Immune Checkpoint Inhibitor-Related Cytopenias: About 68 Cases from the French Pharmacovigilance Database
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
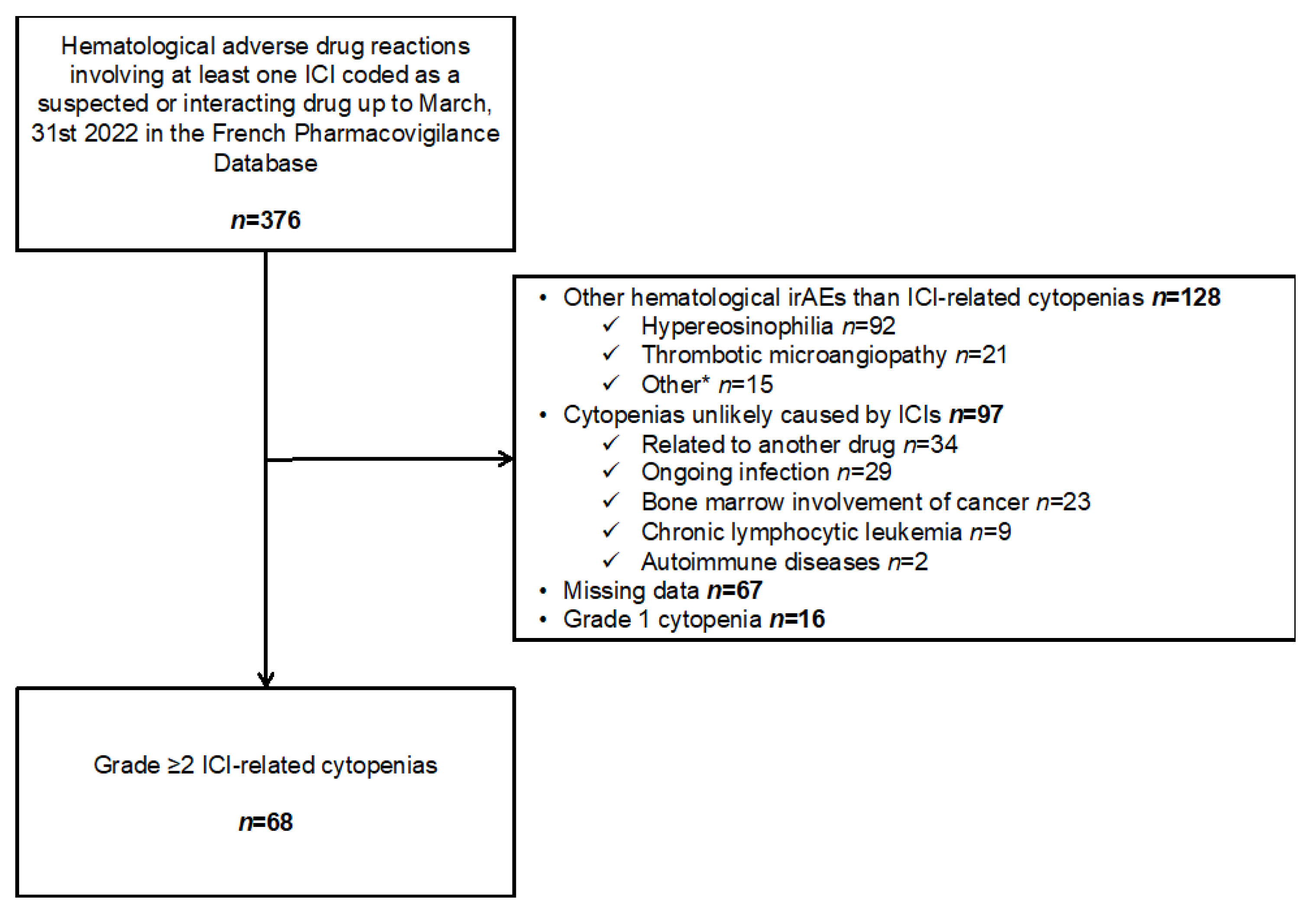
2.2. Data Extraction and Selection
2.3. Data Collection
2.4. Statistical Analysis
2.5. Ethics Approval and Consent
3. Results
3.1. Patient and ICI Treatment Characteristics
3.2. Characteristics of the ICI-Related Cytopenias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Aplastic anemia |
| AIHA | Autoimmune hemolytic anemia |
| AIN | Autoimmune neutropenia |
| CTLA-4 | Cytotoxic T-lymphocyte-associated-4 |
| ESA | Erythropoiesis stimulating agent |
| G-CSF | Granulocyte colony stimulating factor |
| ICI | Immune checkpoint inhibitors |
| irAEs | Immune-related adverse events |
| ITP | Immune thrombocytopenic purpura |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed cell death ligand 1 |
| PRCA | Pure red cell aplasia |
References
- Hirsch, L.; Zitvogel, L.; Eggermont, A.; Marabelle, A. PD-Loma: A Cancer Entity with a Shared Sensitivity to the PD-1/PD-L1 Pathway Blockade. Br. J. Cancer 2019, 120, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-Related Adverse Events of Checkpoint Inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Korman, A.J.; Peggs, K.S.; Allison, J.P. Checkpoint Blockade in Cancer Immunotherapy. Adv. Immunol. 2006, 90, 297–339. [Google Scholar] [CrossRef]
- Zhuang, J.; Du, J.; Guo, X.; Zhou, J.; Duan, L.; Qiu, W.; Si, X.; Zhang, L.; Li, Y.; Liu, X.; et al. Clinical Diagnosis and Treatment Recommendations for Immune Checkpoint Inhibitor-Related Hematological Adverse Events. Thorac. Cancer 2020, 11, 799–804. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Kramer, R.; Zaremba, A.; Moreira, A.; Ugurel, S.; Johnson, D.B.; Hassel, J.C.; Salzmann, M.; Gesierich, A.; Weppler, A.; Spain, L.; et al. Hematological Immune Related Adverse Events after Treatment with Immune Checkpoint Inhibitors. Eur. J. Cancer 2021, 147, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.J.; Salem, J.-E.; Young, A.; Green, J.R.; Ferrell, P.B.; Ancell, K.K.; Lebrun-Vignes, B.; Moslehi, J.J.; Johnson, D.B. Hematologic Complications of Immune Checkpoint Inhibitors. Oncologist 2019, 24, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Tanios, G.E.; Doley, P.B.; Munker, R. Autoimmune Hemolytic Anemia Associated with the Use of Immune Checkpoint Inhibitors for Cancer: 68 Cases from the Food and Drug Administration Database and Review. Eur. J. Haematol. 2019, 102, 157–162. [Google Scholar] [CrossRef]
