Radiotherapy-Related Gene Signature in Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Patients and Samples
2.2. Ethics Approval
2.3. Isolation of RNA
2.4. Oncomine Immune Response Research Assay
2.5. Gene Expression Analysis and Statistics
2.6. TCGA PRAD Analysis
3. Results
3.1. Sample Demographics
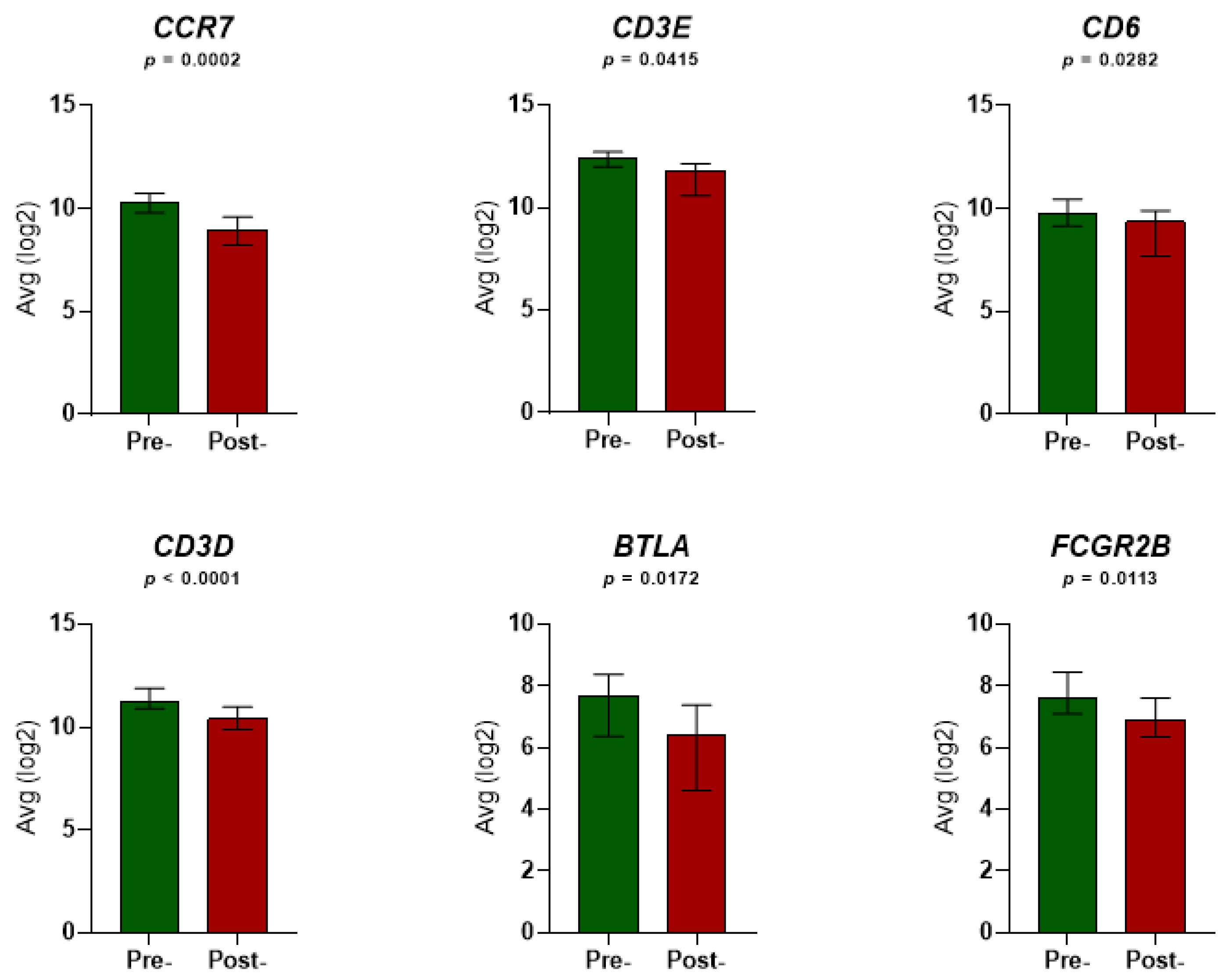
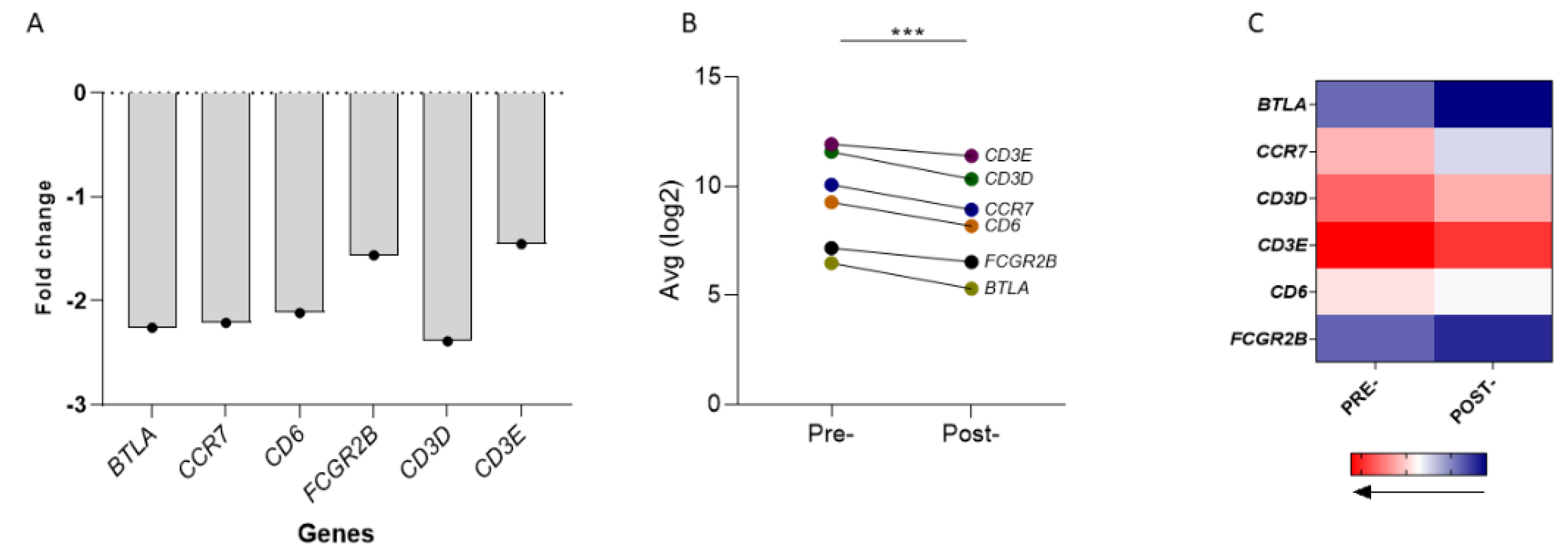
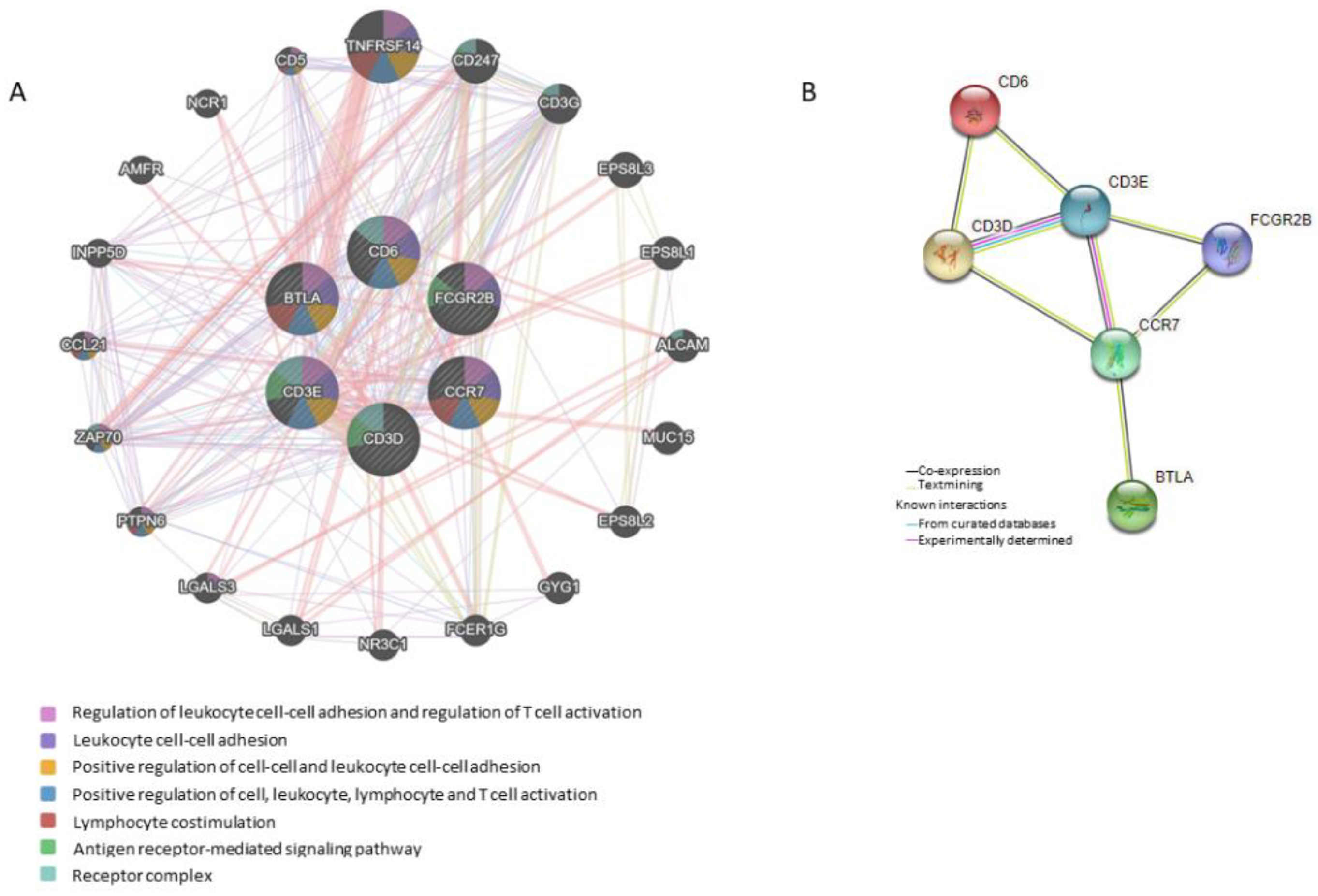
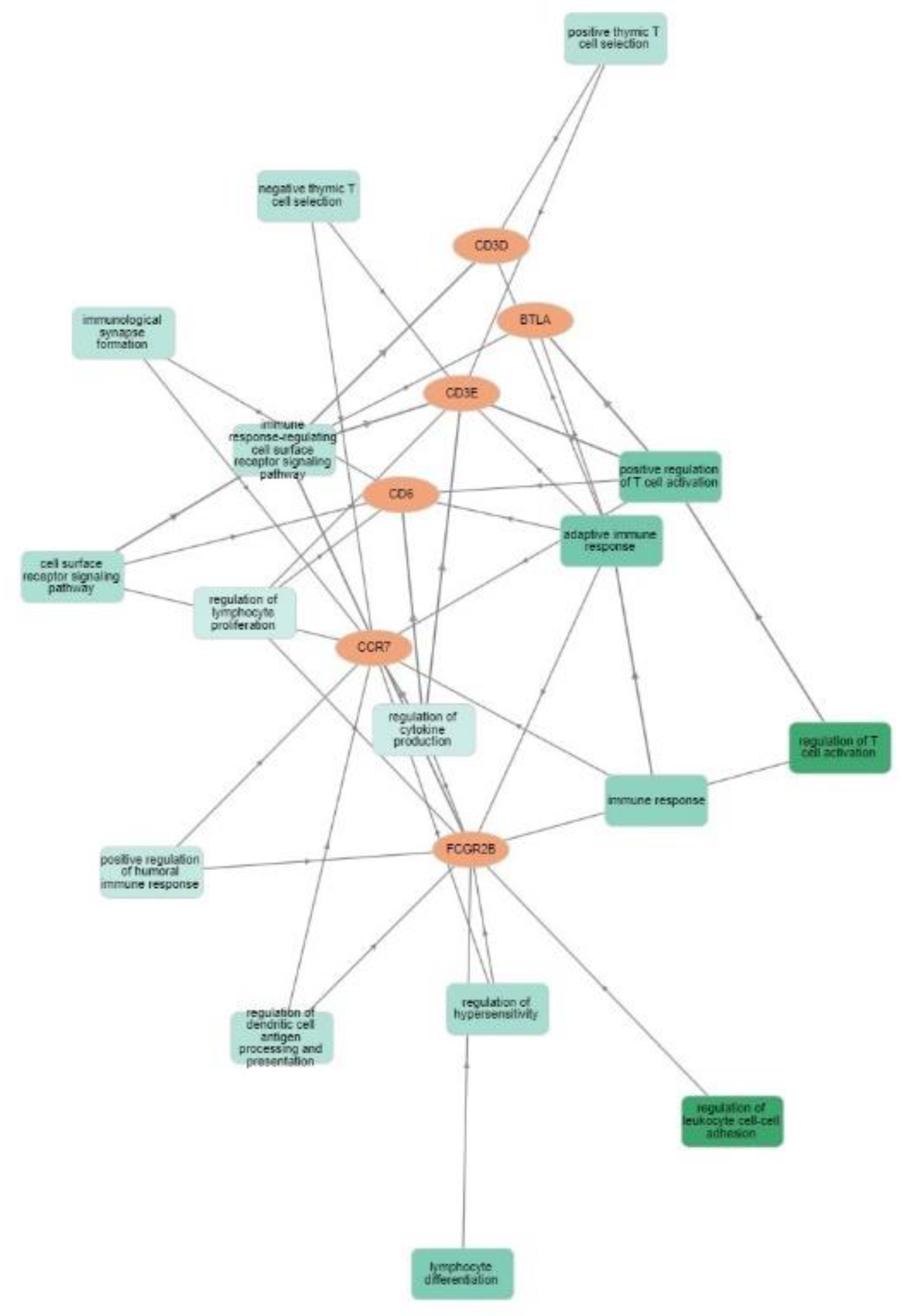
3.2. Gene Expression and Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Gross, S.; Rahal, R.; Stransky, N.; Lengauer, C.; Hoeflich, K.P. Targeting cancer with kinase inhibitors. J. Clin. Invest. 2015, 125, 1780–1789. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Podder, T.K.; Fredman, E.T.; Ellis, R.J. Advances in Radiotherapy for Prostate Cancer Treatment. Adv. Exp. Med. Biol. 2018, 1096, 31–47. [Google Scholar] [CrossRef]
- Twyman-Saint Victor, C.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Pilones, K.A.; Wennerberg, E.; Formenti, S.C.; Demaria, S. In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine 2015, 33, 7415–7422. [Google Scholar] [CrossRef]
- Carvalho, H.A.; Villar, R.C. Radiotherapy and immune response: The systemic effects of a local treatment. Clinics 2018, 73, e557s. [Google Scholar] [CrossRef]
- Derer, A.; Deloch, L.; Rubner, Y.; Fietkau, R.; Frey, B.; Gaipl, U.S. Radio-Immunotherapy-Induced Immunogenic Cancer Cells as Basis for Induction of Systemic Anti-Tumor Immune Responses—Pre-Clinical Evidence and Ongoing Clinical Applications. Front. Immunol. 2015, 6, 505. [Google Scholar] [CrossRef]
- Janopaul-Naylor, J.R.; Shen, Y.; Qian, D.C.; Buchwald, Z.S. The Abscopal Effect: A Review of Pre-Clinical and Clinical Advances. Int. J. Mol. Sci. 2021, 22, 11061. [Google Scholar] [CrossRef]
- Baxevanis, C.N.; Gritzapis, A.D.; Voutsas, I.F.; Batsaki, P.; Goulielmaki, M.; Adamaki, M.; Zoumpourlis, V.; Fortis, S.P. T-Cell Repertoire in Tumor Radiation: The Emerging Frontier as a Radiotherapy Biomarker. Cancers 2022, 14, 2674. [Google Scholar] [CrossRef]
- Wang, X.B.; Wu, D.J.; Chen, W.P.; Liu, J.; Ju, Y.J. Impact of radiotherapy on immunological parameters, levels of inflammatory factors, and clinical prognosis in patients with esophageal cancer. J. Radiat. Res. 2019, 60, 353–363. [Google Scholar] [CrossRef]
