Diagnostic Value of Radio-Guided Sentinel Node Detection in Patients with Prostate Cancer Undergoing Radical Prostatectomy with Modified-Extended Lymphadenectomy
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients and Study Design
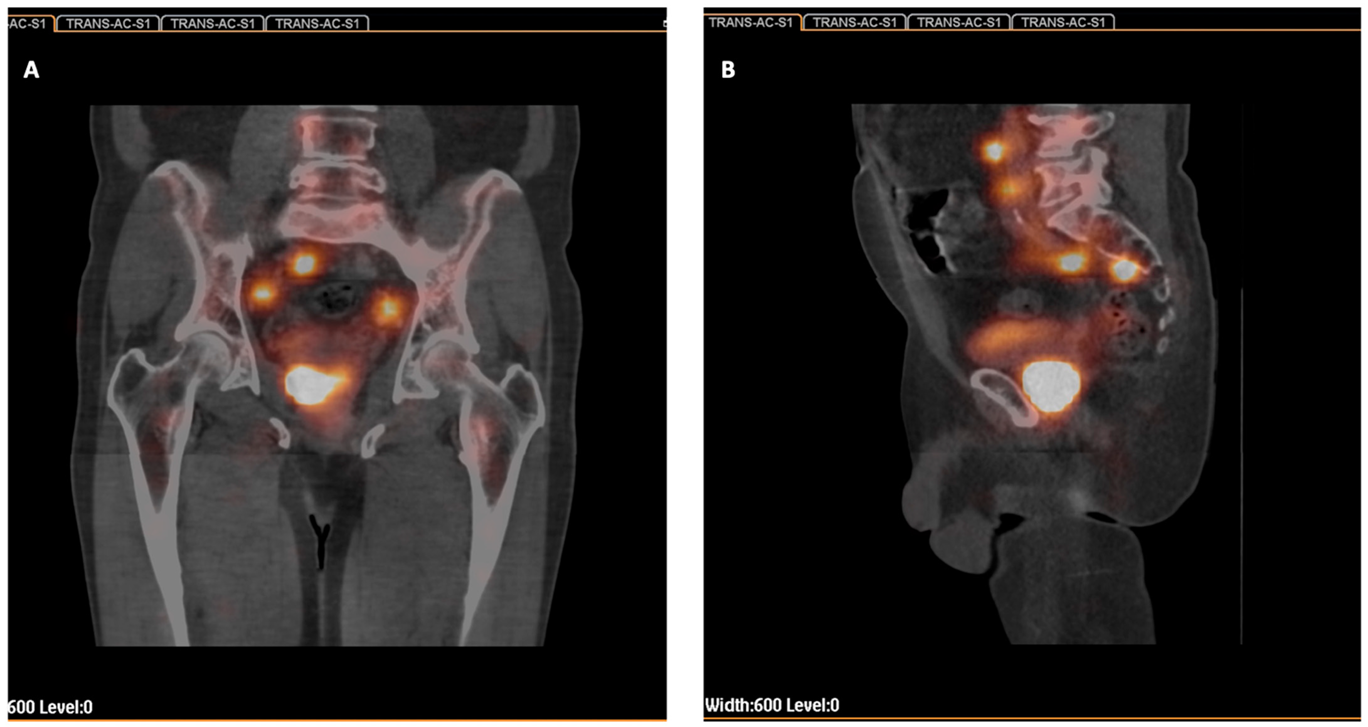
2.2. Radiocolloid Injection and SPECT-CT Imaging
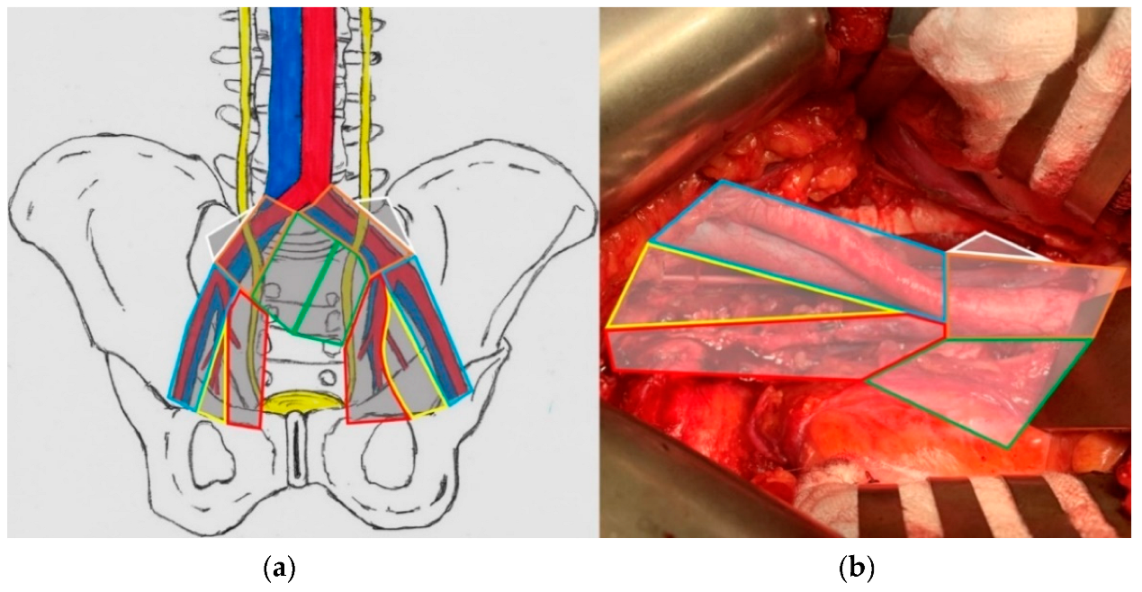
2.3. Surgical Procedure
2.4. Definitions and Statistical Analysis
3. Results
3.1. Basic Clinicopathological Characteristics
3.2. Sentinel Nodes Detected Scintigraphically
3.3. Sentinel Nodes Detected Intraoperatively
3.4. Diagnostic Parameters of the SLN Technique for SPECT-CT and the Gamma-Probe
3.5. Lymphadenectomy and Positive Lymph Nodes
3.6. Morbidity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Van Baelen, A.; Mottet, N.; Spahn, M.; Briganti, A.; Gontero, P.; Joniau, S. Sense and Nonsense of an Extended Pelvic Lymph Node Dissection in Prostate Cancer. Adv. Urol. 2012, 2012, 983058. [Google Scholar] [CrossRef] [PubMed]
- Allaf, M.E.; Palapattu, G.S.; Trock, B.J.; Carter, H.B.; Walsh, P.C. Anatomical Extent of Lymph Node Dissection: Impact on Men with Clinically Localized Prostate Cancer. J. Urol. 2004, 172, 1840–1844. [Google Scholar] [CrossRef] [PubMed]
- Hövels, A.M.; Heesakkers, R.A.M.; Adang, E.M.; Jager, G.J.; Strum, S.; Hoogeveen, Y.L.; Severens, J.L.; Barentsz, J.O. The Diagnostic Accuracy of CT and MRI in the Staging of Pelvic Lymph Nodes in Patients with Prostate Cancer: A Meta-Analysis. Clin. Radiol. 2008, 63, 387–395. [Google Scholar] [CrossRef]
- Stabile, A.; Pellegrino, A.; Mazzone, E.; Cannoletta, D.; de Angelis, M.; Barletta, F.; Scuderi, S.; Cucchiara, V.; Gandaglia, G.; Raggi, D.; et al. Can Negative Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography Avoid the Need for Pelvic Lymph Node Dissection in Newly Diagnosed Prostate Cancer Patients? A Systematic Review and Meta-Analysis with Backup Histology as Reference Standard. Eur. Urol. Oncol. 2022, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Touijer, K.A.; Sjoberg, D.D.; Benfante, N.; Laudone, V.P.; Ehdaie, B.; Eastham, J.A.; Scardino, P.T.; Vickers, A. Limited versus Extended Pelvic Lymph Node Dissection for Prostate Cancer: A Randomized Clinical Trial. Eur. Urol. Oncol. 2021, 4, 532–539. [Google Scholar] [CrossRef]
