Fluorescent Lymphography-Guided Lymphadenectomy during Minimally Invasive Completion Total Gastrectomy for Remnant Gastric Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Endoscopic Injection of ICG
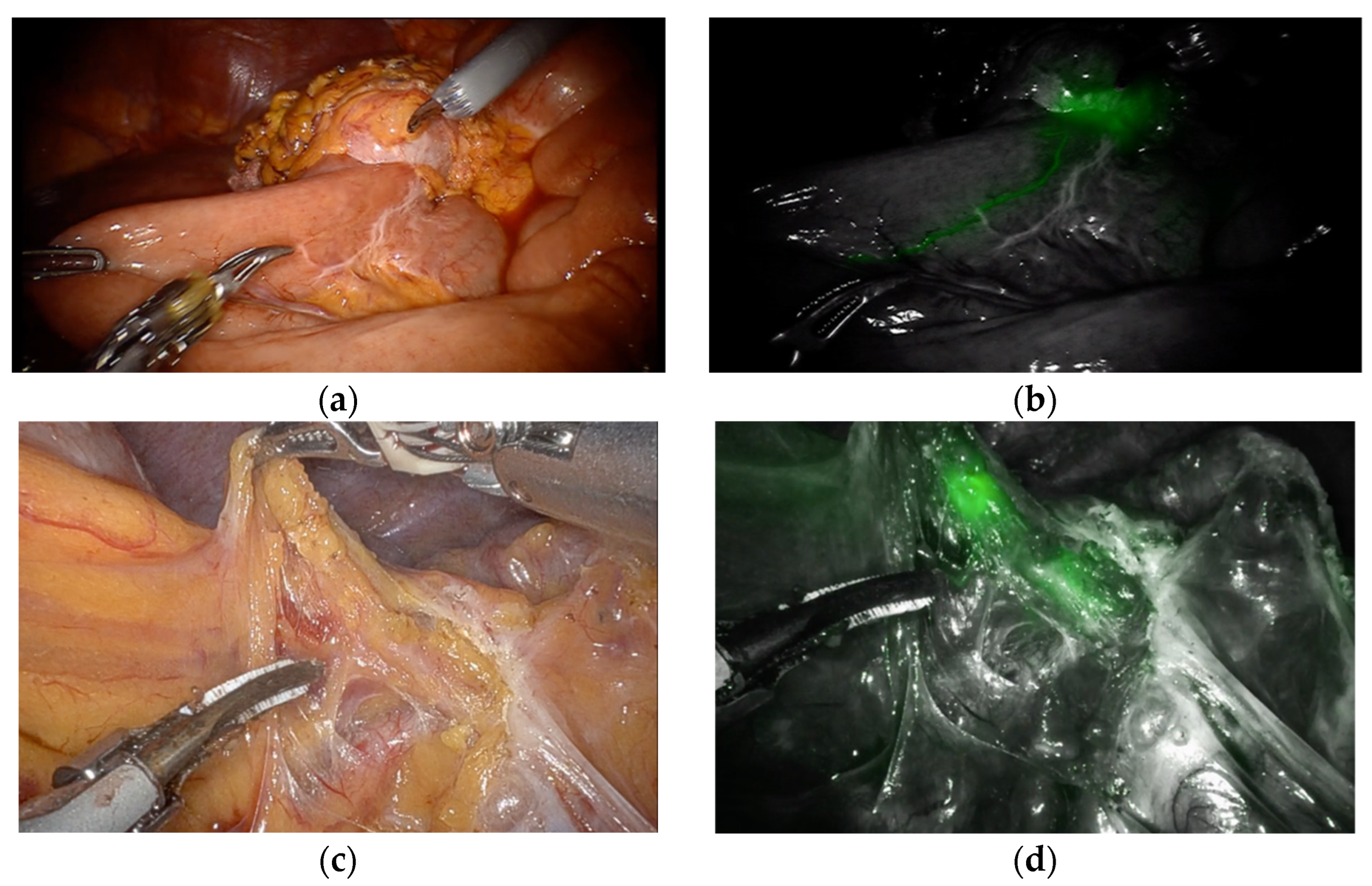
2.3. Surgical Procedures Including Fluorescent Lymphography-Guided Lymphadenectomy
2.4. Lymph Node Retrieval and Examination
2.5. Statistical Analysis
3. Results
- Comparison of perioperative outcomes;
- Comparison of lymph node retrieval;
- Diagnostic accuracy of fluorescent lymphography-guided lymphadenectomy;
- Comparison of survival.
