Correlation of Yes-Associated Protein 1 with Stroma Type and Tumor Stiffness in Hormone-Receptor Positive Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
- Inclusion criteria:
- Patients aged ≥ 20 years
- Invasive breast cancer confirmed by pathological diagnosis
- Preoperative elastography results
- Available YAP1 IHC staining of the resected tissue
- Exclusion criteria:
- Any other carcinoma in situ
- Other cancer history (except for thyroid cancer and carcinoma in situ)
- Inaccessible electronic medical records
- Received Neoadjuvant Chemotherapy (NAC)
- Hormone receptor-positive (HR+) HER2 negative (HER2−): ER and/or PR positive and HER2 negative
- HR+HER2+: ER and/or PR positive and HER2 overexpressed and/or amplified
- HER2: ER and PR negative and HER2 overexpressed and/or amplified
- Triple-negative breast cancer (TNBC): ER, PR, and HER2 negative
2.2. Shear Wave Elastography
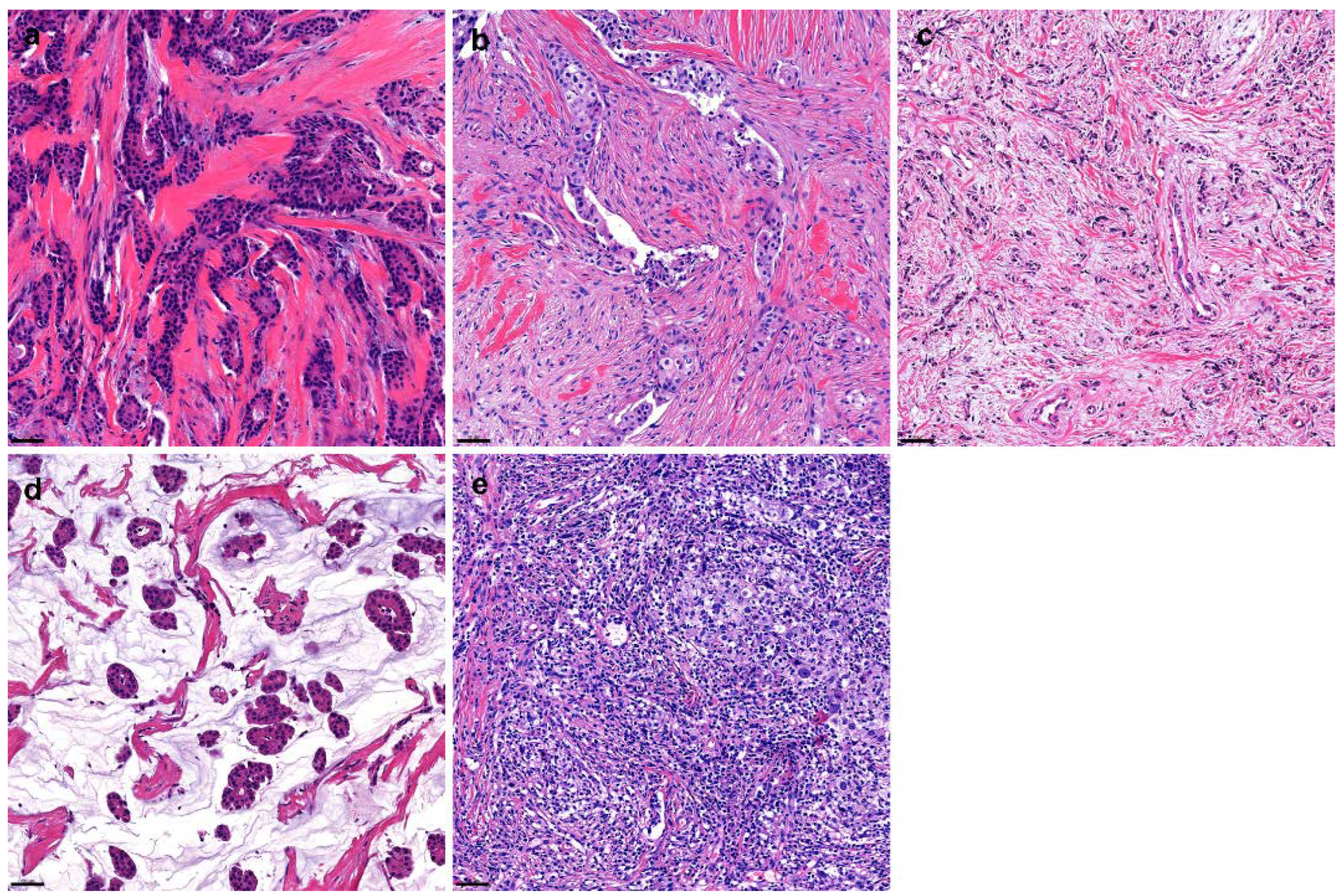
2.3. Evaluation of Tumor–Stroma Ratio and Tumor Stroma Subtyping
- Collagen: dense collagenous fibrosis without cellular components
- Non-collagen: non-collagenous stroma, including mesenchymal, inflammatory, or mucinous components
2.4. YAP1 IHC and Interpretation
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Different Elasticity of Breast Cancer According to Pathologic Parameters
3.3. Association between YAP1 Expression and Elasticity
3.4. Subgroup Analyses: YAP1 Expression and Tumor Stiffness Based on the HR Status and Stroma Type
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variables | Patients (n = 488) |
|---|---|
| Breast surgery | |
| Breast-conserving surgery | 363 (74.4) |
| Mastectomy | 125 (25.6) |
| Axillary surgery | |
| SLNB | 429 (87.9) |
| ALND | 59 (12.1) |
| Chemotherapy | |
| Anthracycline-based | 112 (23.0) |
| Anthracycline and Taxane-based | 114 (23.3) |
| Taxane-based | 23 (4.7) |
| Not done | 239 (49.0) |
| Hormone therapy | |
| Tamoxifen | 207 (42.4) |
| Aromatase inhibitor | 193 (39.5) |
| Not done | 88 (18.1) |
| Trastuzumab | |
| Done | 69 (14.1) |
| Not done | 419 (85.9) |
| Radiotherapy | |
| Done | 389 (79.7) |
| Not done | 99 (20.3) |
| Linear Regression with YAP1 H-Score | Univariate | Multivariate | ||||
| ß | SE | p | ß | SE | p | |
| Elasticity ratio | 0.161 | 0.162 | 0.321 | |||
| Mean elasticity | 0.062 | 0.026 | 0.017 | |||
| Maximal elasticity | 0.058 | 0.024 | 0.015 | 0.058 | 0.024 | 0.015 |
| Minimal elasticity | 0.025 | 0.019 | 0.205 | |||
| Logistic Regression with YAP1 Positivity | Univariate | Multivariate | ||||
| OR | 95% CI | p | OR | 95% CI | p | |
