The Role of Stereotactic Radiosurgery in the Management of Foramen Magnum Meningiomas—A Multicenter Analysis and Review of the Literature
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient and Treatment Characteristics
3.2. Treatment Outcomes
3.3. Literature Review
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro Oncol. 2019, 21, v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Dou, Z.; Wu, J.; Jiang, B.; Iranmanesh, Y.; Yu, X.; Li, J.; Zhou, H.; Zhong, C.; Peng, Y.; et al. The Preferred Locations of Meningioma According to Different Biological Characteristics Based on Voxel-Wise Analysis. Front. Oncol. 2020, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
- Arnautović, K.I.; Al-Mefty, O.; Husain, M. Ventral foramen magnum meninigiomas. J. Neurosurg. 2000, 92, 71–80. [Google Scholar] [CrossRef]
- Bruneau, M.; George, B. Foramen magnum meningiomas: Detailed surgical approaches and technical aspects at Lariboisière Hospital and review of the literature. Neurosurg. Rev. 2008, 31, 19–33. [Google Scholar] [CrossRef]
- Bir, S.C.; Maiti, T.K.; Nanda, A. Foramen magnum meningiomas. Handb. Clin. Neurol. 2020, 170, 167–174. [Google Scholar] [CrossRef]
- Paun, L.; Gondar, R.; Borrelli, P.; Meling, T.R. Foramen magnum meningiomas: A systematic review and meta-analysis. Neurosurg. Rev. 2021, 44, 2583–2596. [Google Scholar] [CrossRef]
- Mehta, G.U.; Zenonos, G.; Patibandla, M.R.; Lin, C.J.; Wolf, A.; Grills, I.; Mathieu, D.; McShane, B.; Lee, J.Y.; Blas, K.; et al. Outcomes of stereotactic radiosurgery for foramen magnum meningiomas: An international multicenter study. J. Neurosurg. 2018, 129, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Kilby, W.; Dooley, J.R.; Kuduvalli, G.; Sayeh, S.; Maurer, C.R., Jr. The CyberKnife Robotic Radiosurgery System in 2010. Technol. Cancer Res. Treat. 2010, 9, 433–452. [Google Scholar] [CrossRef]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 2010, 37, 4078–4101. [Google Scholar] [CrossRef]
- Malone, J.; Gaviolli, E.; Doody, J.; Sinclair, J.; Malone, S. Unresectable Foramen Magnum Meningioma Treated With CyberKnife Robotic Stereotactic Radiosurgery. Cureus 2020, 12, e8409. [Google Scholar] [CrossRef]
- Cheshier, S.H.; Hanft, S.J.; Adler, J.R.; Chang, S.D. CyberKnife Radiosurgery for Lesions of the Foramen Magnum. Technol. Cancer Res. Treat. 2007, 6, 329–335. [Google Scholar] [CrossRef]
