Comprehensive Treatment Algorithms of the Swiss Peritoneal Cancer Group for Peritoneal Cancer of Gastrointestinal Origin
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Review of the Literature and Drafting of Comprehensive Treatment Algorithms
2.2. Internal Validation of the Lake Geneva Algorithms
2.3. External Validation of the SPCG Algorithms
3. Results
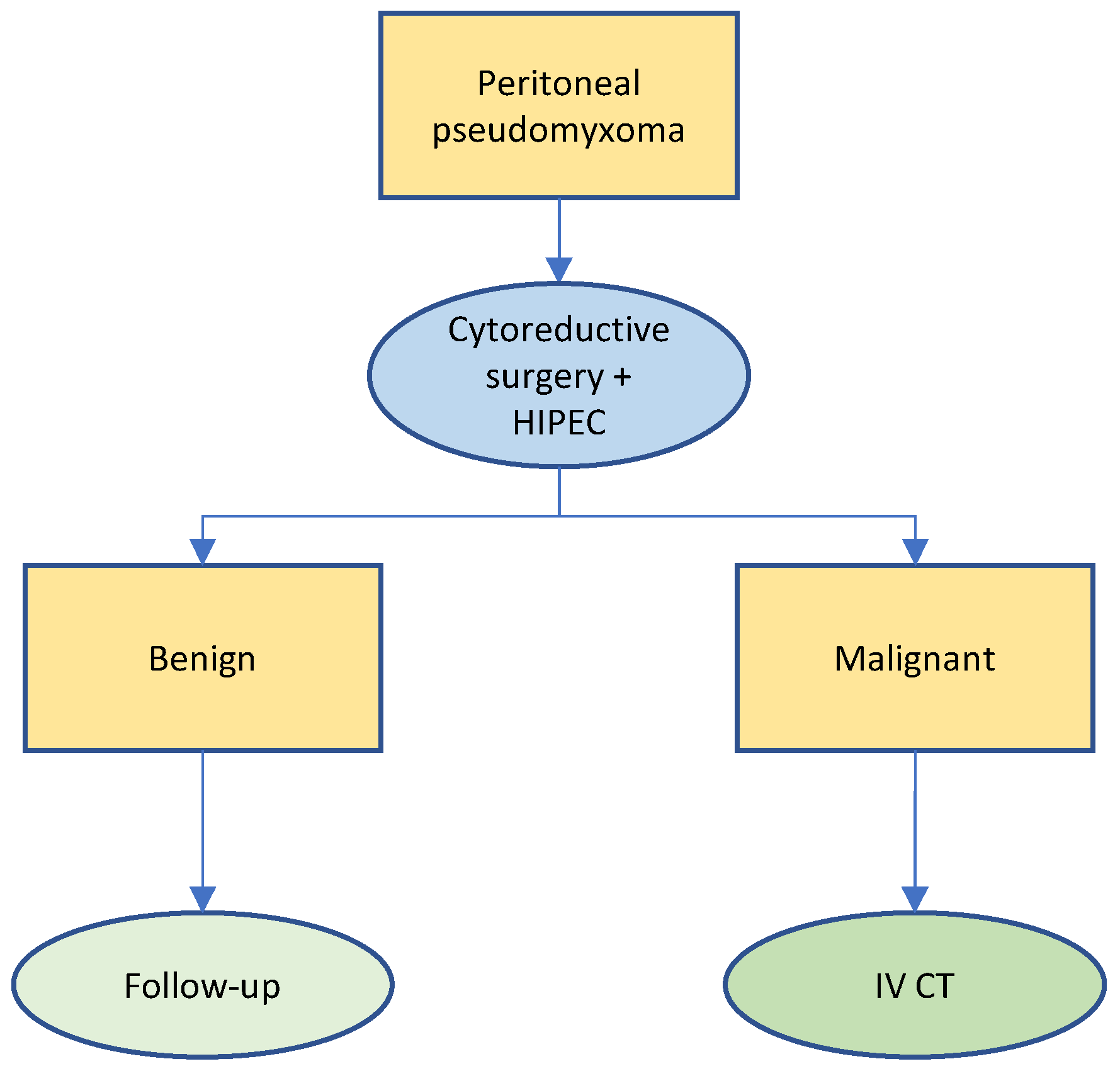
3.1. Pseudomyxoma Peritonei
3.2. Peritoneal Mesothelioma
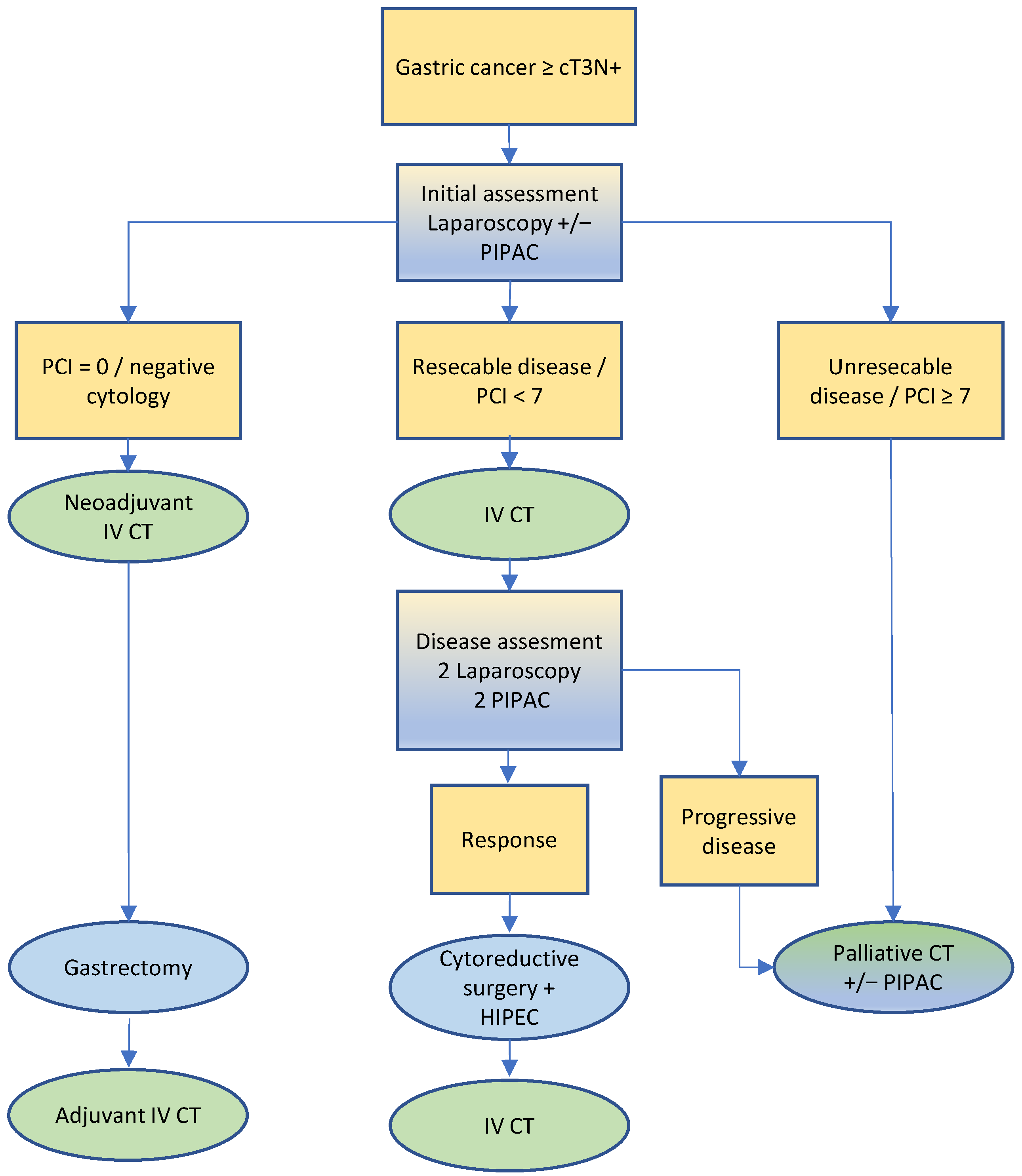
3.3. Gastric Cancer
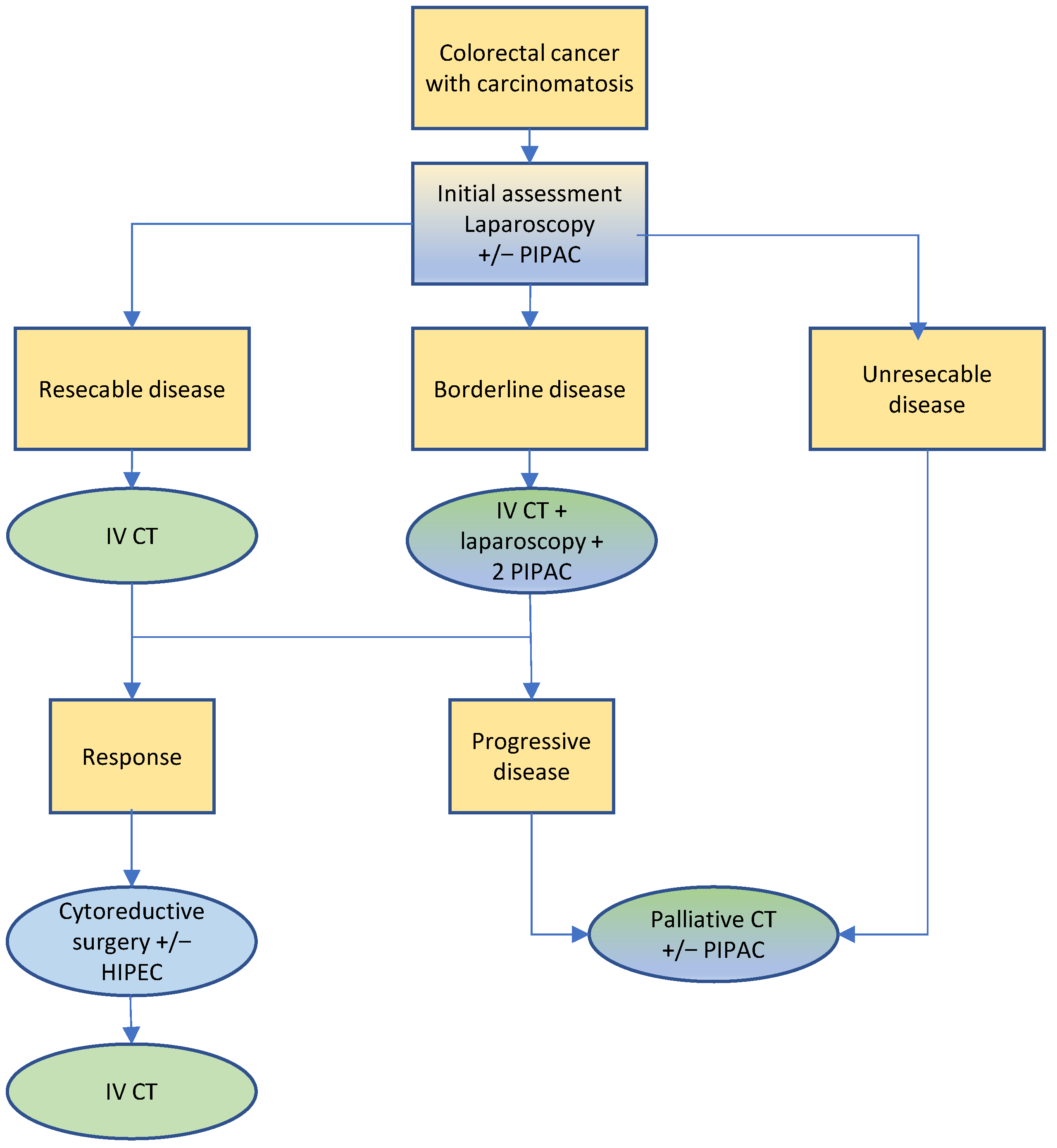
3.4. Colorectal Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Franko, J.; Shi, Q.; Goldman, C.D.; Pockaj, B.A.; Nelson, G.D.; Goldberg, R.M.; Pitot, H.C.; Grothey, A.; Alberts, S.R.; Sargent, D.J. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: A pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J. Clin. Oncol. 2012, 30, 263–267. [Google Scholar] [CrossRef]
- Lemmens, V.E.; Klaver, Y.L.; Verwaal, V.J.; Rutten, H.J.; Coebergh, J.W.; de Hingh, I.H. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: A population-based study. Int. J. Cancer 2011, 128, 2717–2725. [Google Scholar] [CrossRef]
- Di Giorgio, A.; De Iaco, P.; De Simone, M.; Garofalo, A.; Scambia, G.; Pinna, A.D.; Verdecchia, G.M.; Ansaloni, L.; Macri, A.; Cappellini, P.; et al. Cytoreduction (Peritonectomy Procedures) Combined with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Advanced Ovarian Cancer: Retrospective Italian Multicenter Observational Study of 511 Cases. Ann. Surg. Oncol. 2017, 24, 914–922. [Google Scholar] [CrossRef]