| Elasticity ratio | 1.006 | 0.989–1.024 | 0.47 | |||
| Mean elasticity | 1.004 | 1.001–1.007 | 0.014 | |||
| Maximal elasticity | 0.114 | 1.001–1.006 | 0.011 | 1.004 | 1.001–1.007 | 0.006 |
| Minimal elasticity | 1.003 | 1.000–1.007 | 0.033 | |||
| Hormone Receptor Negative | Univariate | Multivariate | ||||
| OR | 95% CI | p | OR | CI | p | |
| Elasticity ratio | 1.017 | 0.979–1.057 | 0.386 | |||
| Mean elasticity | 1.005 | 0.997–1.012 | 0.211 | |||
| Maximal elasticity | 1.005 | 0.998–1.012 | 0.161 | |||
| Minimal elasticity | 1.003 | 0.995–1.010 | 0.459 | |||
| Hormone Receptor Positive | Univariate | Multivariate | ||||
| OR | 95% CI | p | OR | CI | p | |
| Elasticity ratio | 1.003 | 0.984–1.023 | 0.729 | |||
| Mean elasticity | 1.004 | 1.000–1.007 | 0.035 | |||
| Maximal elasticity | 1.003 | 1.000–1.006 | 0.031 | 1.004 | 1.001–1.007 | 0.018 |
| Minimal elasticity | 1.003 | 1.000–1.007 | 0.050 | |||
| Collagen Type Stroma | Univariate | Multivariate | ||||
| OR | 95% CI | p | OR | 95% CI | p | |
| Elasticity ratio | 1.004 | 0.975–1.034 | 0.783 | |||
| Mean elasticity | 1.002 | 0.998–1.007 | 0.302 | |||
| Maximal elasticity | 1.002 | 0.998–1.006 | 0.363 | |||
| Minimal elasticity | 1.004 | 0.999–1.009 | 0.145 | |||
| Non-Collagen Type Stroma | Univariate | Multivariate | ||||
| OR | 95% CI | p | OR | 95% CI | p | |
| Elasticity ratio | 1.008 | 0.986–1.030 | 0.471 | |||
| Mean elasticity | 1.005 | 1.001–1.009 | 0.020 | |||
| Maximal elasticity | 1.005 | 1.001–1.008 | 0.010 | 1.005 | 1.001–1.089 | 0.010 |
| Minimal elasticity | 1.003 | 0.999–1.007 | 0.109 | |||
References
- Kanai, F.; Marignani, P.A.; Sarbassova, D.; Yagi, R.; Hall, R.A.; Donowitz, M.; Hisaminato, A.; Fujiwara, T.; Ito, Y.; Cantley, L.C.; et al. Taz: A novel transcriptional co-activator regulated by interactions with 14-3-3 and pdz domain proteins. EMBO J. 2000, 19, 6778–6791. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.; Barrera, J.; Matthews, K.; Pan, D. The hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating yorkie, the drosophila homolog of yap. Cell 2005, 122, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Pan, D. The hippo signaling pathway in development and cancer. Dev. Cell 2010, 19, 491–505. [Google Scholar] [CrossRef]
- Hall, C.A.; Wang, R.; Miao, J.; Oliva, E.; Shen, X.; Wheeler, T.; Hilsenbeck, S.G.; Orsulic, S.; Goode, S. Hippo pathway effector yap is an ovarian cancer oncogene. Cancer Res. 2010, 70, 8517–8525. [Google Scholar] [CrossRef]