- Lestingi, J.F.P.; Guglielmetti, G.B.; Trinh, Q.D.; Coelho, R.F.; Pontes, J.; Bastos, D.A.; Cordeiro, M.D.; Sarkis, A.S.; Faraj, S.F.; Mitre, A.I.; et al. Extended Versus Limited Pelvic Lymph Node Dissection During Radical Prostatectomy for Intermediate- and High-Risk Prostate Cancer: Early Oncological Outcomes from a Randomized Phase 3 Trial. Eur. Urol. 2021, 79, 595–604. [Google Scholar] [CrossRef]
- Musch, M.; Klevecka, V.; Roggenbuck, U.; Kroepfl, D. Complications of Pelvic Lymphadenectomy in 1,380 Patients Undergoing Radical Retropubic Prostatectomy between 1993 and 2006. J. Urol. 2008, 179, 923–929. [Google Scholar] [CrossRef]
- Grivas, N.; Wit, E.M.K.; Kuusk, T.; KleinJan, G.H.; Donswijk, M.L.; Van Leeuwen, F.W.B.; Van Der Poel, H.G. The Impact of Adding Sentinel Node Biopsy to Extended Pelvic Lymph Node Dissection on Biochemical Recurrence in Prostate Cancer Patients Treated with Robot-Assisted Radical Prostatectomy. J. Nucl. Med. 2018, 59, 204–209. [Google Scholar] [CrossRef]
- Egawa, M.; Fukuda, M.; Takashima, H.; Misaki, T.; Kinuya, K.; Terahata, S. The Sentinel Node Concept in Prostate Cancer: Present Reality and Future Prospects. Indian J. Urol. 2008, 24, 451–456. [Google Scholar] [CrossRef]
- Lee, P.; Francis, K.E.; Solomon, M.J.; Ramsey-Stewart, G.; Austin, K.K.S.; Koh, C. Triangle of Marcille: The Anatomical Gateway to Lateral Pelvic Exenteration. ANZ J. Surg. 2017, 87, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Maderthaner, L.; Furrer, M.A.; Studer, U.E.; Burkhard, F.C.; Thalmann, G.N.; Nguyen, D.P. More Extended Lymph Node Dissection Template at Radical Prostatectomy Detects Metastases in the Common Iliac Region and in the Fossa of Marcille. BJU Int. 2018, 121, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Bastian, P.; Bellmunt, J.; van den Bergh, R.; Bolla, M.; van Casteren, N.; Cornford, P.; Joniau, S.; Matveev, V.; van der Kwast, T.; et al. EAU-EANM-ESTRO-ESUR-SIOG: Guidelines on Prostate Cancer; European Association of Urology: Arnhem, The Netherlands, 2020; pp. 1–182. [Google Scholar]