3.1. Comparison of Perioperative Outcomes
3.2. Comparison of Lymph Node Retrieval
3.3. Diagnostic Accuracy of Fluorescent Lymphography-Guided Lymphadenectomy
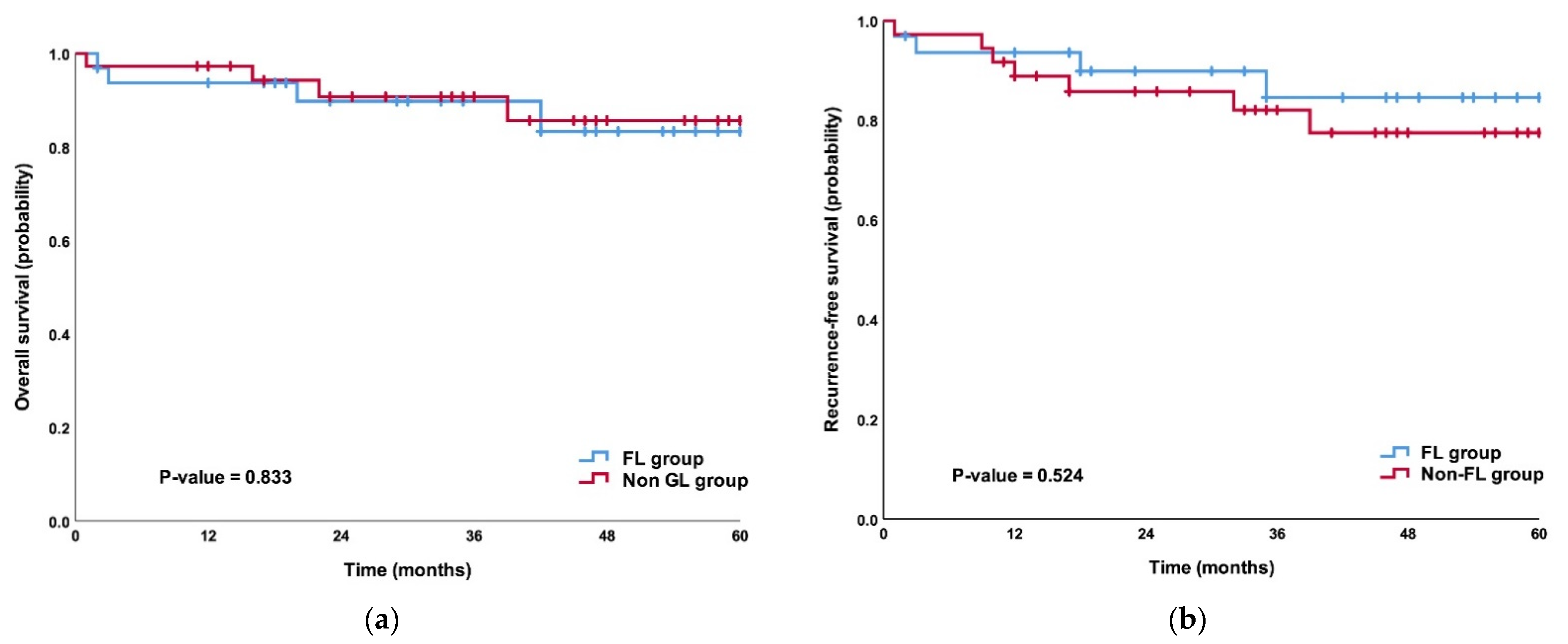
3.4. Comparison of Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mezhir, J.J.; Gonen, M.; Ammori, J.B.; Strong, V.E.; Brennan, M.F.; Coit, D.G. Treatment and outcome of patients with gastric remnant cancer after resection for peptic ulcer disease. Ann. Surg. Oncol. 2011, 18, 670–676. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd english edition. Gastric Cancer 2011, 14, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Kodera, Y.; Yamamura, Y.; Torii, A.; Uesaka, K.; Hirai, T.; Yasui, K.; Morimoto, T.; Kato, T.; Kito, T. Gastric remnant carcinoma after partial gastrectomy for benign and malignant gastric lesions. J. Am. Coll. Surg. 1996, 182, 1–6. [Google Scholar] [PubMed]
- Takeno, S.; Noguchi, T.; Kimura, Y.; Fujiwara, S.; Kubo, N.; Kawahara, K. Early and late gastric cancer arising in the remnant stomach after distal gastrectomy. Eur. J. Surg. Oncol. (EJSO) 2006, 32, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, M.; Katai, H.; Fukagawa, T.; Gotoda, T.; Sano, T.; Sasako, M. Cancer of the gastric stump following distal gastrectomy for cancer. Br. J. Surg. 2007, 94, 92–95. [Google Scholar] [CrossRef]
- Hanyu, T.; Wakai, A.; Ishikawa, T.; Ichikawa, H.; Kameyama, H.; Wakai, T. Carcinoma in the remnant stomach during long-term follow-up after distal gastrectomy for gastric cancer: Analysis of cumulative incidence and associated risk factors. World J. Surg. 2018, 42, 782–787. [Google Scholar] [CrossRef]
- Komatsu, S.; Ichikawa, D.; Okamoto, K.; Ikoma, D.; Tsujiura, M.; Nishimura, Y.; Murayama, Y.; Shiozaki, A.; Ikoma, H.; Kuriu, Y.; et al. Progression of remnant gastric cancer is associated with duration of follow-up following distal gastrectomy. World J. Gastroenterol. 2012, 18, 2832–2836. [Google Scholar]
- Kaneko, K.; Kondo, H.; Saito, D.; Shirao, K.; Yamaguchi, H.; Yokota, T.; Yamao, G.; Sano, T.; Sasako, M.; Yoshida, S. Early gastric stump cancer following distal gastrectomy. Gut 1998, 43, 342–344. [Google Scholar] [CrossRef][Green Version]
- Di Leo, A.; Pedrazzani, C.; Bencivenga, M.; Coniglio, A.; Rosa, F.; Morgani, P.; Marrelli, D.; Marchet, A.; Cozzaglio, L.; Giacopuzzi, S.; et al. Gastric stump cancer after distal gastrectomy for benign disease: Clinicopathological features and surgical outcomes. Ann. Surg. Oncol. 2014, 21, 2594–2600. [Google Scholar] [CrossRef]
- Sinning, C.; Schaefer, N.; Standop, J.; Hirner, A.; Wolff, M. Gastric stump carcinoma—Epidemiology and current concepts in pathogenesis and treatment. Eur. J. Surg. Oncol. 2007, 33, 133–139. [Google Scholar] [CrossRef]