- Zhang, X.; George, J.; Deb, S.; Degoutin, J.L.; Takano, E.A.; Fox, S.B.; Bowtell, D.D.; Harvey, K.F. The hippo pathway transcriptional co-activator, yap, is an ovarian cancer oncogene. Oncogene 2011, 30, 2810–2822. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, Q.; Zhang, Q.; Li, Z.; Wang, E.; Qiu, X. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 2010, 101, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, T.; Imoto, I.; Matsui, T.; Kozaki, K.; Haruki, S.; Sudol, M.; Shimada, Y.; Tsuda, H.; Kawano, T.; Inazawa, J. Yap is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis 2011, 32, 389–398. [Google Scholar] [CrossRef]
- Cha, Y.J.; Bae, S.J.; Kim, D.; Ahn, S.G.; Jeong, J.; Koo, J.S.; Yoo, T.K.; Park, W.C.; Lee, A.; Yoon, C.I. High nuclear expression of yes-associated protein 1 correlates with metastasis in patients with breast cancer. Front. Oncol. 2021, 11, 609743. [Google Scholar] [CrossRef]
- Chen, W.; Bai, Y.; Patel, C.; Geng, F. Autophagy promotes triple negative breast cancer metastasis via yap nuclear localization. Biochem. Biophys. Res. Commun. 2019, 520, 263–268. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of yap/taz in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Noguchi, S.; Saito, A.; Nagase, T. Yap/taz signaling as a molecular link between fibrosis and cancer. Int. J. Mol. Sci. 2018, 19, 3674. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Tomlinson, V.; Lara, R.; Holliday, D.; Chelala, C.; Harada, T.; Gangeswaran, R.; Manson-Bishop, C.; Smith, P.; Danovi, S.A.; et al. Yes-associated protein (yap) functions as a tumor suppressor in breast. Cell Death Differ. 2008, 15, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Rodriguez, Y.; Cerda-Flores, R.M.; Ruiz-Ramos, R.; Lopez-Marquez, F.C.; Calderon-Garciduenas, A.L. Yap expression in normal and neoplastic breast tissue: An immunohistochemical study. Arch. Med. Res. 2014, 45, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Sun, P.-L.; Yao, M.; Jia, M.; Gao, H. Expression of yes-associated protein (yap) and its clinical significance in breast cancer tissues. Hum. Pathol. 2017, 68, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Su, L.; Ou, Q. Yes-associated protein promotes tumour development in luminal epithelial derived breast cancer. Eur. J. Cancer 2012, 48, 1227–1234. [Google Scholar] [CrossRef]
- Maugeri-Saccà, M.; Barba, M.; Pizzuti, L.; Vici, P.; Di Lauro, L.; Dattilo, R.; Vitale, I.; Bartucci, M.; Mottolese, M.; De Maria, R. The hippo transducers taz and yap in breast cancer: Oncogenic activities and clinical implications. Expert Rev. Mol. Med. 2015, 17, e14. [Google Scholar] [CrossRef]