- Sheehan, J.P.; Starke, R.M.; Kano, H.; Barnett, G.H.; Mathieu, D.; Chiang, V.; Yu, J.B.; Hess, J.; McBride, H.L.; Honea, N.; et al. Gamma Knife radiosurgery for posterior fossa meningiomas: A multicenter study. J. Neurosurg. 2015, 122, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, N.; Kondziolka, D.; Lunsford, L.D.; Flickinger, J.C. Stereotactic radiosurgery for anterior foramen magnum meningiomas. Surg. Neurol. 1999, 51, 268–273. [Google Scholar] [CrossRef]
- Akyoldaş, G.; Samancı, Y.; Yılmaz, M.; Şengöz, M.; Peker, S. Long-term results of gamma knife radiosurgery for foramen magnum meningiomas. Neurosurg. Rev. 2020, 44, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.; Bunevicius, A.; Muttikkal Thomas, E.; Druzgal, J.; Sheehan, J.P. Gamma Knife radiosurgery associated worsening of superficial siderosis due to a foramen magnum tumor—A case report. J. Radiosurg. SBRT 2020, 7, 169–172. [Google Scholar]
- Starke, R.M.; Nguyen, J.H.; Reames, D.L.; Rainey, J.; Sheehan, J.P. Gamma knife radiosurgery of meningiomas involving the foramen magnum. J. Craniovertebr. Junction Spine 2010, 1, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Zenonos, G.; Kondziolka, D.; Flickinger, J.C.; Gardner, P.; Lunsford, L.D. Gamma Knife surgery in the treatment paradigm for foramen magnum meningiomas. J. Neurosurg. 2012, 117, 864–873. [Google Scholar] [CrossRef]
- Nicolato, A.; Foroni, R.; Pellegrino, M.; Ferraresi, P.; Alessandrini, F.; Gerosa, M.; Bricolo, A. Gamma knife radiosurgery in meningiomas of the posterior fossa. Experience with 62 treated lesions. Minim. Invasive Neurosurg. 2001, 44, 211–217. [Google Scholar] [CrossRef]
- Lin, C.; Node, Y.; Teramoto, A. Treatment of posterior skull base tumors. Nihon Ika Daigaku Zasshi 1998, 65, 316–319. [Google Scholar] [CrossRef][Green Version]
- Kondziolka, D.; Mathieu, D.; Lunsford, L.D.; Martin, J.J.; Madhok, R.; Niranjan, A.; Flickinger, J.C. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery 2008, 62, 53–60. [Google Scholar] [CrossRef]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016, 17, e383–e391. [Google Scholar] [CrossRef]