- Eckert, F.; Schaedle, P.; Zips, D.; Schmid-Horch, B.; Rammensee, H.G.; Gani, C.; Gouttefangeas, C. Impact of curative radiotherapy on the immune status of patients with localized prostate cancer. Oncoimmunology 2018, 7, e1496881. [Google Scholar] [CrossRef]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38, W214–W220. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Pomaznoy, M.; Ha, B.; Peters, B. GOnet: A tool for interactive Gene Ontology analysis. BMC Bioinform. 2018, 19, 470. [Google Scholar] [CrossRef]
- Tritschler, S.; Ganswindt, U.; Stief, C.G. Localized intermediate- to high-risk prostate cancer. Urol. A 2016, 55, 318–325. [Google Scholar] [CrossRef]
- Potters, L.; Morgenstern, C.; Calugaru, E.; Fearn, P.; Jassal, A.; Presser, J.; Mullen, E. 12-year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer. J. Urol. 2005, 173, 1562–1566. [Google Scholar] [CrossRef]
- Blasko, J.C.; Grimm, P.D.; Sylsvester, J.E.; Cavanagh, W. The role of external beam radiotherapy with I-125/Pd-103 brachytherapy for prostate carcinoma. Radiother. Oncol. 2000, 57, 273–278. [Google Scholar] [CrossRef]
- Liu, Y.; Patel, S.A.; Jani, A.B.; Gillespie, T.W.; Patel, P.R.; Godette, K.D.; Hershatter, B.W.; Shelton, J.W.; McDonald, M.W. Overall Survival After Treatment of Localized Prostate Cancer with Proton Beam Therapy, External-Beam Photon Therapy, or Brachytherapy. Clin. Genitourin. Cancer 2021, 19, 255–266. [Google Scholar] [CrossRef]
- Puche-Sanz, I.; Rodriguez-Martinez, A.; Garrido-Navas, M.C.; Robles-Fernandez, I.; Vazquez-Alonso, F.; Alvarez Cubero, M.J.; Lorente-Acosta, J.A.; Serrano-Fernandez, M.J.; Cozar-Olmo, J.M. Liquid biopsy and prostate cancer. Current evidence applied to clinical practice. Actas Urol. Esp. 2020, 44, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Salas, I.; Athie, A.; Boutros, P.C.; Del Re, M.; Miyamoto, D.T.; Pienta, K.J.; Posadas, E.M.; Sowalsky, A.G.; Stenzl, A.; Wyatt, A.W.; et al. Quantitative and Qualitative Analysis of Blood-based Liquid Biopsies to Inform Clinical Decision-making in Prostate Cancer. Eur. Urol. 2021, 79, 762–771. [Google Scholar] [CrossRef] [PubMed]
- De Kruijff, I.E.; Beije, N.; Martens, J.W.M.; de Wit, R.; Boormans, J.L.; Sleijfer, S. Liquid Biopsies to Select Patients for Perioperative Chemotherapy in Muscle-invasive Bladder Cancer: A Systematic Review. Eur. Urol. Oncol. 2021, 4, 204–214. [Google Scholar] [CrossRef]