- Glehen, O.; Gilly, F.N.; Boutitie, F.; Bereder, J.M.; Quenet, F.; Sideris, L.; Mansvelt, B.; Lorimier, G.; Msika, S.; Elias, D.; et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: A multi-institutional study of 1290 patients. Cancer 2010, 116, 5608–5618. [Google Scholar] [CrossRef]
- Glehen, O.; Gilly, F.N.; Arvieux, C.; Cotte, E.; Boutitie, F.; Mansvelt, B.; Bereder, J.M.; Lorimier, G.; Quenet, F.; Elias, D.; et al. Peritoneal carcinomatosis from gastric cancer: A multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann. Surg. Oncol. 2010, 17, 2370–2377. [Google Scholar] [CrossRef]
- Elias, D.; Gilly, F.; Quenet, F.; Bereder, J.M.; Sideris, L.; Mansvelt, B.; Lorimier, G.; Glehen, O.; Association Française de Chirurgie. Pseudomyxoma peritonei: A French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2010, 36, 456–462. [Google Scholar] [CrossRef]
- Sadeghi, B.; Arvieux, C.; Glehen, O.; Beaujard, A.C.; Rivoire, M.; Baulieux, J.; Fontaumard, E.; Brachet, A.; Caillot, J.L.; Faure, J.L.; et al. Peritoneal carcinomatosis from non-gynecologic malignancies: Results of the EVOCAPE 1 multicentric prospective study. Cancer 2000, 88, 358–363. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures. Available online: http://www.cancer.org (accessed on 18 July 2022).