- Fokas, E.; Henzel, M.; Surber, G.; Hamm, K.; Engenhart-Cabillic, R. Stereotactic radiation therapy for benign meningioma: Long-term outcome in 318 patients. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 569–575. [Google Scholar] [CrossRef]
- Capper, D.; Jones, D.T.W.; Sill, M.; Hovestadt, V.; Schrimpf, D.; Sturm, D.; Koelsche, C.; Sahm, F.; Chavez, L.; Reuss, D.E.; et al. DNA methylation-based classification of central nervous system tumours. Nature 2018, 555, 469–474. [Google Scholar] [CrossRef]
- Sahm, F.; Schrimpf, D.; Stichel, D.; Jones, D.T.W.; Hielscher, T.; Schefzyk, S.; Okonechnikov, K.; Koelsche, C.; Reuss, D.E.; Capper, D.; et al. DNA methylation-based classification and grading system for meningioma: A multicentre, retrospective analysis. Lancet Oncol. 2017, 18, 682–694. [Google Scholar] [CrossRef]
- Ruge, M.I.; Tutunji, J.; Rueß, D.; Celik, E.; Baues, C.; Treuer, H.; Kocher, M.; Grau, S. Stereotactic radiosurgery for treating meningiomas eligible for complete resection. Radiat. Oncol. 2021, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Mayo, C.; Yorke, E.; Merchant, T.E. Radiation associated brainstem injury. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S36–S41. [Google Scholar] [CrossRef]
- Trifiletti, D.M.; Lee, C.C.; Kano, H.; Cohen, J.; Janopaul-Naylor, J.; Alonso-Basanta, M.; Lee, J.Y.K.; Simonova, G.; Liscak, R.; Wolf, A.; et al. Stereotactic Radiosurgery for Brainstem Metastases: An International Cooperative Study to Define Response and Toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Goldbrunner, R.; Stavrinou, P.; Jenkinson, M.D.; Sahm, F.; Mawrin, C.; Weber, D.C.; Preusser, M.; Minniti, G.; Lund-Johansen, M.; Lefranc, F.; et al. EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol. 2021, 23, 1821–1834. [Google Scholar] [CrossRef]
- Yeung, J.T.; Karim, S.A.; Chang, S.D. Multi-session radiosurgery of benign intracranial tumors. Neurosurg. Clin. 2013, 24, 543–551. [Google Scholar] [CrossRef]
- Yenice, K.M.; Narayana, A.; Chang, J.; Gutin, P.H.; Amols, H.I. Intensity-modulated stereotactic radiotherapy (IMSRT) for skull-base meningiomas. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, S95–S101. [Google Scholar] [CrossRef]