- Ohashi, M.; Morita, S.; Fukagawa, T.; Kushima, R.; Katai, H. Surgical treatment of non-early gastric remnant carcinoma developing after distal gastrectomy for gastric cancer. J. Surg. Oncol. 2015, 111, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, R.; Liang, H.; Liu, H.; Quan, J.; Zhao, J. The pattern of lymph node metastasis and the suitability of 7th uicc n stage in predicting prognosis of remnant gastric cancer. J. Cancer Res. Clin. Oncol. 2012, 138, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Ichikawa, D.; Okamoto, K.; Ikoma, D.; Tsujiura, M.; Shiozaki, A.; Fujiwara, H.; Murayama, Y.; Kuriu, Y.; Ikoma, H.; et al. Differences of the lymphatic distribution and surgical outcomes between remnant gastric cancers and primary proximal gastric cancers. J. Gastrointest. Surg. 2012, 16, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, N.; Nomura, E.; Niki, M.; Shinohara, H.; Nishiguchi, K.; Okuzawa, M.; Toyoda, M.; Morita, S. Clinical study to identify specific characteristics of cancer newly developed in the remnant stomach. Gastric Cancer 2002, 5, 23–28. [Google Scholar] [CrossRef]
- Kano, K.; Yamada, T.; Yamamoto, K.; Komori, K.; Watanabe, H.; Takahashi, K.; Maezawa, Y.; Fujikawa, H.; Numata, M.; Aoyama, T.; et al. Evaluation of lymph node staging systems as independent prognosticators in remnant gastric cancer patients with an insufficient number of harvested lymph nodes. Ann. Surg. Oncol. 2021, 28, 2866–2876. [Google Scholar] [CrossRef] [PubMed]
- Son, S.Y.; Kong, S.H.; Ahn, H.S.; Park, Y.S.; Ahn, S.H.; Suh, Y.S.; Park, D.J.; Lee, H.J.; Kim, H.H.; Yang, H.K. The value of n staging with the positive lymph node ratio, and splenectomy, for remnant gastric cancer: A multicenter retrospective study. J. Surg. Oncol. 2017, 116, 884–893. [Google Scholar] [CrossRef]
- Roh, C.K.; Choi, S.; Seo, W.J.; Cho, M.; Son, T.; Kim, H.I.; Hyung, W.J. Indocyanine green fluorescence lymphography during gastrectomy after initial endoscopic submucosal dissection for early gastric cancer. Br. J. Surg. 2020, 107, 712–719. [Google Scholar] [CrossRef]
- Kwon, I.G.; Son, T.; Kim, H.I.; Hyung, W.J. Fluorescent lymphography-guided lymphadenectomy during robotic radical gastrectomy for gastric cancer. JAMA Surg. 2019, 154, 150–158. [Google Scholar] [CrossRef]
- Lee, S.; Song, J.H.; Choi, S.; Cho, M.; Kim, Y.M.; Kim, H.I.; Hyung, W.J. Fluorescent lymphography during minimally invasive total gastrectomy for gastric cancer: An effective technique for splenic hilar lymph node dissection. Surg. Endosc. 2022, 36, 2914–2924. [Google Scholar] [CrossRef]