- Segelman, J.; Granath, F.; Holm, T.; Machado, M.; Mahteme, H.; Martling, A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2012, 99, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.C.; Moran, B.J.; Sugarbaker, P.H.; Levine, E.A.; Glehen, O.; Gilly, F.N.; Baratti, D.; Deraco, M.; Elias, D.; Sardi, A.; et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J. Clin. Oncol. 2012, 30, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Govaerts, K.; Lurvink, R.J.; De Hingh, I.; Van der Speeten, K.; Villeneuve, L.; Kusamura, S.; Kepenekian, V.; Deraco, M.; Glehen, O.; Moran, B.J. Appendiceal tumours and pseudomyxoma peritonei: Literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur. J. Surg. Oncol. 2021, 47, 11–35. [Google Scholar] [CrossRef] [PubMed]
- Kusamura, S.; Kepenekian, V.; Villeneuve, L.; Lurvink, R.J.; Govaerts, K.; De Hingh, I.; Moran, B.J.; Van der Speeten, K.; Deraco, M.; Glehen, O. Peritoneal mesothelioma: PSOGI/EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Eur. J. Surg. Oncol. 2021, 47, 36–59. [Google Scholar] [CrossRef]
- Kepenekian, V.; Péron, J.; Goéré, D.; Sgarbura, O.; Delhorme, J.B.; Eveno, C.; Benzerdjeb, N.; Bonnefoy, I.; Villeneuve, L.; Rousset, P.; et al. Multicystic peritoneal mesothelioma treated with cytoreductive surgery followed or not by hyperthermic intraperitoneal chemotherapy: Results from a large multicentric cohort. Int. J. Hyperth. 2021, 38, 805–814. [Google Scholar] [CrossRef]
- Zahid, A.; Clarke, L.; Carr, N.; Chandrakumaran, K.; Tzivanakis, A.; Dayal, S.; Mohamed, F.; Cecil, T.; Moran, B.J. Outcomes of multicystic peritoneal mesothelioma treatment with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. BJS Open 2021, 5, zraa001. [Google Scholar] [CrossRef]
- Kyziridis, D.; Hristakis, C.; Kalakonas, A.; Vaikos, D.; Pallas, N.; Karamveri, C.; Kyriakopoulos, V.; Tentes, A.A. Ten-year experience with peritoneal mesothelioma. J. Buon. 2019, 24, 391–396. [Google Scholar] [PubMed]
- Chicago Consensus Working Group. The Chicago Consensus on peritoneal surface malignancies: Management of peritoneal mesothelioma. Cancer 2020, 126, 2547–2552. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, J.; Piso, P.; Verwaal, V.; Bachleitner-Hofmann, T.; Glehen, O.; Gonzalez-Moreno, S.; Deraco, M.; Pelz, J.; Alexander, R.; Glockzin, G. American Society of Peritoneal Surface Malignancies opinion statement on defining expectations from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with colorectal cancer. J. Surg. Oncol. 2014, 110, 777–778. [Google Scholar] [CrossRef]
- Levine, E.A.; Stewart, J.H., IV; Shen, P.; Russell, G.B.; Loggie, B.L.; Votanopoulos, K.I. Intraperitoneal chemotherapy for peritoneal surface malignancy: Experience with 1,000 patients. J. Am. Coll. Surg. 2014, 218, 573–585. [Google Scholar] [CrossRef]
- Kusamura, S.; Barretta, F.; Yonemura, Y.; Sugarbaker, P.H.; Moran, B.J.; Levine, E.A.; Goere, D.; Baratti, D.; Nizri, E.; Morris, D.L.; et al. The Role of Hyperthermic Intraperitoneal Chemotherapy in Pseudomyxoma Peritonei After Cytoreductive Surgery. JAMA Surg. 2021, 156, e206363. [Google Scholar] [CrossRef]
- Esquivel, J. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer: Survival outcomes and patient selection. J. Gastrointest. Oncol. 2016, 7, 72–78. [Google Scholar] [CrossRef]
- Verwaal, V.J.; Bruin, S.; Boot, H.; van Slooten, G.; van Tinteren, H. 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann. Surg. Oncol. 2008, 15, 2426–2432. [Google Scholar] [CrossRef] [PubMed]
- Quenet, F.; Elias, D.; Roca, L.; Goere, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Shen, P.; Thai, K.; Stewart, J.H.; Howerton, R.; Loggie, B.W.; Russell, G.B.; Levine, E.A. Peritoneal surface disease from colorectal cancer: Comparison with the hepatic metastases surgical paradigm in optimally resected patients. Ann. Surg. Oncol. 2008, 15, 3422–3432. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Lefevre, J.H.; Chevalier, J.; Brouquet, A.; Marchal, F.; Classe, J.M.; Ferron, G.; Guilloit, J.M.; Meeus, P.; Goere, D.; et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J. Clin. Oncol. 2009, 27, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Kwiatkowski, F.; Sugarbaker, P.H.; Elias, D.; Levine, E.A.; De Simone, M.; Barone, R.; Yonemura, Y.; Cavaliere, F.; Quenet, F.; et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A multi-institutional study. J. Clin. Oncol. 2004, 22, 3284–3292. [Google Scholar] [CrossRef] [PubMed]
- Marano, L.; Marrelli, D.; Sammartino, P.; Biacchi, D.; Graziosi, L.; Marino, E.; Coccolini, F.; Fugazzola, P.; Valle, M.; Federici, O.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Synchronous Peritoneal Metastases: Multicenter Study of ‘Italian Peritoneal Surface Malignancies Oncoteam-S.I.C.O.’. Ann. Surg. Oncol. 2021, 28, 9060–9070. [Google Scholar] [CrossRef]
- Granieri, S.; Bonomi, A.; Frassini, S.; Chierici, A.P.; Bruno, F.; Paleino, S.; Kusamura, S.; Germini, A.; Facciorusso, A.; Deraco, M.; et al. Prognostic impact of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in gastric cancer patients: A meta-analysis of randomized controlled trials. Eur. J. Surg. Oncol. 2021, 47, 2757–2767. [Google Scholar] [CrossRef]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.; van der Velden, J.; Arts, H.J.; Massuger, L.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Ali, Y.M.; Sweeney, J.; Shen, P.; Votanopoulos, K.I.; McQuellon, R.; Duckworth, K.; Perry, K.C.; Russell, G.; Levine, E.A. Effect of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy on Quality of Life in Patients with Peritoneal Mesothelioma. Ann. Surg. Oncol. 2020, 27, 117–123. [Google Scholar] [CrossRef]