- Huang, R.Y.; Bi, W.L.; Weller, M.; Kaley, T.; Blakeley, J.; Dunn, I.; Galanis, E.; Preusser, M.; McDermott, M.; Rogers, L.; et al. Proposed response assessment and endpoints for meningioma clinical trials: Report from the Response Assessment in Neuro-Oncology Working Group. Neuro Oncol. 2019, 21, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Bassiouni, H.; Ntoukas, V.; Asgari, S.; Sandalcioglu, E.I.; Stolke, D.; Seifert, V. Foramen magnum meningiomas: Clinical outcome after microsurgical resection via a posterolateral suboccipital retrocondylar approach. Neurosurgery 2006, 59, 1177–1185. [Google Scholar] [CrossRef] [PubMed]

| Number of patients | 62 | ||||
| Gender (male/female) | 18 | 44 | |||
| % | 29 | 71 | |||
| Median | Mean (±SD) | IQR | Range | ||
| Age (years) | 58.5 | 60.1 (±14.5) | 49.0–72.6 | 33.7–89.6 | |
| Pretreatment Karnofsky Performance Status (%) | 100 | 93.0 (10.3) | 90–100 | 50–100 | |
| Follow-up (months) | 28.9 | 39.0 (±31.6) | 12.3–60 | 5.9–132.2 | |
| RRS indication | Primary treatment | Recurrence | Adjuvant treatment | ||
| Number of patients | 39 | 10 | 13 | ||
| % | 63 | 16 | 21 | ||
| Tumor location | Anterior | Lateral | Posterior | PL | AL |
| Number of patients | 14 | 21 | 4 | 9 | 14 |
| % | 23 | 34 | 6 | 14 | 23 |
| Tumor compartment | Intradural | Intra-extradural | Extradural | ||
| Number of patients | 42 | 16 | 4 | ||
| % | 68 | 26 | 6 | ||
| WHO tumor grading * | I | II | |||
| Number of patients | 22 | 1 | |||
| % | 96 | 4 | |||
| Symptom/Deficit | Number of Patients | % | Number of Patients | % |
|---|---|---|---|---|
| Headaches | 10 | 16 | 10 | 16 |
| Vertigo | 9 | 15 | 9 | 15 |
| Hypoesthesia | 8 | 13 | 7 | 11 |
| Muscle weakness/paralysis extremities | 8 | 13 | 8 | 13 |
| XII neuropathy | 5 | 8 | 2 | 3 |
| Dysphagia | 5 | 8 | 2 | 3 |
| Neck pain | 3 | 5 | 1 | 2 |
| Hearing loss | 3 | 5 | 2 | 3 |
| Ataxia | 3 | 5 | 2 | 3 |
| Partial hearing loss | 3 | 5 | 2 | 3 |
| Dysesthesia | 3 | 5 | 0 | 0 |
| Seizures | 2 | 3 | 3 | 5 |
| XI neuropathy | 1 | 2 | 0 | 0 |
| VII neuropathy | 1 | 2 | 1 | 2 |
| CSF fistula | 1 | 2 | 1 | 2 |
| Tinnitus | 1 | 2 | 0 | 0 |
| Dysarthria | 1 | 2 | 0 | 0 |
| Treatment Variable | Median | Mean (±SD) | IQR | Range |
|---|---|---|---|---|
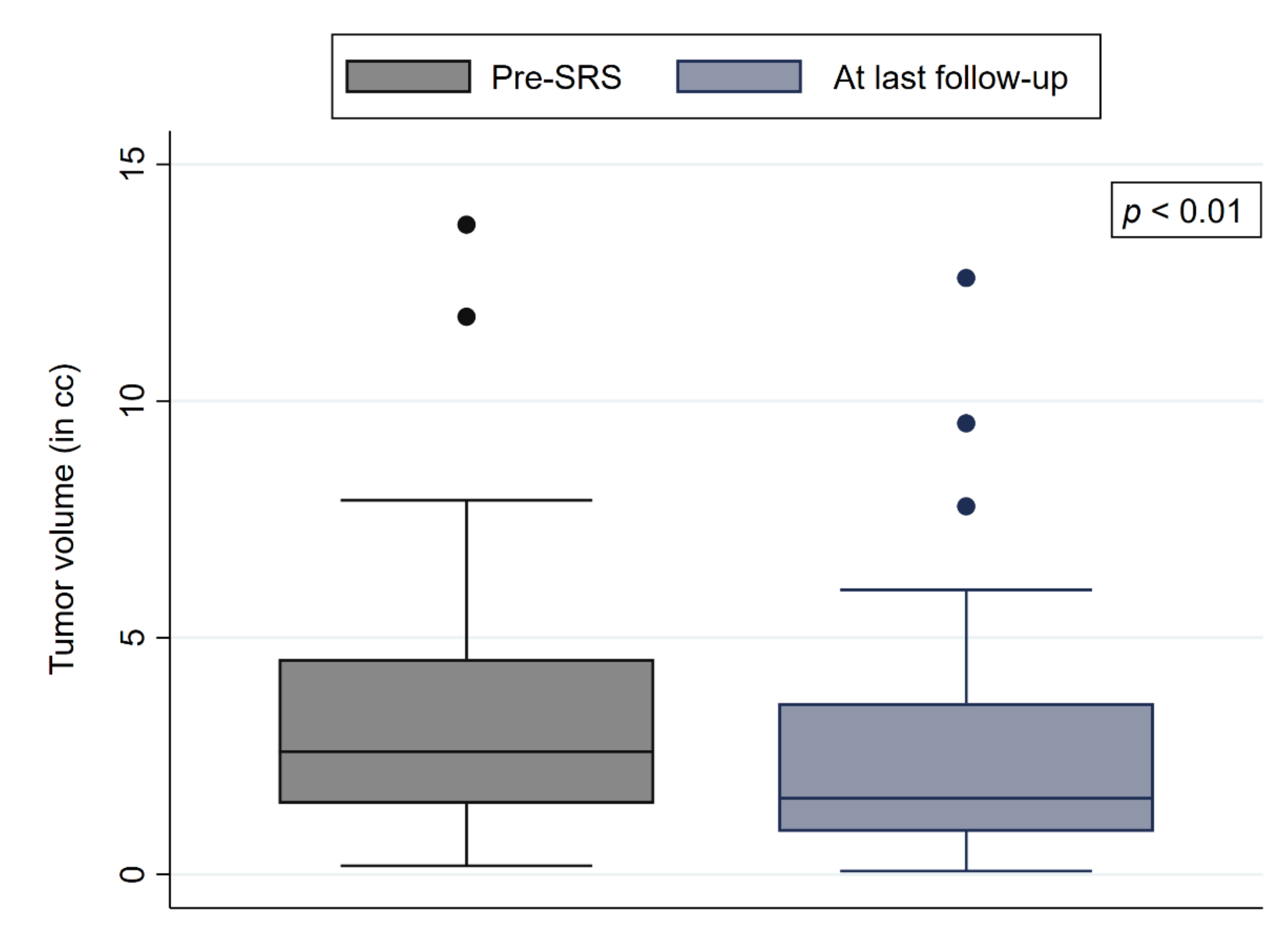
| Tumor volume (cc) | 2.6 | 3.3 (±2.6) | 1.4–4.5 | 0.2–13.7 |
| Prescription dose all treatments (Gy) | 14 | 15.4 (±3.6) | 13.5–15 | 12–25 |
| Prescription dose single-session treatments (Gy) | 14 | 13.8 (±1.0) | 13–14.5 | 12–17 |