- Yazgan, S.C.; Yekeduz, E.; Utkan, G.; Urun, Y. Prognostic role of pan-immune-inflammation value in patients with metastatic castration-resistant prostate cancer treated with androgen receptor-signaling inhibitors. Prostate 2022, 82, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Choueiri, T.K.; McDermott, D.F.; Powles, T.; Vano, Y.A.; Gupta, S.; Yao, J.; Han, C.; Ammar, R.; Papillon-Cavanagh, S.; et al. Biomarker analysis from CheckMate 214: Nivolumab plus ipilimumab versus sunitinib in renal cell carcinoma. J. Immunother. Cancer 2022, 10, e004316. [Google Scholar] [CrossRef] [PubMed]
- Cimadamore, A.; Massari, F.; Santoni, M.; Lopez-Beltran, A.; Cheng, L.; Scarpelli, M.; Montironi, R.; Moch, H. PD1 and PD-L1 Inhibitors for the Treatment of Kidney Cancer: The Role of PD-L1 Assay. Curr. Drug. Targets 2020, 21, 1664–1671. [Google Scholar] [CrossRef]
- Mateo, J.; Carreira, S.; Sandhu, S.; Miranda, S.; Mossop, H.; Perez-Lopez, R.; Nava Rodrigues, D.; Robinson, D.; Omlin, A.; Tunariu, N.; et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl. J. Med. 2015, 373, 1697–1708. [Google Scholar] [CrossRef]
- Cattrini, C.; Espana, R.; Mennitto, A.; Bersanelli, M.; Castro, E.; Olmos, D.; Lorente, D.; Gennari, A. Optimal Sequencing and Predictive Biomarkers in Patients with Advanced Prostate Cancer. Cancers 2021, 13, 4522. [Google Scholar] [CrossRef]
- Claps, F.; Mir, M.C.; Zargar, H. Molecular markers of systemic therapy response in urothelial carcinoma. Asian J. Urol. 2021, 8, 376–390. [Google Scholar] [CrossRef]
- Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Obeid, M.; Ortiz, C.; Criollo, A.; Mignot, G.; Maiuri, M.C.; Ullrich, E.; Saulnier, P.; et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007, 13, 1050–1059. [Google Scholar] [CrossRef]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N.; et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007, 13, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Auh, S.L.; Wang, Y.; Burnette, B.; Wang, Y.; Meng, Y.; Beckett, M.; Sharma, R.; Chin, R.; Tu, T.; et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood 2009, 114, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef]