- Tsilimparis, N.; Bockelmann, C.; Raue, W.; Menenakos, C.; Perez, S.; Rau, B.; Hartmann, J. Quality of life in patients after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: Is it worth the risk? Ann. Surg. Oncol. 2013, 20, 226–232. [Google Scholar] [CrossRef]
- Tan, W.J.; Wong, J.F.; Chia, C.S.; Tan, G.H.; Soo, K.C.; Teo, M.C. Quality of life after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: An Asian perspective. Ann. Surg. Oncol. 2013, 20, 4219–4223. [Google Scholar] [CrossRef]
- Steffens, D.; Koh, C.; Ansari, N.; Solomon, M.J.; Brown, K.; McBride, K.; Young, J.; Young, C.J.; Moran, B. Quality of Life After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Early Results from a Prospective Cohort Study of 115 Patients. Ann. Surg. Oncol. 2020, 27, 3986–3994. [Google Scholar] [CrossRef]
- Koole, S.N.; Kieffer, J.M.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.; de Hingh, I.H.; van der Velden, J.; Arts, H.J.; van Ham, M.; et al. Health-related quality of life after interval cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with stage III ovarian cancer. Eur. J. Surg. Oncol. 2021, 47, 101–107. [Google Scholar] [CrossRef]
- Dodson, R.M.; McQuellon, R.P.; Mogal, H.D.; Duckworth, K.E.; Russell, G.B.; Votanopoulos, K.I.; Shen, P.; Levine, E.A. Quality-of-Life Evaluation After Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2016, 23, 772–783. [Google Scholar] [CrossRef]
- Moaven, O.; Votanopoulos, K.I.; Shen, P.; Mansfield, P.; Bartlett, D.L.; Russell, G.; McQuellon, R.; Stewart, J.H.; Levine, E.A. Health-Related Quality of Life After Cytoreductive Surgery/HIPEC for Mucinous Appendiceal Cancer: Results of a Multicenter Randomized Trial Comparing Oxaliplatin and Mitomycin. Ann. Surg. Oncol. 2020, 27, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Grass, F.; Vuagniaux, A.; Teixeira-Farinha, H.; Lehmann, K.; Demartines, N.; Hubner, M. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br. J. Surg. 2017, 104, 669–678. [Google Scholar] [CrossRef]
- Tempfer, C.B.; Winnekendonk, G.; Solass, W.; Horvat, R.; Giger-Pabst, U.; Zieren, J.; Rezniczek, G.A.; Reymond, M.A. Pressurized intraperitoneal aerosol chemotherapy in women with recurrent ovarian cancer: A phase 2 study. Gynecol. Oncol. 2015, 137, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Nadiradze, G.; Giger-Pabst, U.; Zieren, J.; Strumberg, D.; Solass, W.; Reymond, M.A. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Low-Dose Cisplatin and Doxorubicin in Gastric Peritoneal Metastasis. J. Gastrointest. Surg. 2016, 20, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Demtroder, C.; Solass, W.; Zieren, J.; Strumberg, D.; Giger-Pabst, U.; Reymond, M.A. Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin in colorectal peritoneal metastasis. Colorectal. Dis. 2016, 18, 364–371. [Google Scholar] [CrossRef]
- Odendahl, K.; Solass, W.; Demtroder, C.; Giger-Pabst, U.; Zieren, J.; Tempfer, C.; Reymond, M.A. Quality of life of patients with end-stage peritoneal metastasis treated with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC). Eur. J. Surg. Oncol. 2015, 41, 1379–1385. [Google Scholar] [CrossRef]
- Hubner, M.; Teixeira Farinha, H.; Grass, F.; Wolfer, A.; Mathevet, P.; Hahnloser, D.; Demartines, N. Feasibility and Safety of Pressurized Intraperitoneal Aerosol Chemotherapy for Peritoneal Carcinomatosis: A Retrospective Cohort Study. Gastroenterol. Res. Pract. 2017, 2017, 6852749. [Google Scholar] [CrossRef]
- Teixeira Farinha, H.; Grass, F.; Kefleyesus, A.; Achtari, C.; Romain, B.; Montemurro, M.; Demartines, N.; Hubner, M. Impact of Pressurized Intraperitoneal Aerosol Chemotherapy on Quality of Life and Symptoms in Patients with Peritoneal Carcinomatosis: A Retrospective Cohort Study. Gastroenterol. Res. Pract. 2017, 2017, 4596176. [Google Scholar] [CrossRef]
- Winkler, C.S.; Sandhu, J.; Pettke, E.; Merchea, A.; Fong, Y.; Kumara, H.; Whelan, R.L. Pressurized Intraperitoneal Aerosol Chemotherapy, a Palliative Treatment Approach for Patients with Peritoneal Carcinomatosis: Description of Method and Systematic Review of Literature. Dis. Colon Rectum. 2020, 63, 242–255. [Google Scholar] [CrossRef]
- Solass, W.; Sempoux, C.; Carr, N.J.; Bibeau, F.; Neureiter, D.; Jager, T.; Di Caterino, T.; Brunel, C.; Klieser, E.; Fristrup, C.W.; et al. Reproducibility of the peritoneal regression grading score for assessment of response to therapy in peritoneal metastasis. Histopathology 2019, 74, 1014–1024. [Google Scholar] [CrossRef]
- Schmucker, C.M.; Blumle, A.; Schell, L.K.; Schwarzer, G.; Oeller, P.; Cabrera, L.; von Elm, E.; Briel, M.; Meerpohl, J.J.; on behalf of the OPEN Consortium. Systematic review finds that study data not published in full text articles have unclear impact on meta-analyses results in medical research. PLoS ONE 2017, 12, e0176210. [Google Scholar] [CrossRef]
- Mittal, R.; Chandramohan, A.; Moran, B. Pseudomyxoma peritonei: Natural history and treatment. Int. J. Hyperth. 2017, 33, 511–519. [Google Scholar] [CrossRef]
- Lin, Y.L.; Xu, D.Z.; Li, X.B.; Yan, F.C.; Xu, H.B.; Peng, Z.; Li, Y. Consensuses and controversies on pseudomyxoma peritonei: A review of the published consensus statements and guidelines. Orphanet J. Rare Dis. 2021, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Smeenk, R.M.; van Velthuysen, M.L.; Verwaal, V.J.; Zoetmulder, F.A. Appendiceal neoplasms and pseudomyxoma peritonei: A population based study. Eur. J. Surg. Oncol. 2008, 34, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Delhorme, J.B.; Severac, F.; Averous, G.; Glehen, O.; Passot, G.; Bakrin, N.; Marchal, F.; Pocard, M.; Lo Dico, R.; Eveno, C.; et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei of appendicular and extra-appendicular origin. Br. J. Surg. 2018, 105, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Mercier, F.; Dagbert, F.; Pocard, M.; Goere, D.; Quenet, F.; Wernert, R.; Dumont, F.; Brigand, C.; Passot, G.; Glehen, O.; et al. Recurrence of pseudomyxoma peritonei after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. BJS Open 2019, 3, 195–202. [Google Scholar] [CrossRef]