| Prescription dose multisession treatments (Gy) | 21 | 22.0 (±2.6) | 19.5–25 | 19.5–26 |
| Prescription isodose line (%) | 70 | 70.6 (±4.3) | 70–70 | 61–80 |
| Number of fractions | 1 | 1.5 (±1.2) | 1–1 | 1–5 |
| Max tumor dose (Gy) | 20.0 | 21.8 (±5.4) | 18.6–23.0 | 15.0–37.8 |
| Mean tumor dose (Gy) | 17.0 | 18.4 (±4.3) | 15.8–18.7 | 13.4–31.5 |
| Min tumor dose (Gy) | 12.7 | 13.4 (±3.0) | 11.8–13.9 | 8.9–21.1 |
| Max dose medulla oblongata/brainstem (Gy) | 13.5 | 14.0 (±4.2) | 11.6–15.1 | 6.7–26.5 |
| Conformity index | 1.24 | 1.27 (±0.17) | 1.17–1.36 | 1.05–1.94 |
| Homogeneity index | 1.43 | 1.42 (±0.09) | 1.41–1.43 | 1.25–1.82 |
| Coverage (%) | 98.7 | 97.8 (±2.3) | 97.1–99.2 | 87.4–100 |
| Author | Year | Number of Patients | Number of Primarily Treated FMMs | Treatment Modality | Median Follow-Up in Months | Median Tumor Volume in cc | Dose/Fractions | Clinical Outcome | Radiographic Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Mehta et al. [7] * | 2017 | 57 | 37 | GK | Radiographic: 36, clinical: 53 | 2.9 | Median margin dose: 12.5 Gy, one fraction | Improvement: 52% | LC: 93% |
| Sheehan et al. [12] *, ** | 2015 | 18 | NR | GK | 60.1 (all patients) | 6.5 (all tumors) | Median margin dose: 13.6 Gy, one fraction | Improvement or stable: 91% (all patients) | LC: 91% (all tumors) |
| Malone et al. [10] | 2020 | 1 | 1 | CK | 97 | 14.2 | 30 Gy, five fractions | Improvement | LC: 100% |
| Cheshier et al. [11] *** | 2007 | 7 | NR | CK | Mean radiographic: 15.4, mean clinical: 8.9 | 1.5 | 18 Gy, two fractions | Improvement: 29%, stable: 46%, worse: 25% (for 24 patients) | LC: 83% for 23 patients (all tumors) |
| Muthukumar et al. [13] | 1999 | 5 | 3 | GK | 36 | 10.5 | Median margin dose: 14 Gy, one fraction | Stable: 100% | LC: 100% |
| Akyoldaş et al. [14] | 2020 | 37 | 25 | GK | Median radiographic: 84, median clinical: 80 | 3.3 | Median margin dose: 12 Gy, one fraction | Improvement: 73%, Stable: 27% | LC: 97.3% |
| Mohammed et al. [15] * | 2020 | 1 | 1 | GK | 61 | 5.8 | 13.5 Gy in one fraction | Stable | LC: 100% |
| Starke et al. [16] * | 2010 | 5 | 2 | GK | 72 | 6.8 | Median margin dose: 12 Gy, one fraction | Improvement: 60%, stable: 20%, worse: 20% | LC: 80% |
| Zenonos et al. [17] * | 2012 | 21 | 15 | GK | 47 | 4.1 | Median margin dose: 13 Gy, one fraction | Improvement: 48%, stable: 52% | LC: 100% |