- Bégaud, B.; Evreux, J.C.; Jouglard, J.; Lagier, G. Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France. Therapie 1985, 40, 111–118. [Google Scholar] [PubMed]
- Brown, E.G.; Wood, L.; Wood, S. The Medical Dictionary for Regulatory Activities (MedDRA). Drug Saf. 1999, 20, 109–117. [Google Scholar] [CrossRef]
- Michel, M.; Terriou, L.; Roudot-Thoraval, F.; Hamidou, M.; Ebbo, M.; Le Guenno, G.; Galicier, L.; Audia, S.; Royer, B.; Morin, A.-S.; et al. A Randomized and Double-Blind Controlled Trial Evaluating the Safety and Efficacy of Rituximab for Warm Auto-Immune Hemolytic Anemia in Adults (the RAIHA Study). Am. J. Hematol. 2017, 92, 23–27. [Google Scholar] [CrossRef]
- Rodeghiero, F.; Stasi, R.; Gernsheimer, T.; Michel, M.; Provan, D.; Arnold, D.M.; Bussel, J.B.; Cines, D.B.; Chong, B.H.; Cooper, N.; et al. Standardization of Terminology, Definitions and Outcome Criteria in Immune Thrombocytopenic Purpura of Adults and Children: Report from an International Working Group. Blood 2009, 113, 2386–2393. [Google Scholar] [CrossRef] [PubMed]
- Akhtari, M.; Curtis, B.; Waller, E.K. Autoimmune Neutropenia in Adults. Autoimmun. Rev. 2009, 9, 62–66. [Google Scholar] [CrossRef]
- Means, R.T., Jr. Pure Red Cell Aplasia. Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Camitta, B.M. Criteria for Severe Aplastic Anaemia. Lancet 1988, 1, 303–304. [Google Scholar] [CrossRef]
- Scheinberg, P.; Nunez, O.; Weinstein, B.; Scheinberg, P.; Biancotto, A.; Wu, C.O.; Young, N.S. Horse versus Rabbit Antithymocyte Globulin in Acquired Aplastic Anemia. N. Engl. J. Med. 2011, 365, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Molad, Y. Hematological Manifestations among Patients with Rheumatic Diseases. Acta Haematol. 2021, 144, 403–412. [Google Scholar] [CrossRef] [PubMed]
- De Back, T.R.; Kater, A.P.; Tonino, S.H. Autoimmune Cytopenias in Chronic Lymphocytic Leukemia: A Concise Review and Treatment Recommendations. Expert Rev. Hematol. 2018, 11, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Flaujac, C.; Boukour, S.; Cramer-Bordé, E. Platelets and Viruses: An Ambivalent Relationship. Cell. Mol. Life Sci. 2010, 67, 545–556. [Google Scholar] [CrossRef]
- Johansson, D.; Rasmussen, M.; Inghammar, M. Thrombocytopenia in Bacteraemia and Association with Bacterial Species. Epidemiol. Infect. 2018, 146, 1312–1317. [Google Scholar] [CrossRef]