- Kong, J.C.; Flood, M.P.; Guerra, G.R.; Liesegang, A.; Wong, W.J.; Mitchell, C.; Warrier, S.K.; Naidu, S.; Meade, B.; Lutton, N.; et al. Appendiceal pseudomyxoma peritonei: Predictors of recurrence and iterative surgery. Colorectal. Dis. 2021, 23, 2368–2375. [Google Scholar] [CrossRef] [PubMed]
- Lord, A.C.; Shihab, O.; Chandrakumaran, K.; Mohamed, F.; Cecil, T.D.; Moran, B.J. Recurrence and outcome after complete tumour removal and hyperthermic intraperitoneal chemotherapy in 512 patients with pseudomyxoma peritonei from perforated appendiceal mucinous tumours. Eur. J. Surg. Oncol. 2015, 41, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Vassos, N.; Fortsch, T.; Aladashvili, A.; Hohenberger, W.; Croner, R.S. Repeated cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with recurrent peritoneal carcinomatosis. World J. Surg. Oncol. 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Chicago Consensus Working Group. The Chicago Consensus on peritoneal surface malignancies: Management of appendiceal neoplasms. Cancer 2020, 126, 2525–2533. [Google Scholar] [CrossRef]
- Carr, N.J.; Cecil, T.D.; Mohamed, F.; Sobin, L.H.; Sugarbaker, P.H.; Gonzalez-Moreno, S.; Taflampas, P.; Chapman, S.; Moran, B.J. A Consensus for Classification and Pathologic Reporting of Pseudomyxoma Peritonei and Associated Appendiceal Neoplasia: The Results of the Peritoneal Surface Oncology Group International (PSOGI) Modified Delphi Process. Am. J. Surg. Pathol. 2016, 40, 14–26. [Google Scholar] [CrossRef]
- Sgarbura, O.; Hubner, M.; Alyami, M.; Eveno, C.; Gagniere, J.; Pache, B.; Pocard, M.; Bakrin, N.; Quenet, F. Oxaliplatin use in pressurized intraperitoneal aerosol chemotherapy (PIPAC) is safe and effective: A multicenter study. Eur. J. Surg. Oncol. 2019, 45, 2386–2391. [Google Scholar] [CrossRef]
- Kurtz, F.; Struller, F.; Horvath, P.; Solass, W.; Bosmuller, H.; Konigsrainer, A.; Reymond, M.A. Feasibility, Safety, and Efficacy of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) for Peritoneal Metastasis: A Registry Study. Gastroenterol. Res. Pract. 2018, 2018, 2743985. [Google Scholar] [CrossRef] [PubMed]
- Salo, S.A.S.; Ilonen, I.; Laaksonen, S.; Myllarniemi, M.; Salo, J.A.; Rantanen, T. Epidemiology of malignant peritoneal mesothelioma: A population-based study. Cancer Epidemiol. 2017, 51, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P. Epidemiology of peritoneal mesothelioma: A review. Ann. Oncol. 2007, 18, 985–990. [Google Scholar] [CrossRef]
- Kitadai, R.; Shimoi, T.; Sudo, K.; Noguchi, E.; Nagata, Y.; Sawada, R.; Takashima, A.; Boku, N.; Yonemori, K. Efficacy of second-line treatment and prognostic factors in patients with advanced malignant peritoneal mesothelioma: A retrospective study. BMC Cancer 2021, 21, 294. [Google Scholar] [CrossRef]
- Girshally, R.; Demtroder, C.; Albayrak, N.; Zieren, J.; Tempfer, C.; Reymond, M.A. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J. Surg. Oncol. 2016, 14, 253. [Google Scholar] [CrossRef] [Green Version]
- Robella, M.; Vaira, M.; De Simone, M. Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: An innovative approach to treat peritoneal carcinomatosis. World J. Surg. Oncol. 2016, 14, 128. [Google Scholar] [CrossRef]
- Alyami, M.; Mercier, F.; Siebert, M.; Bonnot, P.E.; Laplace, N.; Villeneuve, L.; Passot, G.; Glehen, O.; Bakrin, N.; Kepenekian, V. Unresectable peritoneal metastasis treated by pressurized intraperitoneal aerosol chemotherapy (PIPAC) leading to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2021, 47, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Alyami, M.; Gagniere, J.; Sgarbura, O.; Cabelguenne, D.; Villeneuve, L.; Pezet, D.; Quenet, F.; Glehen, O.; Bakrin, N.; Passot, G. Multicentric initial experience with the use of the pressurized intraperitoneal aerosol chemotherapy (PIPAC) in the management of unresectable peritoneal carcinomatosis. Eur. J. Surg. Oncol. 2017, 43, 2178–2183. [Google Scholar] [CrossRef]
- Giger-Pabst, U.; Demtroder, C.; Falkenstein, T.A.; Ouaissi, M.; Gotze, T.O.; Rezniczek, G.A.; Tempfer, C.B. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) for the treatment of malignant mesothelioma. BMC Cancer 2018, 18, 442. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D.; Committee, E.G. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef]
- Pentheroudakis, G.; Committee, E.G. Recent eUpdates to the ESMO Clinical Practice Guidelines on hepatocellular carcinoma, cancer of the pancreas, soft tissue and visceral sarcomas, cancer of the prostate and gastric cancer. Ann. Oncol. 2019, 30, 1395–1397. [Google Scholar] [CrossRef] [PubMed]
- Montori, G.; Coccolini, F.; Ceresoli, M.; Catena, F.; Colaianni, N.; Poletti, E.; Ansaloni, L. The treatment of peritoneal carcinomatosis in advanced gastric cancer: State of the art. Int. J. Surg. Oncol. 2014, 2014, 912418. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, F.; von Rahden, B.H.; Miras, A.D.; Gasser, M.; Maeder, U.; Kunzmann, V.; Germer, C.T.; Pelz, J.; Kerscher, A.G. Incidence, time course and independent risk factors for metachronous peritoneal carcinomatosis of gastric origin—A longitudinal experience from a prospectively collected database of 1108 patients. BMC Cancer 2015, 15, 73. [Google Scholar] [CrossRef]