- Fossati, N.; Willemse, P.P.M.; Van den Broeck, T.; van den Bergh, R.C.N.; Yuan, C.Y.; Briers, E.; Bellmunt, J.; Bolla, M.; Cornford, P.; De Santis, M.; et al. The Benefits and Harms of Different Extents of Lymph Node Dissection During Radical Prostatectomy for Prostate Cancer: A Systematic Review. Eur. Urol. 2017, 72, 84–109. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Cornford, P.; van den Bergh, R.C.N.; Briers, E.; De Santis, M.; Gillessen, S.; Grummet, J.; Henry, A.M.; van der Kwast, T.H.; Lam, T.B.; et al. EAU-EANM-ESTRO-ESUR-ISUP_SIOG Guidelines on Prostate Cancer 2022. Eur. Urol. 2022, 79, 243–262. [Google Scholar] [CrossRef]
- Wagner, M.; Sokoloff, M.; Daneshmand, S. The Role of Pelvic Lymphadenectomy for Prostate Cancer-Therapeutic? J. Urol. 2008, 179, 408–413. [Google Scholar] [CrossRef]
- Chalouhy, C.; Gurram, S.; Ghavamian, R. Current Controversies on the Role of Lymphadenectomy for Prostate Cancer. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Joniau, S.; Van Den Bergh, L.; Lerut, E.; Deroose, C.M.; Haustermans, K.; Oyen, R.; Budiharto, T.; Ameye, F.; Bogaerts, K.; Van Poppel, H. Mapping of Pelvic Lymph Node Metastases in Prostate Cancer. Eur. Urol. 2013, 63, 450–458. [Google Scholar] [CrossRef]
- Gandaglia, G.; Mazzone, E.; Pellegrino, A.; Fossati, N.; Stabile, A.; Barletta, F.; Scuderi, S.; Leni, R.; Robesti, D.; D’Ambrosio, L.; et al. V11-02 99M-Technetium-Psma Radio-Guided Surgery to Detect Nodal Metastases in Prostate Cancer Patients Undergoing Radical Prostatectomy and Extended Pelvic Lymph Node Dissection: A Phase 2 Prospective, Single-Institution Study. J. Urol. 2022, 207, e921. [Google Scholar] [CrossRef]
- Munbauhal, G.; Seisen, T.; Gomez, F.D.; Peyronnet, B.; Cussenot, O.; Shariat, S.F.; Rouprêt, M. Current Perspectives of Sentinel Lymph Node Dissection at the Time of Radical Surgery for Prostate Cancer. Cancer Treat. Rev. 2016, 50, 228–239. [Google Scholar] [CrossRef]
- Wit, E.M.K.; Acar, C.; Grivas, N.; Yuan, C.; Horenblas, S.; Liedberg, F.; Valdes Olmos, R.A.; van Leeuwen, F.W.B.; van den Berg, N.S.; Winter, A.; et al. Sentinel Node Procedure in Prostate Cancer: A Systematic Review to Assess Diagnostic Accuracy. Eur. Urol. 2017, 71, 596–605. [Google Scholar] [CrossRef]