- Youk, J.H.; Gweon, H.M.; Son, E.J. Shear-wave elastography in breast ultrasonography: The state of the art. Ultrasonography 2017, 36, 300–309. [Google Scholar] [CrossRef]
- Chang, J.M.; Moon, W.K.; Cho, N.; Yi, A.; Koo, H.R.; Han, W.; Noh, D.Y.; Moon, H.G.; Kim, S.J. Clinical application of shear wave elastography (swe) in the diagnosis of benign and malignant breast diseases. Breast Cancer Res. Treat. 2011, 129, 89–97. [Google Scholar] [CrossRef]
- Yoon, J.H.; Ko, K.H.; Jung, H.K.; Lee, J.T. Qualitative pattern classification of shear wave elastography for breast masses: How it correlates to quantitative measurements. Eur. J. Radiol. 2013, 82, 2199–2204. [Google Scholar] [CrossRef]
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of american pathologists clinical practice guideline focused update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (tils) in breast cancer: Recommendations by an international tils working group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Abuhadra, N.; Hess, K.R.; Litton, J.K.; Rauch, G.M.; Thompson, A.M.; Lim, B.; Adrada, B.E.; Mittendorf, E.A.; Damodaran, S.; Candelaria, R.P.; et al. Beyond tils: Predictors of pathologic complete response (pcr) in triple-negative breast cancer (tnbc) patients with moderate tumor-infiltrating lymphocytes (til) receiving neoadjuvant therapy. J. Clin. Oncol. 2019, 37, 572. [Google Scholar] [CrossRef]
- Kemi, N.; Eskuri, M.; Herva, A.; Leppänen, J.; Huhta, H.; Helminen, O.; Saarnio, J.; Karttunen, T.J.; Kauppila, J.H. Tumour-stroma ratio and prognosis in gastric adenocarcinoma. Br. J. Cancer 2018, 119, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Tufail, R.; Jorda, M.; Zhao, W.; Reis, I.; Nawaz, Z. Loss of yes-associated protein (yap) expression is associated with estrogen and progesterone receptors negativity in invasive breast carcinomas. Breast Cancer Res. Treat. 2012, 131, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, Y.; Luo, J.; Zheng, J.; Shao, G. Yap1 overexpression is associated with poor prognosis of breast cancer patients and induces breast cancer cell growth by inhibiting pten. FEBS Open Bio. 2019, 9, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Totaro, A.; Panciera, T.; Piccolo, S. Yap/taz upstream signals and downstream responses. Nat. Cell Biol. 2018, 20, 888–899. [Google Scholar] [CrossRef]
- Yi, A.; Moon, W.K.; Cho, N.; Chang, J.M.; Bae, M.S.; Kim, S.J.; Han, W.; Park, I.A. Association of tumour stiffness on sonoelastography with axillary nodal status in t1 breast carcinoma patients. Eur. Radiol. 2013, 23, 2979–2987. [Google Scholar] [CrossRef]