- Wang, R.; Song, S.; Harada, K.; Ghazanfari Amlashi, F.; Badgwell, B.; Pizzi, M.P.; Xu, Y.; Zhao, W.; Dong, X.; Jin, J.; et al. Multiplex profiling of peritoneal metastases from gastric adenocarcinoma identified novel targets and molecular subtypes that predict treatment response. Gut 2020, 69, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, A.; Mishra, D.; Brandl, A.; Yonemura, Y. Gastric Cancer with Peritoneal Metastasis—A Comprehensive Review of Current Intraperitoneal Treatment Modalities. Front. Oncol. 2022, 12, 864647. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, A.; Schena, C.A.; El Halabieh, M.A.; Abatini, C.; Vita, E.; Strippoli, A.; Inzani, F.; Rodolfino, E.; Romano, B.; Pacelli, F.; et al. Systemic chemotherapy and pressurized intraperitoneal aerosol chemotherapy (PIPAC): A bidirectional approach for gastric cancer peritoneal metastasis. Surg. Oncol. 2020, 34, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Takahashi, M.; Mutou, T.; Kobayashi, K.; Toyosawa, T. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer 1999, 85, 529–534. [Google Scholar] [CrossRef]
- Yang, X.J.; Huang, C.Q.; Suo, T.; Mei, L.J.; Yang, G.L.; Cheng, F.L.; Zhou, Y.F.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann. Surg. Oncol. 2011, 18, 1575–1581. [Google Scholar] [CrossRef]
- Bonnot, P.E.; Lintis, A.; Mercier, F.; Benzerdjeb, N.; Passot, G.; Pocard, M.; Meunier, B.; Bereder, J.M.; Abboud, K.; Marchal, F.; et al. Prognosis of poorly cohesive gastric cancer after complete cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (CYTO-CHIP study). Br. J. Surg. 2021, 108, 1225–1235. [Google Scholar] [CrossRef]
- Rau, B.; Brandl, A.; Piso, P.; Pelz, J.; Busch, P.; Demtroder, C.; Schule, S.; Schlitt, H.J.; Roitman, M.; Tepel, J.; et al. Peritoneal metastasis in gastric cancer: Results from the German database. Gastric Cancer 2020, 23, 11–22. [Google Scholar] [CrossRef]
- Rau, B.; Lang, H.; Königsrainer, A.; Gockel, I.; Rau, H.G.; Seeliger, H.; Lerchenmüller, C.; Reim, D.; Wahba, R.; Angele, M.; et al. 1376O The effect of hyperthermic intraperitoneal chemotherapy (HIPEC) upon cytoreductive surgery (CRS) in gastric cancer (GC) with synchronous peritoneal metastasis (PM): A randomized multicentre phase III trial (GASTRIPEC-I-trial). Ann. Oncol. 2021, 32, S1040. [Google Scholar] [CrossRef]
- Alyami, M.; Bonnot, P.E.; Mercier, F.; Laplace, N.; Villeneuve, L.; Passot, G.; Bakrin, N.; Kepenekian, V.; Glehen, O. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for unresectable peritoneal metastasis from gastric cancer. Eur. J. Surg. Oncol. 2021, 47, 123–127. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Chia, C.S.; You, B.; Decullier, E.; Vaudoyer, D.; Lorimier, G.; Abboud, K.; Bereder, J.M.; Arvieux, C.; Boschetti, G.; Glehen, O.; et al. Patients with Peritoneal Carcinomatosis from Gastric Cancer Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Is Cure a Possibility? Ann. Surg. Oncol. 2016, 23, 1971–1979. [Google Scholar] [CrossRef]
- Rosa, F.; Galiandro, F.; Ricci, R.; Di Miceli, D.; Longo, F.; Quero, G.; Tortorelli, A.P.; Alfieri, S. Survival advantage of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for advanced gastric cancer: Experience from a Western tertiary referral center. Langenbeck’s Arch. Surg. 2021, 406, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Badgwell, B.; Ikoma, N.; Murphy, M.B.; Wang, X.; Estrella, J.; Roy-Chowdhuri, S.; Das, P.; Minsky, B.D.; Lano, E.; Song, S.; et al. A Phase II Trial of Cytoreduction, Gastrectomy, and Hyperthermic Intraperitoneal Perfusion with Chemotherapy for Patients with Gastric Cancer and Carcinomatosis or Positive Cytology. Ann. Surg. Oncol. 2021, 28, 258–264. [Google Scholar] [CrossRef]
- Bonnot, P.E.; Piessen, G.; Kepenekian, V.; Decullier, E.; Pocard, M.; Meunier, B.; Bereder, J.M.; Abboud, K.; Marchal, F.; Quenet, F.; et al. Cytoreductive Surgery with or without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J. Clin. Oncol. 2019, 37, 2028–2040. [Google Scholar] [CrossRef]
- Ukegjini, K.; Putora, P.M.; Guidi, M.; Suveg, K.; Cihoric, N.; Widmann, B.; Steffen, T. Pressurized Intraperitoneal Aerosol Chemotherapy-Related Clinical Trials in the Treatment of Peritoneal Metastases. Oncology 2021, 99, 601–610. [Google Scholar] [CrossRef]
- Eveno, C.; Jouvin, I.; Pocard, M. PIPAC EstoK 01: Pressurized IntraPeritoneal Aerosol Chemotherapy with cisplatin and doxorubicin (PIPAC C/D) in gastric peritoneal metastasis: A randomized and multicenter phase II study. Pleura Peritoneum 2018, 3, 20180116. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Colorectal cancer: Prevention and management of metastatic disease. BioMed Res. Int. 2014, 2014, 782890. [Google Scholar] [CrossRef]
- Massalou, D.; Benizri, E.; Chevallier, A.; Duranton-Tanneur, V.; Pedeutour, F.; Benchimol, D.; Bereder, J.M. Peritoneal carcinomatosis of colorectal cancer: Novel clinical and molecular outcomes. Am. J. Surg. 2017, 213, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Quere, P.; Facy, O.; Manfredi, S.; Jooste, V.; Faivre, J.; Lepage, C.; Bouvier, A.M. Epidemiology, Management, and Survival of Peritoneal Carcinomatosis from Colorectal Cancer: A Population-Based Study. Dis. Colon Rectum. 2015, 58, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Hugen, N.; van de Velde, C.J.H.; de Wilt, J.H.W.; Nagtegaal, I.D. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann. Oncol. 2014, 25, 651–657. [Google Scholar] [CrossRef]