- Bhatta Dhar, N.; Burkhard, F.C.; Studer, U.E. Role of Lymphadenectomy in Clinically Organ-Confined Prostate Cancer. World J. Urol. 2007, 25, 39–44. [Google Scholar] [CrossRef] [PubMed][Green Version]
- García-Perdomo, H.A.; Correa-Ochoa, J.J.; Contreras-García, R.; Daneshmand, S. Effectiveness of Extended Pelvic Lymphadenectomy in the Survival of Prostate Cancer: A Systematic Review and Meta-Analysis. Cent. Eur. J. Urol. 2018, 71, 262–269. [Google Scholar] [CrossRef]
- Nguyen, D.P.; Huber, P.M.; Metzger, T.A.; Genitsch, V.; Schudel, H.H.; Thalmann, G.N. A Specific Mapping Study Using Fluorescence Sentinel Lymph Node Detection in Patients with Intermediate- and High-Risk Prostate Cancer Undergoing Extended Pelvic Lymph Node Dissection. Eur. Urol. 2016, 70, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, A.B.; Cacciamani, G.E.; Sebben, M.; Tafuri, A.; Processali, T.; Rizzetto, R.; De Luyk, N.; Pirozzi, M.; Amigoni, N.; Corsi, P.; et al. Lymph Nodes Invasion of Marcille’s Fossa Associates with High Metastatic Load in Prostate Cancer Patients Undergoing Extended Pelvic Lymph Node Dissection: The Role of “Marcillectomy”. Urol. Int. 2019, 103, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Backhaus, M.; Mira Moreno, A.; Gómez Ferrer, A.; Calatrava Fons, A.; Casanova, J.; Solsona Narbón, E.; Ortiz Rodríguez, I.M.; Rubio Briones, J. Indocyanine Green Guided Pelvic Lymph Node Dissection: An Efficient Technique to Classify the Lymph Node Status of Patients with Prostate Cancer Who Underwent Radical Prostatectomy. J. Urol. 2016, 196, 1429–1435. [Google Scholar] [CrossRef]


| Patients N0 = 33 | Patients N+ = 10 | All Patients = 43 | |
|---|---|---|---|
| Age mean (years); median (range) | 64 years; median 66 (50–71) | 61.4 years; median 61 (49–70) | 63.4 years; median 66 (49–71) |
| Preoperative PSA mean (ng/mL); median (range) | 13 ng/mL; median 12 (2.41–48.2) | 16 ng/mL; median 9 (6.28–33.52) | 14.02 ng/mL; median 11.65 (2.41–48.2) |
| Clinical T Stage, n (%) | |||
| cT1 | 14 | 4 | 18 (41.9%) |
| cT2 | 16 | 3 | 19 (44.1%) |
| cT3a | 2 | 0 | 2 (4.6%) |