- Evans, A.; Whelehan, P.; Thomson, K.; McLean, D.; Brauer, K.; Purdie, C.; Baker, L.; Jordan, L.; Rauchhaus, P.; Thompson, A. Invasive breast cancer: Relationship between shear-wave elastographic findings and histologic prognostic factors. Radiology 2012, 263, 673–677. [Google Scholar] [CrossRef]
- Youk, J.H.; Gweon, H.M.; Son, E.J.; Kim, J.A.; Jeong, J. Shear-wave elastography of invasive breast cancer: Correlation between quantitative mean elasticity value and immunohistochemical profile. Breast Cancer Res. Treat. 2013, 138, 119–126. [Google Scholar] [CrossRef]
- Valkenburg, K.C.; de Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Cukierman, E. Stromal dynamic reciprocity in cancer: Intricacies of fibroblastic-ecm interactions. Curr. Opin. Cell Biol. 2016, 42, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar]
- Qin, X.; Lv, X.; Li, P.; Yang, R.; Xia, Q.; Chen, Y.; Peng, Y.; Li, L.; Li, S.; Li, T.; et al. Matrix stiffness modulates ilk-mediated yap activation to control the drug resistance of breast cancer cells. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165625. [Google Scholar] [CrossRef]
- Murakami, S.; Shahbazian, D.; Surana, R.; Zhang, W.; Chen, H.; Graham, G.T.; White, S.M.; Weiner, L.M.; Yi, C. Yes-associated protein mediates immune reprogramming in pancreatic ductal adenocarcinoma. Oncogene 2017, 36, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Bristow, C.A.; Dey, P.; Rai, K.; Perets, R.; Ramirez-Cardenas, A.; Malasi, S.; Huang-Hobbs, E.; Haemmerle, M.; Wu, S.Y.; et al. Prkci promotes immune suppression in ovarian cancer. Genes Dev. 2017, 31, 1109–1121. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, Y.; Yan, H.; Yang, Y.; Shen, S.; Dai, X.; Ji, X.; Ji, F.; Gong, X.G.; Li, L.; et al. Single tumor-initiating cells evade immune clearance by recruiting type ii macrophages. Genes Dev. 2017, 31, 247–259. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, H.; Wang, J.; Liu, Y.; Luo, T.; Hua, H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J. Hematol. Oncol. 2022, 15, 34. [Google Scholar] [CrossRef]
- Chakraborty, M.; Chu, K.; Shrestha, A.; Revelo, X.S.; Zhang, X.; Gold, M.J.; Khan, S.; Lee, M.; Huang, C.; Akbari, M.; et al. Mechanical stiffness controls dendritic cell metabolism and function. Cell Rep. 2021, 34, 108609. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chang, J.K.; Dominguez, A.A.; Lee, H.P.; Nam, S.; Chang, J.; Varma, S.; Qi, L.S.; West, R.B.; Chaudhuri, O. Yap-independent mechanotransduction drives breast cancer progression. Nat. Commun. 