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.A.; Koopman, M.; Tournigand, C.; et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef]
- Tol, J.; Koopman, M.; Cats, A.; Rodenburg, C.J.; Creemers, G.J.; Schrama, J.G.; Erdkamp, F.L.; Vos, A.H.; van Groeningen, C.J.; Sinnige, H.A.; et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N. Engl. J. Med. 2009, 360, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Gilly, F.; Boutitie, F.; Quenet, F.; Bereder, J.M.; Mansvelt, B.; Lorimier, G.; Dube, P.; Glehen, O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: Retrospective analysis of 523 patients from a multicentric French study. J. Clin. Oncol. 2010, 28, 63–68. [Google Scholar] [CrossRef]
- Arjona-Sanchez, A.; Medina-Fernandez, F.J.; Munoz-Casares, F.C.; Casado-Adam, A.; Sanchez-Hidalgo, J.M.; Rufian-Pena, S. Peritoneal metastases of colorectal origin treated by cytoreduction and HIPEC: An overview. World J. Gastrointest. Oncol. 2014, 6, 407–412. [Google Scholar] [CrossRef]
- Mirnezami, R.; Mehta, A.M.; Chandrakumaran, K.; Cecil, T.; Moran, B.J.; Carr, N.; Verwaal, V.J.; Mohamed, F.; Mirnezami, A.H. Cytoreductive surgery in combination with hyperthermic intraperitoneal chemotherapy improves survival in patients with colorectal peritoneal metastases compared with systemic chemotherapy alone. Br. J. Cancer 2014, 111, 1500–1508. [Google Scholar] [CrossRef]
- Elias, D.; Mariani, A.; Cloutier, A.S.; Blot, F.; Goere, D.; Dumont, F.; Honore, C.; Billard, V.; Dartigues, P.; Ducreux, M. Modified selection criteria for complete cytoreductive surgery plus HIPEC based on peritoneal cancer index and small bowel involvement for peritoneal carcinomatosis of colorectal origin. Eur. J. Surg. Oncol. 2014, 40, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Ljunggren, M.; Nordenvall, C.; Palmer, G. Direct surgery with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for patients with colorectal peritoneal metastases. Eur. J. Surg. Oncol. 2021, 47, 2865–2872. [Google Scholar] [CrossRef]
- Goere, D.; Souadka, A.; Faron, M.; Cloutier, A.S.; Viana, B.; Honore, C.; Dumont, F.; Elias, D. Extent of colorectal peritoneal carcinomatosis: Attempt to define a threshold above which HIPEC does not offer survival benefit: A comparative study. Ann. Surg. Oncol. 2015, 22, 2958–2964. [Google Scholar] [CrossRef]
- Goere, D.; Malka, D.; Tzanis, D.; Gava, V.; Boige, V.; Eveno, C.; Maggiori, L.; Dumont, F.; Ducreux, M.; Elias, D. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann. Surg. 2013, 257, 1065–1071. [Google Scholar] [CrossRef]
- Quenet, F.; Roca, L.; de Forges, H.; Glehen, O.; Goere, D. Limitations of the PRODIGE 7 trial—Authors’ reply. Lancet Oncol. 2021, 22, e179–e180. [Google Scholar] [CrossRef]
- Simkens, G.A.; Razenberg, L.G.; Lemmens, V.E.; Rutten, H.J.; Creemers, G.J.; de Hingh, I.H. Histological subtype and systemic metastases strongly influence treatment and survival in patients with synchronous colorectal peritoneal metastases. Eur. J. Surg. Oncol. 2016, 42, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, R.; Cercek, A.; Chou, J.F.; Sylvester, B.E.; Kemeny, N.E.; Hechtman, J.F.; Ladanyi, M.; Rosen, N.; Weiser, M.R.; Capanu, M.; et al. BRAF mutation predicts for poor outcomes after metastasectomy in patients with metastatic colorectal cancer. Cancer 2014, 120, 2316–2324. [Google Scholar] [CrossRef]
- Larsen, S.G.; Goscinski, M.A.; Dueland, S.; Steigen, S.E.; Hofsli, E.; Torgunrud, A.; Lund-Iversen, M.; Dagenborg, V.J.; Flatmark, K.; Sorbye, H. Impact of KRAS, BRAF and microsatellite instability status after cytoreductive surgery and HIPEC in a national cohort of colorectal peritoneal metastasis patients. Br. J. Cancer 2022, 126, 726–735. [Google Scholar] [CrossRef]
- Breuer, E.; Hebeisen, M.; Schneider, M.A.; Roth, L.; Pauli, C.; Frischer-Ordu, K.; Eden, J.; Pache, B.; Steffen, T.; Hübner, M.; et al. Site of Recurrence and Survival after Surgery for Colorectal Peritoneal Metastasis. J. Natl. Cancer Inst. 2021, 113, 1027–1035. [Google Scholar] [CrossRef]
- Schneider, M.A.; Eden, J.; Pache, B.; Laminger, F.; Lopez-Lopez, V.; Steffen, T.; Hübner, M.; Kober, F.; Roka, S.; Campos, P.C.; et al. Mutations of RAS/RAF Proto-oncogenes Impair Survival After Cytoreductive Surgery and HIPEC for Peritoneal Metastasis of Colorectal Origin. Ann. Surg. 2018, 268, 845–853. [Google Scholar] [CrossRef]
- Krebsgesellschaft, D. S3-Leitlinie Kolorektales Karzinom, Langversion 2.1; Leitlinienprogramm Onkologie: Berlin, Germany, 2019. [Google Scholar]
- The National Institute for Health and Care Excellence. Colorectal cancer. In NICE Guideline; The National Institute for Health and Care Excellence: London, UK, 2020. [Google Scholar]
- National Comprehensive Cancer Network. Colon Cancer. In NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines); NCCN: Plymouth Meeting, PA, USA, 2021; pp. MS-28–MS-30. [Google Scholar]
- Brind’Amour, A.; Dube, P.; Tremblay, J.F.; Soucisse, M.L.; Mack, L.; Bouchard-Fortier, A.; McCart, J.A.; Govindarajan, A.; Bischof, D.; Haase, E.; et al. Canadian guidelines on the management of colorectal peritoneal metastases. Curr. Oncol. 2020, 27, e621–e631. [Google Scholar] [CrossRef] [PubMed]
- Kamada, Y.; Hida, K.; Yonemura, Y.; Sugarbaker, P.H.; Ghabra, S.; Ishihara, S.; Nagata, H.; Murono, K.; Goi, T.; Katayama, K.; et al. The Characteristics of 206 Long-Term Survivors with Peritoneal Metastases from Colorectal Cancer Treated with Curative Intent Surgery: A Multi-Center Cohort from PSOGI. Cancers 2021, 13, 2964. [Google Scholar] [CrossRef] [PubMed]