- Wu, C.Y.; Yang, L.H.; Yang, H.Y.; Knoff, J.; Peng, S.; Lin, Y.H.; Wang, C.; Alvarez, R.D.; Pai, S.I.; Roden, R.B.; et al. Enhanced cancer radiotherapy through immunosuppressive stromal cell destruction in tumors. Clin. Cancer Res. 2014, 20, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.G.; Wanjari, U.R.; Prabakaran, D.S.; Ganesan, R.; Renu, K.; Dey, A.; Vellingiri, B.; Kandasamy, S.; Ramesh, T.; Gopalakrishnan, A.V. The Cellular and Molecular Immunotherapy in Prostate Cancer. Vaccines 2022, 10, 1370. [Google Scholar] [CrossRef]
- Lindner, D.; Arndt, C.; Loureiro, L.R.; Feldmann, A.; Kegler, A.; Koristka, S.; Berndt, N.; Mitwasi, N.; Bergmann, R.; Frenz, M.; et al. Combining Radiation- with Immunotherapy in Prostate Cancer: Influence of Radiation on T Cells. Int. J. Mol. Sci. 2022, 23, 7922. [Google Scholar] [CrossRef]
- Sharabi, A.B.; Lim, M.; DeWeese, T.L.; Drake, C.G. Radiation and checkpoint blockade immunotherapy: Radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015, 16, e498–e509. [Google Scholar] [CrossRef]
- Crittenden, M.; Kohrt, H.; Levy, R.; Jones, J.; Camphausen, K.; Dicker, A.; Demaria, S.; Formenti, S. Current clinical trials testing combinations of immunotherapy and radiation. Semin. Radiat. Oncol. 2015, 25, 54–64. [Google Scholar] [CrossRef]
- D’Auria, F.; Statuto, T.; Rago, L.; Montagna, A.; Castaldo, G.; Schiro, I.; Zeccola, A.; Virgilio, T.; Bianchino, G.; Traficante, A.; et al. Modulation of Peripheral Immune Cell Subpopulations After RapidArc/Moderate Hypofractionated Radiotherapy for Localized Prostate Cancer: Findings and Comparison With 3D Conformal/Conventional Fractionation Treatment. Front. Oncol. 2022, 12, 829812. [Google Scholar] [CrossRef]
- McGee, H.M.; Daly, M.E.; Azghadi, S.; Stewart, S.L.; Oesterich, L.; Schlom, J.; Donahue, R.; Schoenfeld, J.D.; Chen, Q.; Rao, S.; et al. Stereotactic Ablative Radiation Therapy Induces Systemic Differences in Peripheral Blood Immunophenotype Dependent on Irradiated Site. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1259–1270. [Google Scholar] [CrossRef]
- Chen, Y.; Shao, Z.; Jiang, E.; Zhou, X.; Wang, L.; Wang, H.; Luo, X.; Chen, Q.; Liu, K.; Shang, Z. CCL21/CCR7 interaction promotes EMT and enhances the stemness of OSCC via a JAK2/STAT3 signaling pathway. J. Cell Physiol. 2020, 235, 5995–6009. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Grochans, S.; Gutowska, I.; Barczak, K.; Baranowska-Bosiacka, I. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of Receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 Ligands. Int. J. Mol. Sci. 2020, 21, 7619. [Google Scholar] [CrossRef]
