immune checkpoint molecules (PD-L1, B7.1, B7.2, PD-1, CD28) and infiltration of cytotoxic NK and T-cells (p-value not provided). Combining ICI treatment (αPD-L1) with exercise enhanced intratumoural expression of immune checkpoint molecules and receptors compared to exercise alone (p-value not provided). | [37] 1 |
| Bay et al. | Expression of immune checkpoint molecules, spleen weight, killing capacity of PBMCs | Exercise alone significantly increased intratumoural expression of immune checkpoint molecules (PD1, PD-L1, PD-L2, B7.1, B7.2 and CD28) in the melanoma mouse model. Only PD-L1 and CD28 were upregulated in the LLC model, and none were upregulated in the breast cancer model. Combining ICI treatment (αPD-1 or αPD-L1) with exercise showed no significant difference in spleen weight or PMBC killing capacity compared to ICI treatment or exercise alone. | [40] |
| Buss et al. | TIME infiltration (NK cells and T-cells), hypoxia, tumour perfusion | Combining ICI treatment (αPD-1) with exercise significantly reduced absolute numbers of tumour-infiltrating CD8+ cells (in breast tumours) but significantly increased the relative number of CD8+ cells (in melanomas) compared to treatment with ICIs alone. | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handford, J.; Chen, M.; Rai, R.; Moss, C.L.; Enting, D.; Peat, N.; Karagiannis, S.N.; Van Hemelrijck, M.; Russell, B. Is There a Role for Exercise When Treating Patients with Cancer with Immune Checkpoint Inhibitors? A Scoping Review. Cancers 2022, 14, 5039. https://doi.org/10.3390/cancers14205039
Handford J, Chen M, Rai R, Moss CL, Enting D, Peat N, Karagiannis SN, Van Hemelrijck M, Russell B. Is There a Role for Exercise When Treating Patients with Cancer with Immune Checkpoint Inhibitors? A Scoping Review. Cancers. 2022; 14(20):5039. https://doi.org/10.3390/cancers14205039
Chicago/Turabian StyleHandford, Jasmine, Miaoqi Chen, Ridesh Rai, Charlotte L. Moss, Deborah Enting, Nicola Peat, Sophia N. Karagiannis, Mieke Van Hemelrijck, and Beth Russell. 2022. "Is There a Role for Exercise When Treating Patients with Cancer with Immune Checkpoint Inhibitors? A Scoping Review" Cancers 14, no. 20: 5039. https://doi.org/10.3390/cancers14205039
APA StyleHandford, J., Chen, M., Rai, R., Moss, C. L., Enting, D., Peat, N., Karagiannis, S. N., Van Hemelrijck, M., & Russell, B. (2022). Is There a Role for Exercise When Treating Patients with Cancer with Immune Checkpoint Inhibitors? A Scoping Review. Cancers, 14(20), 5039. https://doi.org/10.3390/cancers14205039