- Jung, M.K.; Cho, M.; Roh, C.K.; Seo, W.J.; Choi, S.; Son, T.; Kim, H.I.; Hyung, W.J. Assessment of diagnostic value of fluorescent lymphography-guided lymphadenectomy for gastric cancer. Gastric Cancer 2021, 24, 515–525. [Google Scholar] [CrossRef]
- Baiocchi, G.L.; Molfino, S.; Molteni, B.; Quarti, L.; Arcangeli, G.; Manenti, S.; Arru, L.; Botticini, M.; Gheza, F. Fluorescence-guided lymphadenectomy in gastric cancer: A prospective western series. Updates Surg. 2020, 72, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Cianchi, F.; Indennitate, G.; Paoli, B.; Ortolani, M.; Lami, G.; Manetti, N.; Tarantino, O.; Messeri, S.; Foppa, C.; Badii, B.; et al. The clinical value of fluorescent lymphography with indocyanine green during robotic surgery for gastric cancer: A matched cohort study. J. Gastrointest. Surg. 2020, 24, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.G.; Cho, I.; Guner, A.; Choi, Y.Y.; Shin, H.B.; Kim, H.I.; An, J.Y.; Cheong, J.H.; Noh, S.H.; Hyung, W.J. Minimally invasive surgery for remnant gastric cancer: A comparison with open surgery. Surg. Endosc. 2014, 28, 2452–2458. [Google Scholar] [CrossRef] [PubMed]
- Alhossaini, R.M.; Altamran, A.A.; Cho, M.; Roh, C.K.; Seo, W.J.; Choi, S.; Son, T.; Kim, H.I.; Hyung, W.J. Lower rate of conversion using robotic-assisted surgery compared to laparoscopy in completion total gastrectomy for remnant gastric cancer. Surg. Endosc. 2020, 34, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kim, J.Y.; Kim, S.; Choi, W.H.; Cheong, J.H.; Hyung, W.J.; Choi, S.H.; Noh, S.H. Laparoscopic completion total gastrectomy in remnant gastric cancer: Technical detail and experience of two cases. Hepatogastroenterology 2009, 56, 1249–1252. [Google Scholar]
- Son, T.; Lee, J.H.; Kim, Y.M.; Kim, H.-I.; Noh, S.H.; Hyung, W.J. Robotic spleen-preserving total gastrectomy for gastric cancer: Comparison with conventional laparoscopic procedure. Surg. Endosc. 2014, 28, 2606–2615. [Google Scholar] [CrossRef]
- Kim, Y.M.; Son, T.; Kim, H.I.; Noh, S.H.; Hyung, W.J. Robotic d2 lymph node dissection during distal subtotal gastrectomy for gastric cancer: Toward procedural standardization. Ann. Surg. Oncol. 2016, 23, 2409–2410. [Google Scholar] [CrossRef]
- Song, J.; Oh, S.J.; Kang, W.H.; Hyung, W.J.; Choi, S.H.; Noh, S.H. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: Lessons learned from an initial 100 consecutive procedures. Ann. Surg. 2009, 249, 927–932. [Google Scholar] [CrossRef]