| cT3b | 1 | 3 | 4 (9.4%) |
| Biopsy Gleason grade group, n (%) | |||
| 1 (3 + 3) | 10 | 3 | 13 (30.2%) |
| 2 (3 + 4) | 11 | 3 | 14 (32.5%) |
| 3 (4 + 3) | 6 | 1 | 7 (16.3%) |
| 4 (4 + 4) | 3 | 2 | 5 (11.6%) |
| 5 (4 + 5 or 5 + 4) | 3 | 1 | 4 (9.4%) |
| Pathologic T Score, n (%) | |||
| pT2a | 1 | 0 | 1 (2.3%) |
| pT2c | 19 | 0 | 19 (44.1%) |
| pT3a | 10 | 5 | 15 (35.0%) |
| pT3b | 3 | 5 | 8 (18.6%) |
| Pathological Gleason grade group, n (%) | |||
| 1 (3 + 3) | 4 | 0 | 4 (9.4%) |
| 2 (3 + 4) | 17 | 4 | 21 (48.7%) |
| 3 (4 + 3) | 9 | 4 | 13 (30.2%) |
| 4 (4 + 4) | 0 | 1 | 1 (2.3%) |
| 5 (4 + 5 or 5 + 4) | 3 | 1 | 4 (9.4%) |
| Briganti nomogram, mean (%); median (range) | 12.35%; median 8 (2–48) | 22%; median 22 (3–49) | 14.36%; median 9 (2–49) |
| Nodes removed per patient (no.); median (range) | 25; median 25 (14–52) | 26; median 28 (16–33) | 26; median 26 (14–52) |
| Diagnostic Test Parameter | Gamma-Probe | SPECT-CT |
|---|---|---|
| sensitivity | 90% | 90% |
| specificity | 6.06% | 6.06% |
| PPV | 22.5% | 22.5% |
| NPV | 66.67% | 66.67% |
| ACC | 25.58% | 25.58% |
| FN rate | 10% | 10% |
| Variant of Lymphadenectomy | N+ Patients Correctly Staged, No. (%) | N+ Patients with All Metastases Removed No. (%) | N+ Lymph Nodes Removed, No. (%) | Lymph Nodes Removed, No. |
|---|---|---|---|---|
| obturator | 4 (40%) | 2 (20%) | 9 (47.37%) | 474 |
| limited lymphadenectomy: obturator + external iliac | 9 (90%) | 8 (80%) | 17 (89.47%) | 776 |
| standard lymphadenectomy: obturator + external and internal iliac | 10 (100%) | 10 (100%) | 19 (100%) | 805 |
| extended lymphadenectomy: obturator + external and internal iliac + common iliac | 10 (100%) | 10 (100%) | 19 (100%) | 906 |
| modified-extended lymphadenectomy: above mentioned + presacral + Marcille’s fossa | 10 (100%) | 10 (100%) | 19 (100%) | 1097 |
| sentinel lymph node dissection only | 9 (90%) | 4 (40%) | 13 (68.42%) | 118 |
| Anatomical Region | No. of Patients | SLN | % of All LN from Station | SLN+ Count | %SLN+/SLN from Station |
|---|---|---|---|---|---|
| obturator left (OL) | 17 | 23 | 10.60% | 2 | 8.70% |
| obturator right (OR) | 16 | 21 | 8.17% | 3 | 14.29% |