- Michalak, S.S.; Olewicz-Gawlik, A.; Rupa-Matysek, J.; Wolny-Rokicka, E.; Nowakowska, E.; Gil, L. Autoimmune Hemolytic Anemia: Current Knowledge and Perspectives. Immun. Ageing 2020, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Schoettler, M.L.; Nathan, D.G. The Pathophysiology of Acquired Aplastic Anemia: Current Concepts Revisited. Hematol. Oncol. Clin. N. Am. 2018, 32, 581–594. [Google Scholar] [CrossRef]
- Allouchery, M.; Lombard, T.; Martin, M.; Rouby, F.; Sassier, M.; Bertin, C.; Atzenhoffer, M.; Miremont-Salame, G.; Perault-Pochat, M.-C.; Puyade, M.; et al. Safety of Immune Checkpoint Inhibitor Rechallenge after Discontinuation for Grade ≥2 Immune-Related Adverse Events in Patients with Cancer. J. Immunother. Cancer 2020, 8, e001622. [Google Scholar] [CrossRef] [PubMed]
- Delanoy, N.; Michot, J.-M.; Comont, T.; Kramkimel, N.; Lazarovici, J.; Dupont, R.; Champiat, S.; Chahine, C.; Robert, C.; Herbaux, C.; et al. Haematological Immune-Related Adverse Events Induced by Anti-PD-1 or Anti-PD-L1 Immunotherapy: A Descriptive Observational Study. Lancet Haematol. 2019, 6, e48–e57. [Google Scholar] [CrossRef]
- Michot, J.M.; Lazarovici, J.; Tieu, A.; Champiat, S.; Voisin, A.L.; Ebbo, M.; Godeau, B.; Michel, M.; Ribrag, V.; Lambotte, O. Haematological Immune-Related Adverse Events with Immune Checkpoint Inhibitors, How to Manage? Eur. J. Cancer 2019, 122, 72–90. [Google Scholar] [CrossRef] [PubMed]
- Gnanapandithan, K.; Kharel, P.; Grimshaw, A.; Giri, S. Hematologic Immune-Related Adverse Events from Immune Checkpoint Inhibitors: A Systematic Review of Case-Reports and Case-Series. Blood 2019, 134, 3606. [Google Scholar] [CrossRef]
- Haddad, T.C.; Zhao, S.; Li, M.; Patel, S.H.; Johns, A.; Grogan, M.; Lopez, G.; Miah, A.; Wei, L.; Tinoco, G.; et al. Immune Checkpoint Inhibitor-Related Thrombocytopenia: Incidence, Risk Factors and Effect on Survival. Cancer Immunol. Immunother. 2022, 71, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Spain, L.; Diem, S.; Larkin, J. Management of Toxicities of Immune Checkpoint Inhibitors. Cancer Treat. Rev. 2016, 44, 51–60. [Google Scholar] [CrossRef]
- Haanen, J.; Ernstoff, M.; Wang, Y.; Menzies, A.; Puzanov, I.; Grivas, P.; Larkin, J.; Peters, S.; Thompson, J.; Obeid, M. Rechallenge Patients with Immune Checkpoint Inhibitors Following Severe Immune-Related Adverse Events: Review of the Literature and Suggested Prophylactic Strategy. J. Immunother. Cancer 2020, 8, e000604. [Google Scholar] [CrossRef]