- Kunert, A.; Basak, E.A.; Hurkmans, D.P.; Balcioglu, H.E.; Klaver, Y.; van Brakel, M.; Oostvogels, A.A.M.; Lamers, C.H.J.; Bins, S.; Koolen, S.L.W.; et al. CD45RA(+)CCR7(-) CD8 T cells lacking co-stimulatory receptors demonstrate enhanced frequency in peripheral blood of NSCLC patients responding to nivolumab. J. Immunother. Cancer 2019, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Czystowska, M.; Gooding, W.; Szczepanski, M.J.; Lopez-Abaitero, A.; Ferris, R.L.; Johnson, J.T.; Whiteside, T.L. The immune signature of CD8(+)CCR7(+) T cells in the peripheral circulation associates with disease recurrence in patients with HNSCC. Clin. Cancer Res. 2013, 19, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Chi, B.J.; Du, C.L.; Fu, Y.F.; Zhang, Y.N.; Wang, R.W. Silencing of CCR7 inhibits the growth, invasion and migration of prostate cancer cells induced by VEGFC. Int. J. Clin. Exp. Pathol. 2015, 8, 12533–12540. [Google Scholar]
- Lim, S.H.; Vaughan, A.T.; Ashton-Key, M.; Williams, E.L.; Dixon, S.V.; Chan, H.T.; Beers, S.A.; French, R.R.; Cox, K.L.; Davies, A.J.; et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood 2011, 118, 2530–2540. [Google Scholar] [CrossRef]
- Farley, C.R.; Morris, A.B.; Tariq, M.; Bennion, K.B.; Potdar, S.; Kudchadkar, R.; Lowe, M.C.; Ford, M.L. FcgammaRIIB is a T cell checkpoint in antitumor immunity. JCI Insight 2021, 6, e135623. [Google Scholar] [CrossRef]
- Malissen, N.; Macagno, N.; Granjeaud, S.; Granier, C.; Moutardier, V.; Gaudy-Marqueste, C.; Habel, N.; Mandavit, M.; Guillot, B.; Pasero, C.; et al. HVEM has a broader expression than PD-L1 and constitutes a negative prognostic marker and potential treatment target for melanoma. Oncoimmunology 2019, 8, e1665976. [Google Scholar] [CrossRef]
- Migita, K.; Sho, M.; Shimada, K.; Yasuda, S.; Yamato, I.; Takayama, T.; Matsumoto, S.; Wakatsuki, K.; Hotta, K.; Tanaka, T.; et al. Significant involvement of herpesvirus entry mediator in human esophageal squamous cell carcinoma. Cancer 2014, 120, 808–817. [Google Scholar] [CrossRef]