2019, 10, 1848. [Google Scholar] [CrossRef]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and yap-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Bonnefoi, H.; Litière, S.; Piccart, M.; MacGrogan, G.; Fumoleau, P.; Brain, E.; Petit, T.; Rouanet, P.; Jassem, J.; Moldovan, C.; et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: A landmark and two-step approach analyses from the eortc 10994/big 1-00 phase iii trial. Ann. Oncol. 2014, 25, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Kuroi, K.; Toi, M.; Ohno, S.; Nakamura, S.; Iwata, H.; Masuda, N.; Sato, N.; Tsuda, H.; Kurosumi, M.; Akiyama, F. Prognostic significance of subtype and pathologic response in operable breast cancer; a pooled analysis of prospective neoadjuvant studies of jbcrg. Breast Cancer 2015, 22, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Hess, K.R.; Pusztai, L.; Buzdar, A.U.; Hortobagyi, G.N. Estrogen receptors and distinct patterns of breast cancer relapse. Breast Cancer Res. Treat. 2003, 78, 105–118. [Google Scholar] [CrossRef]


| Variables | Patients (n = 488) |
|---|---|
| Age (years, median, range) | 52 (24–91) |
| Histologic type | |
| Invasive ductal carcinoma | 429 (85.9) |
| Invasive lobular carcinoma | 21 (4.3) |
| Others | 38 (7.8) |
| Tumor size (cm, mean ± SD) | 1.9 ± 1.2 |
| pT stage, % | |
| T1 | 307 (62.9) |
| T2-3 | 181 (37.1) |
| Molecular subtypes | |
| HR + HER2− | 363 (74.4) |
| HR + HER2+ | 45 (9.2) |
| HER2 | 34 (7.0) |
| TNBC | 46 (9.4) |
| LN metastasis | |
| Absent | 381 (78.1) |
| Present | 107 (21.9) |
| Histological grade | |
| I | 101 (20.7) |
| II | 305 (62.5) |
| III | 82 (16.8) |
| TILs (%, mean ± SD) | 19.3 ± 23.1 |
| Tumor-stroma ratio (%, median, IQR) | 70.0 (40.0–80.0) |
| Stroma type | |
| Collagen | 197 (40.4) |
| Non-collagen | 291 (59.6) |
| Ki67 labeling index (%, mean ± SD) | 18.8 ± 21.9 |
| Elasticity value (kPa, mean ± SD) | |
| Mean | 155.0± 69.4 |
| Minimal | 122.9± 95.2 |
| Maximal | 176.1± 76.3 |
| Ratio | 13.5 ± 11.6 |
| FU (months, median, IQR) | 19.1 (12.1–30.2) |
| Death | 0 (0.0) |
| Recurrence | 2 (0.4) |
| Elasticity Values (kPa, Mean ± SD) | Elasticity Ratio | p | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | p | Maximal | p | Minimal | p | |||
| Histologic subtype | ||||||||
| IDC (n = 429) | 153.5 ± 68.6 | 0.525 | 175.1 ± 75.5 | 0.530 | 122.3 ± 96.7 | 0.943 | 13.3 ± 10.9 | 0.027 |
| ILC (n = 21) | 145.8 ± 67.7 | 165.0 ± 74.3 | 119.1 ± 63.6 | 9.3 ± 5.8 | ||||
| Other † (n = 38) | 164.9 ± 65.7 | 186.7 ± 74.8 | 127 ± 60 | 17.7 ± 18.0 | ||||
| Molecular subtype | ||||||||