| Nicolato et al. [18] † | 2001 | 1 | NR | GK | 28.7 | Mean: 5.9 | Mean margin dose: 15.2 Gy, one fraction | Improvement or stable: 100% (all patients) | LC: 95% (all tumors) |
| Lin et al. [19] | 1998 | 1 | 0 | GK | NR | NR | NR | Stable | NR |
| Kondziolka et al. [20] *, ‡ | 2008 | 22 | NR | GK | 48 (all patients) | Mean: 7.4 (all tumors) | Mean margin dose: 14 Gy, one fraction (all patients) | Improvement or stable: 91%/93% for WHO grade I/primary treatments (all patients) | LC: 93%/97% at the median follow-up for WHO grade I/primary treatments (all tumors) |
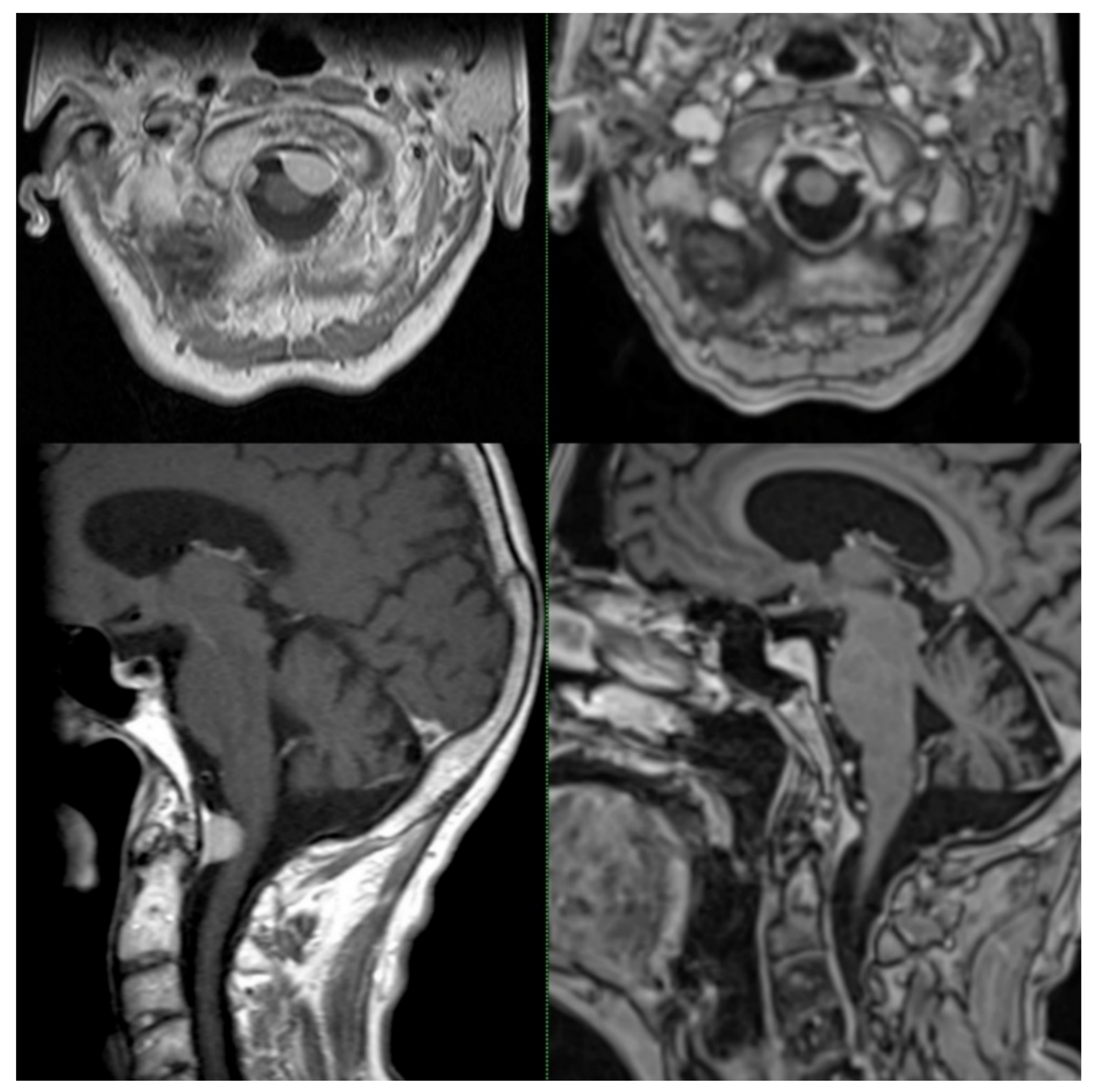
| This series | 2021 | 62 | 39 | CK | 28 | 2.6 | 14 Gy, one fraction | Improvement: 21%, Stable: 47%, Worse: 3% No symptoms before and after SRS: 29% | LC: 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehret, F.; Kufeld, M.; Fürweger, C.; Haidenberger, A.; Fichte, S.; Lehrke, R.; Senger, C.; Kaul, D.; Bleif, M.; Becker, G.; et al. The Role of Stereotactic Radiosurgery in the Management of Foramen Magnum Meningiomas—A Multicenter Analysis and Review of the Literature. Cancers 2022, 14, 341. https://doi.org/10.3390/cancers14020341
Ehret F, Kufeld M, Fürweger C, Haidenberger A, Fichte S, Lehrke R, Senger C, Kaul D, Bleif M, Becker G, et al. The Role of Stereotactic Radiosurgery in the Management of Foramen Magnum Meningiomas—A Multicenter Analysis and Review of the Literature. Cancers. 2022; 14(2):341. https://doi.org/10.3390/cancers14020341
Chicago/Turabian StyleEhret, Felix, Markus Kufeld, Christoph Fürweger, Alfred Haidenberger, Susanne Fichte, Ralph Lehrke, Carolin Senger, David Kaul, Martin Bleif, Gerd Becker, and et al. 2022. "The Role of Stereotactic Radiosurgery in the Management of Foramen Magnum Meningiomas—A Multicenter Analysis and Review of the Literature" Cancers 14, no. 2: 341. https://doi.org/10.3390/cancers14020341
APA StyleEhret, F., Kufeld, M., Fürweger, C., Haidenberger, A., Fichte, S., Lehrke, R., Senger, C., Kaul, D., Bleif, M., Becker, G., Rueß, D., Ruge, M., Schichor, C., Tonn, J.-C., & Muacevic, A. (2022). The Role of Stereotactic Radiosurgery in the Management of Foramen Magnum Meningiomas—A Multicenter Analysis and Review of the Literature. Cancers, 14(2), 341. https://doi.org/10.3390/cancers14020341