- Lan, X.; Li, S.; Gao, H.; Nanding, A.; Quan, L.; Yang, C.; Ding, S.; Xue, Y. Increased BTLA and HVEM in gastric cancer are associated with progression and poor prognosis. Onco. Targets Ther. 2017, 10, 919–926. [Google Scholar] [CrossRef]
- Hokuto, D.; Sho, M.; Yamato, I.; Yasuda, S.; Obara, S.; Nomi, T.; Nakajima, Y. Clinical impact of herpesvirus entry mediator expression in human hepatocellular carcinoma. Eur. J. Cancer 2015, 51, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Aubert, N.; Brunel, S.; Olive, D.; Marodon, G. Blockade of HVEM for Prostate Cancer Immunotherapy in Humanized Mice. Cancers 2021, 13, 3009. [Google Scholar] [CrossRef] [PubMed]
- Derre, L.; Rivals, J.P.; Jandus, C.; Pastor, S.; Rimoldi, D.; Romero, P.; Michielin, O.; Olive, D.; Speiser, D.E. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J. Clin. Invest. 2010, 120, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.H. CD6 as a Cell Surface Receptor and As a Target for Regulating Immune Responses. Curr. Drug. Targets 2016, 17, 619–629. [Google Scholar] [CrossRef]
- Ruth, J.H.; Gurrea-Rubio, M.; Athukorala, K.S.; Rasmussen, S.M.; Weber, D.P.; Randon, P.M.; Gedert, R.J.; Lind, M.E.; Amin, M.A.; Campbell, P.L.; et al. CD6 is a target for cancer immunotherapy. JCI Insight 2021, 6, e145662. [Google Scholar] [CrossRef]
- Liang, C.; Peng, L.; Zeng, S.; Zhao, Q.; Tang, L.; Jiang, X.; Zhang, J.; Yan, N.; Chen, Y. Investigation of indoleamine 2,3-dioxygenase 1 expression in uveal melanoma. Exp. Eye Res. 2019, 181, 112–119. [Google Scholar] [CrossRef]
- Holmgaard, R.B.; Zamarin, D.; Li, Y.; Gasmi, B.; Munn, D.H.; Allison, J.P.; Merghoub, T.; Wolchok, J.D. Tumor-Expressed IDO Recruits and Activates MDSCs in a Treg-Dependent Manner. Cell Rep. 2015, 13, 412–424. [Google Scholar] [CrossRef]
- Saligan, L.N.; Lukkahatai, N.; Zhang, Z.J.; Cheung, C.W.; Wang, X.M. Altered Cd8+ T lymphocyte Response Triggered by Arginase 1: Implication for Fatigue Intensification during Localized Radiation Therapy in Prostate Cancer Patients. Neuropsychiatry 2018, 8, 1249–1262. [Google Scholar] [CrossRef]
- Gaffney, S.G.; Perry, E.B.; Chen, P.M.; Greenstein, A.; Kaech, S.M.; Townsend, J.P. The landscape of novel and complementary targets for immunotherapy: An analysis of gene expression in the tumor microenvironment. Oncotarget 2019, 10, 4532–4545. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Zhang, P.; Xu, C.; Liu, Z.; He, C.; Liu, Y.; Kang, Z. CXCL12 and CD3E as Indicators for Tumor Microenvironment Modulation in Bladder Cancer and Their Correlations with Immune Infiltration and Molecular Subtypes. Front. Oncol. 2021, 11, 636870. [Google Scholar] [CrossRef]