- Katai, H.; Ishikawa, T.; Akazawa, K.; Fukagawa, T.; Isobe, Y.; Miyashiro, I.; Oda, I.; Tsujitani, S.; Ono, H.; Tanabe, S.; et al. Optimal extent of lymph node dissection for remnant advanced gastric carcinoma after distal gastrectomy: A retrospective analysis of more than 3000 patients from the nationwide registry of the japanese gastric cancer association. Gastric Cancer 2020, 23, 1091–1101. [Google Scholar] [CrossRef]
- Sugita, H.; Oda, E.; Hirota, M.; Ishikawa, S.; Tomiyasu, S.; Tanaka, H.; Arita, T.; Yagi, Y.; Baba, H. Significance of lymphadenectomy with splenectomy in radical surgery for advanced (pt3/pt4) remnant gastric cancer. Surgery 2016, 159, 1082–1089. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef] [PubMed]

| FL Group (n = 32) | Non-FL Group (n = 36) | p-Value | |
|---|---|---|---|
| Age (years), mean (SD) | 62.5 (14.7) | 59.9 (14.1) | 0.452 |
| Sex | 0.265 | ||
| Male | 21 (65.6) | 28 (77.8) | |
| Female | 11 (34.4) | 8 (22.2) | |
| BMI (kg/m2), median (IQR) | 21.9 (6.3) | 21.4 (3.9) | 0.636 |
| ASA classification | 0.622 | ||
| I | 2 (6.3) | 6 (16.7) | |
| II | 17 (53.1) | 17 (47.2) | |
| III | 11 (34.4) | 11 (30.6) | |
| IV | 2 (6.3) | 2 (5.6) | |
| Prior gastrectomy | 0.890 | ||
| STG with BI | 10 (31.3) | 11 (30.6) | |
| STG with BII | 21 (65.6) | 25 (69.4) | |
| STG with RY GJ | 1 (3.1) | 0 | |
| Operation method of prior gastrectomy | 0.226 | ||
| Open | 13 (40.6) | 18 (50.0) | |
| Laparoscopy | 9 (28.1) | 13 (36.1) | |
| Robot | 10 (31.3) | 5 (13.9) | |
| Cause of prior gastrectomy | 0.245 | ||
| Cancer | 24 (75.0) | 31 (86.1) | |
| Peptic ulcer | 8 (25.0) | 5 (13.9) | |
| Clinical T classification † | 0.829 | ||
| T1 | 25 (78.1) | 31 (86.1) | |
| T2 | 4 (12.5) | 3 (8.3) | |
| T3 | 2 (6.3) | 1 (2.8) | |
| T4 | 1 (3.1) | 1 (2.8) | |
| Clinical N classification † | 0.660 | ||
| N0 | 29 (90.6) | 34 (94.4) | |
| N+ | 3 (9.4) | 2 (5.6) | |
| Tumor size (mm), mean (SD) | 35.7 (19.5) | 31.2 (18.9) | 0.338 |
| Pathologic T stage † | 0.754 | ||
| T1 | 21(65.6) | 20(55.6) | |
| T2 | 4(12.5) | 5(13.9) | |
| T3 | 2(6.3) | 5 (13.9) | |
| T4a | 5(15.6) | 6(16.7) | |
| Pathologic N stage † | 0.541 | ||
| N0 | 28 (87.5) | 28 (77.8) | |
| N1 | 3 (9.4) | 3 (8.3) | |
| N2 | 1 (3.1) | 2 (5.6) | |
| N3 | 0 | 3 (8.3) |
| FL Group (n = 32) | Non-FL Group (n = 36) | p-Value | |
|---|---|---|---|
| Operation method | 0.001 | ||
| Laparoscopy | 12 (37.5) | 28 (77.8) | |
| Robot | 20 (62.5) | 8 (22.2) | |
| Combined resection | 0.660 | ||
| No | 29(90.6) | 34 (94.4) | |
| Yes | 3 (9.4) | 2 (5.6) | |
| Operation time (minutes), mean (SD) | 273.8 (77.0) | 252.0 (58.2) | 0.190 |
| Estimated blood loss (mL), median (IQR) | 100 (52–154) | 115 (85–211) | 0.224 |
| Postoperative complications | 0.392 | ||
| Absent | 12 (37.5) | 10 (27.8) | |
| Present | 20 (62.5) | 26 (72.2) | |
| Clavien–Dindo Classification | 0.466 | ||