- Michot, J.-M.; Vargaftig, J.; Leduc, C.; Quere, G.; Burroni, B.; Lazarovici, J.; Champiat, S.; Ribrag, V.; Lambotte, O. Immune-Related Bone Marrow Failure Following Anti-PD1 Therapy. Eur. J. Cancer 2017, 80, 128–136. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, Y.; Xu, C.; Qu, X. Increased T Cell Immunoglobulin Mucin-3 and Its Ligand in Acquired Aplastic Anemia. Eur. J. Haematol. 2008, 81, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, Y.; Zhang, P.; Yang, J.; Zhang, L.; He, A.; Zhang, W.; Hideto, T. High Programmed Death 1 Expression on T Cells in Aplastic Anemia. Immunol. Lett. 2017, 183, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Kroll, M.H.; Rojas-Hernandez, C.; Yee, C. Hematologic Complications of Immune Checkpoint Inhibitors. Blood 2022, 139, 3594–3604. [Google Scholar] [CrossRef] [PubMed]
- Perdigoto, A.L.; Kluger, H.; Herold, K.C. Adverse Events Induced by Immune Checkpoint Inhibitors. Curr. Opin. Immunol. 2021, 69, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Hazell, L.; Shakir, S.A.W. Under-Reporting of Adverse Drug Reactions: A Systematic Review. Drug Saf. 2006, 29, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gonzalez, E.; Herdeiro, M.T.; Figueiras, A. Determinants of Under-Reporting of Adverse Drug Reactions: A Systematic Review. Drug Saf. 2009, 32, 19–31. [Google Scholar] [CrossRef] [PubMed]
| N = 68 | ||
|---|---|---|
| Age, years, median (IQR) | 63.0 | (58.0–70.5) |
| Male gender | 43 | (63.2) |
| Body mass index, kg/m2, median (IQR) (n = 36) | 24.8 | (21.6–30.3) |
| History of hematologic malignancies † | 5 | (7.4) |
| Monoclonal gammopathy of undetermined significance | 1 | (1.5) |
| Myelodysplastic syndrome | 2 | (2.9) |
| Multiple myeloma | 1 | (1.5) |
| Diffuse large B cell lymphoma in remission | 1 | (1.5) |
| History of cancer | 6 | (8.8) |
| History of autoimmune disease (n = 66) | 7 | (10.3) |
| Cured ITP | 2 | (3.0) |
| Crohn’s disease | 1 | (1.5) |
| Hypothyroidism | 2 | (3.0) |
| Sarcoidosis | 1 | (1.5) |
| Overlap Sjogren’s syndrome/Behçet’s disease | 1 | (1.5) |
| ICI-treated cancer type | ||
| Lung cancer | 32 | (47.1) |
| Melanoma | 22 | (32.4) |
| Renal cell carcinoma | 4 | (5.8) |
| Head and neck squamous cell carcinoma | 3 | (4.4) |
| Urinary cancer | 2 | (2.9) |
| Other | 5 | (7.4) |