- Benonisson, H.; Altintas, I.; Sluijter, M.; Verploegen, S.; Labrijn, A.F.; Schuurhuis, D.H.; Houtkamp, M.A.; Verbeek, J.S.; Schuurman, J.; van Hall, T. CD3-Bispecific Antibody Therapy Turns Solid Tumors into Inflammatory Sites but Does Not Install Protective Memory. Mol. Cancer Ther. 2019, 18, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, C.; Xu, W.; Wu, Y.; Wang, J.; Chen, S.; Zhang, H.; Huang, H.; Huang, H.; Liu, W. Malignant Tumor Purity Reveals the Driven and Prognostic Role of CD3E in Low-Grade Glioma Microenvironment. Front. Oncol. 2021, 11, 676124. [Google Scholar] [CrossRef] [PubMed]
- Snyder, K.M.; Stock, R.G.; Hong, S.M.; Lo, Y.C.; Stone, N.N. Defining the risk of developing grade 2 proctitis following 125I prostate brachytherapy using a rectal dose-volume histogram analysis. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 335–341. [Google Scholar] [CrossRef]
- Deutsch, I.; Zelefsky, M.J.; Zhang, Z.; Mo, Q.; Zaider, M.; Cohen, G.; Cahlon, O.; Yamada, Y. Comparison of PSA relapse-free survival in patients treated with ultra-high-dose IMRT versus combination HDR brachytherapy and IMRT. Brachytherapy 2010, 9, 313–318. [Google Scholar] [CrossRef]
- Ishiyama, H.; Satoh, T.; Kitano, M.; Tabata, K.; Komori, S.; Ikeda, M.; Soda, I.; Kurosaka, S.; Sekiguchi, A.; Kimura, M.; et al. High-dose-rate brachytherapy and hypofractionated external beam radiotherapy combined with long-term hormonal therapy for high-risk and very high-risk prostate cancer: Outcomes after 5-year follow-up. J. Radiat. Res. 2014, 55, 509–517. [Google Scholar] [CrossRef]
- Makino, T.; Mizokami, A.; Namiki, M. Clinical outcomes of patients with localized and locally advanced prostate cancer undergoing high-dose-rate brachytherapy with external-beam radiotherapy at our institute. Anticancer Res. 2015, 35, 1723–1728. [Google Scholar]


| Patients with Localized PCa (n = 23) | |
|---|---|
| Characteristics of Initial PCa Staging | |
| Median Age at Diagnosis (Years) (Range) | 73 (53–81) |
| PSA | |
| Mean | 18.74 ng/mL |
| SD | 20.85 ng/mL |