- Steffen, T.; Eden, J.; Bijelic, L.; Glatzer, M.; Glehen, O.; Goere, D.; de Hingh, I.; Li, Y.; Moran, B.; Morris, D.; et al. Patient Selection for Hyperthermic Intraperitoneal Chemotherapy in Patients with Colorectal Cancer: Consensus on Decision Making among International Experts. Clin. Colorectal Cancer 2020, 19, 277–284. [Google Scholar] [CrossRef]
- Quenet, F.; Goere, D.; Mehta, S.S.; Roca, L.; Dumont, F.; Hessissen, M.; Saint-Aubert, B.; Elias, D. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann. Surg. 2011, 254, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Verwaal, V.J.; Boot, H.; Aleman, B.M.; van Tinteren, H.; Zoetmulder, F.A. Recurrences after peritoneal carcinomatosis of colorectal origin treated by cytoreduction and hyperthermic intraperitoneal chemotherapy: Location, treatment, and outcome. Ann. Surg. Oncol. 2004, 11, 375–379. [Google Scholar] [CrossRef]
- van Oudheusden, T.R.; Nienhuijs, S.W.; Luyer, M.D.; Nieuwenhuijzen, G.A.; Lemmens, V.E.; Rutten, H.J.; de Hingh, I.H. Incidence and treatment of recurrent disease after cytoreductive surgery and intraperitoneal chemotherapy for peritoneally metastasized colorectal cancer: A systematic review. Eur. J. Surg. Oncol. 2015, 41, 1269–1277. [Google Scholar] [CrossRef]
- Alzahrani, N.A.; Valle, S.J.; Fisher, O.M.; Sugarbaker, P.H.; Yonemura, Y.; Glehen, O.; Goere, D.; Honore, C.; Brigand, C.; de Hingh, I.; et al. Iterative cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastases: A multi-institutional experience. J. Surg. Oncol. 2019, 119, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Iversen, L.H.; Rasmussen, P.C.; Laurberg, S. Value of laparoscopy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Br. J. Surg. 2013, 100, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Steffen, T.; Putora, P.M.; Hübner, M.; Gloor, B.; Lehmann, K.; Kettelhack, C.; Adamina, M.; Peterli, R.; Schmidt, J.; Ris, F.; et al. Diagnostic Nodes of Patient Selection for Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Among Colorectal Cancer Patients: A Swiss National Multicenter Survey. Clin. Colorectal Cancer 2019, 18, e335–e342. [Google Scholar] [CrossRef] [PubMed]
- Lurvink, R.J.; Rauwerdink, P.; Rovers, K.P.; Wassenaar, E.C.E.; Deenen, M.J.; Nederend, J.; Huysentruyt, C.J.R.; van’t Erve, I.; Fijneman, R.J.A.; van der Hoeven, E.; et al. First-line palliative systemic therapy alternated with electrostatic pressurised intraperitoneal aerosol chemotherapy (oxaliplatin) for isolated unresectable colorectal peritoneal metastases: Protocol of a multicentre, single-arm, phase II study (CRC-PIPAC-II). BMJ Open 2021, 11, e044811. [Google Scholar] [CrossRef] [PubMed]
- Alyami, M.; Hubner, M.; Grass, F.; Bakrin, N.; Villeneuve, L.; Laplace, N.; Passot, G.; Glehen, O.; Kepenekian, V. Pressurised intraperitoneal aerosol chemotherapy: Rationale, evidence, and potential indications. Lancet Oncol. 2019, 20, e368–e377. [Google Scholar] [CrossRef]
- Raspe, C.; Flother, L.; Schneider, R.; Bucher, M.; Piso, P. Best practice for perioperative management of patients with cytoreductive surgery and HIPEC. Eur. J. Surg. Oncol. 2016, 43, 1013–1027. [Google Scholar] [CrossRef]
- Kuijpers, A.M.; Hauptmann, M.; Aalbers, A.G.; Nienhuijs, S.W.; de Hingh, I.H.; Wiezer, M.J.; van Ramshorst, B.; van Ginkel, R.J.; Havenga, K.; Verwaal, V.J. Cytoreduction and hyperthermic intraperitoneal chemotherapy: The learning curve reassessed. Eur. J. Surg. Oncol. 2016, 42, 244–250. [Google Scholar] [CrossRef]
- Kuijpers, A.M.; Aalbers, A.G.; Nienhuijs, S.W.; de Hingh, I.H.; Wiezer, M.J.; van Ramshorst, B.; van Ginkel, R.J.; Havenga, K.; Heemsbergen, W.D.; Hauptmann, M.; et al. Implementation of a standardized HIPEC protocol improves outcome for peritoneal malignancy. World J. Surg. 2015, 39, 453–460. [Google Scholar] [CrossRef]
- Kuijpers, A.M.; Mirck, B.; Aalbers, A.G.; Nienhuijs, S.W.; de Hingh, I.H.; Wiezer, M.J.; van Ramshorst, B.; van Ginkel, R.J.; Havenga, K.; Bremers, A.J.; et al. Cytoreduction and HIPEC in the Netherlands: Nationwide long-term outcome following the Dutch protocol. Ann. Surg. Oncol. 2013, 20, 4224–4230. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamina, M.; Warlaumont, M.; Berger, M.D.; Däster, S.; Delaloye, R.; Digklia, A.; Gloor, B.; Fritsch, R.; Koeberle, D.; Koessler, T.; et al. Comprehensive Treatment Algorithms of the Swiss Peritoneal Cancer Group for Peritoneal Cancer of Gastrointestinal Origin. Cancers 2022, 14, 4275. https://doi.org/10.3390/cancers14174275
Adamina M, Warlaumont M, Berger MD, Däster S, Delaloye R, Digklia A, Gloor B, Fritsch R, Koeberle D, Koessler T, et al. Comprehensive Treatment Algorithms of the Swiss Peritoneal Cancer Group for Peritoneal Cancer of Gastrointestinal Origin. Cancers. 2022; 14(17):4275. https://doi.org/10.3390/cancers14174275
Chicago/Turabian StyleAdamina, Michel, Maxime Warlaumont, Martin D. Berger, Silvio Däster, Raphaël Delaloye, Antonia Digklia, Beat Gloor, Ralph Fritsch, Dieter Koeberle, Thibaud Koessler, and et al. 2022. "Comprehensive Treatment Algorithms of the Swiss Peritoneal Cancer Group for Peritoneal Cancer of Gastrointestinal Origin" Cancers 14, no. 17: 4275. https://doi.org/10.3390/cancers14174275
APA StyleAdamina, M., Warlaumont, M., Berger, M. D., Däster, S., Delaloye, R., Digklia, A., Gloor, B., Fritsch, R., Koeberle, D., Koessler, T., Lehmann, K., Müller, P., Peterli, R., Ris, F., Steffen, T., Weisshaupt, C. S., & Hübner, M. (2022). Comprehensive Treatment Algorithms of the Swiss Peritoneal Cancer Group for Peritoneal Cancer of Gastrointestinal Origin. Cancers, 14(17), 4275. https://doi.org/10.3390/cancers14174275