| ICI as first-line treatment (n = 53) | 25 | (47.2) |
| ICI | ||
| Anti-PD-1 | 55 | (80.9) |
| Pembrolizumab | 32 | (47.1) |
| Nivolumab | 23 | (33.8) |
| Anti-PD-1 + anti-CTLA-4 (nivolumab and ipilimumab) | 6 | (8.8) |
| Anti-PD-L1 | 5 | (7.4) |
| Atezolizumab | 3 | (4.4) |
| Durvalumab | 1 | (1.5) |
| Avelumab | 1 | (1.5) |
| Anti-CTLA-4 (ipilimumab) | 2 | (2.9) |
| Concomitant chemotherapy (n = 67) | 5 | (7.4) |
| Death from immune-related cytopenia | 3 | (4.4) |
| ICI resumption after immune-related cytopenia ‡ | 10 | (14.7) |
| All N = 75 | ITP N = 38 | AIHA N = 19 | AIN N = 10 † | PRCA N = 6 | AA N = 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICI | ||||||||||||
| Anti-PD-1 | 60 | (80.0) | 32 | (84.2) | 13 | (68.4) | 9 | (90.0) | 5 | (83.7) | 1 | (50.0) |
| Anti-PD-L1 | 6 | (8.0) | 4 | (10.5) | 2 | (10.5) | 0 | - | 0 | - | 0 | - |
| Anti-CTLA-4 | 2 | (2.7) | 1 | (2.6) | 1 | (5.2) | 0 | - | 0 | 0 | - | |
| Anti-PD-1 + anti-CTLA-4 | 7 | (9.3) | 1 | (2.6) | 3 | (15.8) | 1 | (10.0) | 1 | (16.7) | 1 | (50.0) |
| Time to immune-related cytopenia, months, median (IQR) | 2.0 | (1.0–4.5) | 1.8 | (1.0–4.8) | 1.4 | (1.0–2.8) | 2.4 | (1.2–4.5) | 2.1 | (1.4–2.8) | 4.7 | NA |
| Time to immune-related cytopenia, treatment cycles, median (IQR) | 3.0 | (2.0–6.0) | 3 | (1.0–5.0) | 2.0 | (1.0–4.0) | 3.5 | (2.0–6.0) | 3.0 | (2.0–7.0) | 7.0 | NA |
| Maximum severity (n = 74) | ||||||||||||
| Grade 2 | 9/74 | (12.2) | 3/38 | (7.9) | 6/18 | (33.3) | 0/10 | - | 0/6 | - | 0/2 | - |
| Grade 3 | 29/74 | (39.2) | 9/38 | (23.6) | 9/18 | (50.0) | 5/10 | (50.0) | 5/6 | (83.3) | 0/2 | - |
| Grade 4 | 33/74 | (44.6) | 24/38 | (63.2) | 2/18 | (11.1) | 5/10 | (50.0) | 1/6 | (16.7) | 2/2 | (100) |
| Grade 5 | 3/74 | (4.0) | 2/38 | (5.3) | 1/18 | (5.6) | 0/10 | - | 0/6 | - | 0/2 | - |
| Treatment of immune-related cytopenia (n = 72) | ||||||||||||
| Number of lines, median (IQR) | 1.0 | (1.0–1.0) | 1.0 | (1.0–1.0) | 1.0 | (1.0–1.0) | 1.0 | (1.0–1.0) | 1.5 | (1.0–2.0) | 1.0 | (1.0–1.0) |
| Glucocorticoids, | 56/72 | (77.8) | 32/36 | (88.9) | 16/19 | (84.2) | 2/10 | (20.0) | 5/5 | (100.0) | 1/2 | (50.0) |
| Intravenous immunoglobulins | 12/72 | (16.7) | 9/36 | (25.0) | 0/19 | - | 1/10 | (10.0) | 2/5 | (40.0) | 0/2 | - |
| G-CSF | 5/72 | (6.9) | 0/36 | - | 0/19 | - | 5/10 | (50.0) | 0/5 | - | 0/2 | - |
| Thrombopoietin agonist | 3/72 | (4.2) | 2/36 | (5.6) | 0/19 | - | 0/10 | - | 0/5 | - | 1/2 | (50.0) |