| HR + HER2− (n = 363) | 153.2 ± 66.8 | 0.128 | 175.4 ± 74.4 | 0.919 | 123.2 ± 100.8 | 0.973 | 12.7 ± 10.2 | 0.899 |
| HR + HER2+ (n = 45) | 153.9 ± 79.0 | 174.3 ± 83.3 | 112.8 ± 75.6 | 16.5 ± 17.9 | ||||
| HER2 (n = 34) | 161.8 ± 75.3 | 181.1 ± 81.4 | 126.2 ± 62.2 | 14.9 ± 11.6 | ||||
| TNBC (n = 46) | 155.1 ± 65.4 | 173.36 ± 71.6 | 124.9 ± 63.2 | 15.0 ± 13.1 | ||||
| HR status | ||||||||
| HR- (n = 81) | 159.7 ± 70.8 | 0.417 | 178.3 ± 76.3 | 0.716 | 127.6 ± 65.0 | 0.593 | 15.0 ± 12.3 | 0.200 |
| HR+ (n = 407) | 153.0 ± 67.8 | 175.0 ± 75.2 | 121.5 ± 97.8 | 13.1 ± 11.3 | ||||
| Stroma type | ||||||||
| Collagenous (n = 197) | 156.9 ± 68.1 | 0.457 | 180.0 ± 76.3 | 0.281 | 120.6 ± 64.3 | 0.705 | 12.9 ± 10.8 | 0.403 |
| Non-collagenous (n = 191) | 152.2 ± 68.4 | 172.5 ± 74.6 | 123.9 ± 108.2 | 13.8 ± 12.0 | ||||
| YAP1 positivity | ||||||||
| YAP1-positive (n = 119) | 166.9 ± 73.3 | 0.013 | 190.1 ± 77.5 | 0.010 | 141.5 ± 153.6 | 0.075 | 14.1 ± 10.3 | 0.470 |
| YAP1-negative (n = 357) | 149.1 ± 66.2 | 169.9 ± 74.1 | 115.7 ± 59.9 | 13.2 ± 12.0 | ||||
| TIL level | ||||||||
| Low-TIL (n = 378) | 163.6 ± 67.8 | <0.001 | 131.6 ± 100.2 | <0.001 | 186.2 ± 74.5 | <0.001 | 14.18 ± 12.2 | 0.002 |
| High-TIL (n = 110) | 121.2 ± 59.4 | 91.5 ± 51.7 | 139.0 ± 66.2 | 10.9 ± 8.5 | ||||
| TSR | ||||||||
| Low-TSR (n = 139) | 141.2 ± 69.9 | 0.008 | 161.1 ± 77.2 | 0.031 | 108.0 ± 62.6 | 0.008 | 12.7 ± 10.8 | 0.364 |
| High-TSR (n = 349) | 159.2 ± 67.1 | 181.3 ± 73.8 | 128.3 ± 102.1 | 13.8 ± 11.8 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Bae, S.J.; Eun, N.L.; Ahn, S.G.; Jeong, J.; Cha, Y.J. Correlation of Yes-Associated Protein 1 with Stroma Type and Tumor Stiffness in Hormone-Receptor Positive Breast Cancer. Cancers 2022, 14, 4971. https://doi.org/10.3390/cancers14204971
Lee Y, Bae SJ, Eun NL, Ahn SG, Jeong J, Cha YJ. Correlation of Yes-Associated Protein 1 with Stroma Type and Tumor Stiffness in Hormone-Receptor Positive Breast Cancer. Cancers. 2022; 14(20):4971. https://doi.org/10.3390/cancers14204971
Chicago/Turabian StyleLee, Yangkyu, Soong June Bae, Na Lae Eun, Sung Gwe Ahn, Joon Jeong, and Yoon Jin Cha. 2022. "Correlation of Yes-Associated Protein 1 with Stroma Type and Tumor Stiffness in Hormone-Receptor Positive Breast Cancer" Cancers 14, no. 20: 4971. https://doi.org/10.3390/cancers14204971
APA StyleLee, Y., Bae, S. J., Eun, N. L., Ahn, S. G., Jeong, J., & Cha, Y. J. (2022). Correlation of Yes-Associated Protein 1 with Stroma Type and Tumor Stiffness in Hormone-Receptor Positive Breast Cancer. Cancers, 14(20), 4971. https://doi.org/10.3390/cancers14204971