| Grade I | 8 (40.0) | 6 (23.1) | |
| Grade II | 9 (45.0) | 16 (61.5) | |
| ≥Grade IIIa | 3 (15.0) | 4 (15.4) | |
| Postoperative mortality | 0 | 0 | 0 |
| Hospital stays (days), median (IQR) | 6 (5–10.5) | 7.5 (6–15) | 0.099 |
| Patients | FL Group (n = 32) | Non-FL Group (n = 36) | p-Value |
|---|---|---|---|
| Number of retrieved LN, median (IQR) | 17 (9.3–23.5) | 12.5 (4–17.8) | 0.016 |
| Number of retrieved LN in the patients who underwent prior gastrectomy due to cancer, median (IQR) | 15 (7.3–18) (n = 24) | 11 (4–15) (n = 31) | 0.056 |
| Number of retrieved LN in the patients who underwent prior gastrectomy due to peptic ulcer, median (IQR) | 28.5 (21–52.3) (n = 8) | 19 (17.5–24) (n = 5) | 0.067 |
| Number of cases that show total retrieved lymph nodes ≥ 16 (percentage) | 19 (59.4%) | 12 (33.3%) | 0.031 |
| Total retrieved lymph nodes <16 and metastatic lymph nodes ≥1 (percentage) | 0 | 6 (16.7%) | 0.026 |
| Total Number | Number of Metastasis | Number of Non-Metastasis | Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) | |
|---|---|---|---|---|---|---|---|
| Total LN stations | 146 | ||||||
| Fluorescent station | 114 | 3 | 111 | 75 | 21.8 | 2.6 | 96.9 |
| Non-fluorescent station | 32 | 1 | 31 | ||||
| Total LNs | 630 | ||||||
| Fluorescent LNs | 455 | 4 | 451 | 44.4 | 27.4 | 0.9 | 97.1 |
| Non-fluorescent LNs | 175 | 5 | 170 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrashidi, N.; Kim, K.-Y.; Park, S.H.; Lee, S.; Cho, M.; Kim, Y.M.; Kim, H.-I.; Hyung, W.J. Fluorescent Lymphography-Guided Lymphadenectomy during Minimally Invasive Completion Total Gastrectomy for Remnant Gastric Cancer Patients. Cancers 2022, 14, 5037. https://doi.org/10.3390/cancers14205037
Alrashidi N, Kim K-Y, Park SH, Lee S, Cho M, Kim YM, Kim H-I, Hyung WJ. Fluorescent Lymphography-Guided Lymphadenectomy during Minimally Invasive Completion Total Gastrectomy for Remnant Gastric Cancer Patients. Cancers. 2022; 14(20):5037. https://doi.org/10.3390/cancers14205037
Chicago/Turabian StyleAlrashidi, Nasser, Ki-Yoon Kim, Sung Hyun Park, Sejin Lee, Minah Cho, Yoo Min Kim, Hyoung-Il Kim, and Woo Jin Hyung. 2022. "Fluorescent Lymphography-Guided Lymphadenectomy during Minimally Invasive Completion Total Gastrectomy for Remnant Gastric Cancer Patients" Cancers 14, no. 20: 5037. https://doi.org/10.3390/cancers14205037
APA StyleAlrashidi, N., Kim, K.-Y., Park, S. H., Lee, S., Cho, M., Kim, Y. M., Kim, H.-I., & Hyung, W. J. (2022). Fluorescent Lymphography-Guided Lymphadenectomy during Minimally Invasive Completion Total Gastrectomy for Remnant Gastric Cancer Patients. Cancers, 14(20), 5037. https://doi.org/10.3390/cancers14205037