| Cyclosporine | 3/72 | (4.2) | 0/36 | - | 1/19 | (5.3) | 0/10 | - | 1/5 | (20.0) | 1/2 | (50.0) |
| ESA | 2/72 | (2.8) | 0/36 | - | 0/19 | - | 0/10 | - | 2/5 | (40.0) | 0/2 | - |
| Rituximab | 2/72 | (2.8) | 0/36 | - | 2/19 | (10.5) | 0/10 | - | 0/5 | - | 0/2 | - |
| Cyclophosphamide | 1/72 | (1.4) | 0/36 | - | 0/19 | - | 0/10 | - | 1/5 | (20.0) | 0/2 | - |
| Treatment response of immune-related cytopenia (n = 61) | ||||||||||||
| Complete | 30 | (49.2) | 16/32 | (50.0) | 6/14 | (42.9) | 7/9 | (77.8) | 1/5 | (20.0) | 0/2 | - |
| Partial | 14 | (23.0) | 8/32 | (25.0) | 4/14 | (28.6) | 0/9 | - | 1/5 | (20.0) | 1/2 | (50.0) |
| Stable disease | 17 | (27.9) | 8/32 | (25.0) | 4/14 | (28.6) | 2/9 | (22.2) | 3/5 | (60.0) | 0/2 | - |
| ICI resumption | 10 | (13.3) | 5 | (13.2) | 2 | (10.5) | 3 | (30.0) | 0 | - | 0 | - |
| Relapse of immune cytopenia after ICI resumption | 3 | (33.3) | 2 | (40.0) | 0 | - | 1 | (33.3) | 0 | - | 0 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, M.; Nguyen, H.-M.; Beuvon, C.; Bene, J.; Palassin, P.; Atzenhoffer, M.; Rouby, F.; Sassier, M.; Pérault-Pochat, M.-C.; Roblot, P.; et al. Immune Checkpoint Inhibitor-Related Cytopenias: About 68 Cases from the French Pharmacovigilance Database. Cancers 2022, 14, 5030. https://doi.org/10.3390/cancers14205030
Martin M, Nguyen H-M, Beuvon C, Bene J, Palassin P, Atzenhoffer M, Rouby F, Sassier M, Pérault-Pochat M-C, Roblot P, et al. Immune Checkpoint Inhibitor-Related Cytopenias: About 68 Cases from the French Pharmacovigilance Database. Cancers. 2022; 14(20):5030. https://doi.org/10.3390/cancers14205030
Chicago/Turabian StyleMartin, Mickaël, Hoan-My Nguyen, Clément Beuvon, Johana Bene, Pascale Palassin, Marina Atzenhoffer, Franck Rouby, Marion Sassier, Marie-Christine Pérault-Pochat, Pascal Roblot, and et al. 2022. "Immune Checkpoint Inhibitor-Related Cytopenias: About 68 Cases from the French Pharmacovigilance Database" Cancers 14, no. 20: 5030. https://doi.org/10.3390/cancers14205030
APA StyleMartin, M., Nguyen, H.-M., Beuvon, C., Bene, J., Palassin, P., Atzenhoffer, M., Rouby, F., Sassier, M., Pérault-Pochat, M.-C., Roblot, P., Allouchery, M., & Puyade, M., on behalf of the French Network of Regional Pharmacovigilance Centers. (2022). Immune Checkpoint Inhibitor-Related Cytopenias: About 68 Cases from the French Pharmacovigilance Database. Cancers, 14(20), 5030. https://doi.org/10.3390/cancers14205030