| external iliac left (EIL) | 14 | 17 | 11.64% | 5 | 29.41% |
| external iliac right (EIR) | 12 | 16 | 10.26% | 2 | 12.5% |
| internal iliac left (IIL) | 4 | 4 | 33.33% | 1 | 25% |
| internal iliac right (IIR) | 3 | 3 | 17.65% | 0 | 0% |
| presacral left (PSL) | 3 | 4 | 16% | 0 | 0% |
| presacral right (PSR) | 13 | 14 | 23.73% | 0 | 0% |
| fossa of Marcille left (FML) | 2 | 2 | 3.17% | 0 | 0% |
| fossa of Marcille right (FMR) | 3 | 3 | 6.82% | 0 | 0% |
| common iliac left (CIL) | 4 | 4 | 9.52% | 0 | 0% |
| common iliac right (CIR) | 5 | 7 | 11.86% | 0 | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małkiewicz, B.; Bugla, B.; Czarnecki, M.; Karwacki, J.; Długosz, P.; Gurwin, A.; Kiełb, P.; Lemiński, A.; Krajewski, W.; Jędrzejuk, D.; et al. Diagnostic Value of Radio-Guided Sentinel Node Detection in Patients with Prostate Cancer Undergoing Radical Prostatectomy with Modified-Extended Lymphadenectomy. Cancers 2022, 14, 5012. https://doi.org/10.3390/cancers14205012
Małkiewicz B, Bugla B, Czarnecki M, Karwacki J, Długosz P, Gurwin A, Kiełb P, Lemiński A, Krajewski W, Jędrzejuk D, et al. Diagnostic Value of Radio-Guided Sentinel Node Detection in Patients with Prostate Cancer Undergoing Radical Prostatectomy with Modified-Extended Lymphadenectomy. Cancers. 2022; 14(20):5012. https://doi.org/10.3390/cancers14205012
Chicago/Turabian StyleMałkiewicz, Bartosz, Błażej Bugla, Maciej Czarnecki, Jakub Karwacki, Paulina Długosz, Adam Gurwin, Paweł Kiełb, Artur Lemiński, Wojciech Krajewski, Diana Jędrzejuk, and et al. 2022. "Diagnostic Value of Radio-Guided Sentinel Node Detection in Patients with Prostate Cancer Undergoing Radical Prostatectomy with Modified-Extended Lymphadenectomy" Cancers 14, no. 20: 5012. https://doi.org/10.3390/cancers14205012
APA StyleMałkiewicz, B., Bugla, B., Czarnecki, M., Karwacki, J., Długosz, P., Gurwin, A., Kiełb, P., Lemiński, A., Krajewski, W., Jędrzejuk, D., Bolanowski, M., Hałoń, A., & Szydełko, T. (2022). Diagnostic Value of Radio-Guided Sentinel Node Detection in Patients with Prostate Cancer Undergoing Radical Prostatectomy with Modified-Extended Lymphadenectomy. Cancers, 14(20), 5012. https://doi.org/10.3390/cancers14205012