| Range | 5.51–100.00 ng/mL |
| Gleason Score | |
| Mean | 7 |
| SD | 1 |
| Range | 6–9 |
| T | |
| T1c | 3 (13.0%) |
| T2a, T2b, T2c | 12 (52.2%) |
| T3a, T3b | 8 (34.8%) |
| Type of radiation therapy | |
| Primary | 17 (73.9%) |
| Adjuvant | 6 (26.1%) |
| External beam radiation therapy (EBRT) characteristics | |
| 3D Conformal Radiotherapy | |
| Median daily dose; Gy (range) | 2 (1.8–2.2) |
| Median total dose; Gy (range) | 70 (66–72) |
| Median Radiation treatment schedule; days (range) | 37 (35–38) |
| Univariate | PFI | ||
|---|---|---|---|
| p | Hazard Ratio | HR (95.0% CI) | |
| T status | 0.005 | 2.032 | 1.233–3.349 |
| Gleason Score | <0.001 | 2.389 | 1.621 |
| 6-gene Signature | 0.003 | 1.891 | 1.234–2.898 |
| Multivariate | PFI | ||
| p | Hazard Ratio | HR (95.0% CI) | |
| Model Before Stepwise Selection | |||
| T status | 0.312 | 1.324 | 0.768–2.281 |
| Gleason Score | <0.001 | 2.137 | 1.405–3.250 |
| 6-gene Signature | 0.007 | 1.808 | 1.179–2.772 |
| Model after Stepwise Selection | |||
| Gleason Score | <0.001 | 2.337 | 1.588–3.439 |
| 6-gene Signature | 0.006 | 1.828 | 1.193–2.802 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kogionou, P.; Fortis, S.P.; Goulielmaki, M.; Aubert, N.; Batsaki, P.; Ouzounis, S.; Cavouras, D.; Marodon, G.; Stokidis, S.; Gritzapis, A.D.; et al. Radiotherapy-Related Gene Signature in Prostate Cancer. Cancers 2022, 14, 5032. https://doi.org/10.3390/cancers14205032
Kogionou P, Fortis SP, Goulielmaki M, Aubert N, Batsaki P, Ouzounis S, Cavouras D, Marodon G, Stokidis S, Gritzapis AD, et al. Radiotherapy-Related Gene Signature in Prostate Cancer. Cancers. 2022; 14(20):5032. https://doi.org/10.3390/cancers14205032
Chicago/Turabian StyleKogionou, Paraskevi, Sotirios P. Fortis, Maria Goulielmaki, Nicolas Aubert, Panagiota Batsaki, Sotirios Ouzounis, Dionisis Cavouras, Gilles Marodon, Savvas Stokidis, Angelos D. Gritzapis, and et al. 2022. "Radiotherapy-Related Gene Signature in Prostate Cancer" Cancers 14, no. 20: 5032. https://doi.org/10.3390/cancers14205032
APA StyleKogionou, P., Fortis, S. P., Goulielmaki, M., Aubert, N., Batsaki, P., Ouzounis, S., Cavouras, D., Marodon, G., Stokidis, S., Gritzapis, A. D., & Baxevanis, C. N. (2022). Radiotherapy-Related Gene Signature in Prostate Cancer. Cancers, 14(20), 5032. https://doi.org/10.3390/cancers14205032







