Risks and Benefits of Fiducial Marker Placement in Tumor Lesions for Robotic Radiosurgery: Technical Outcomes of 357 Implantations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Implantation Procedure
2.3. CyberKnife Fiducial Tracking
2.4. Assessment of Technical Outcomes
2.5. Statistical Analysis
3. Results
3.1. Patient, Lesion, and FM Implantation Characteristics
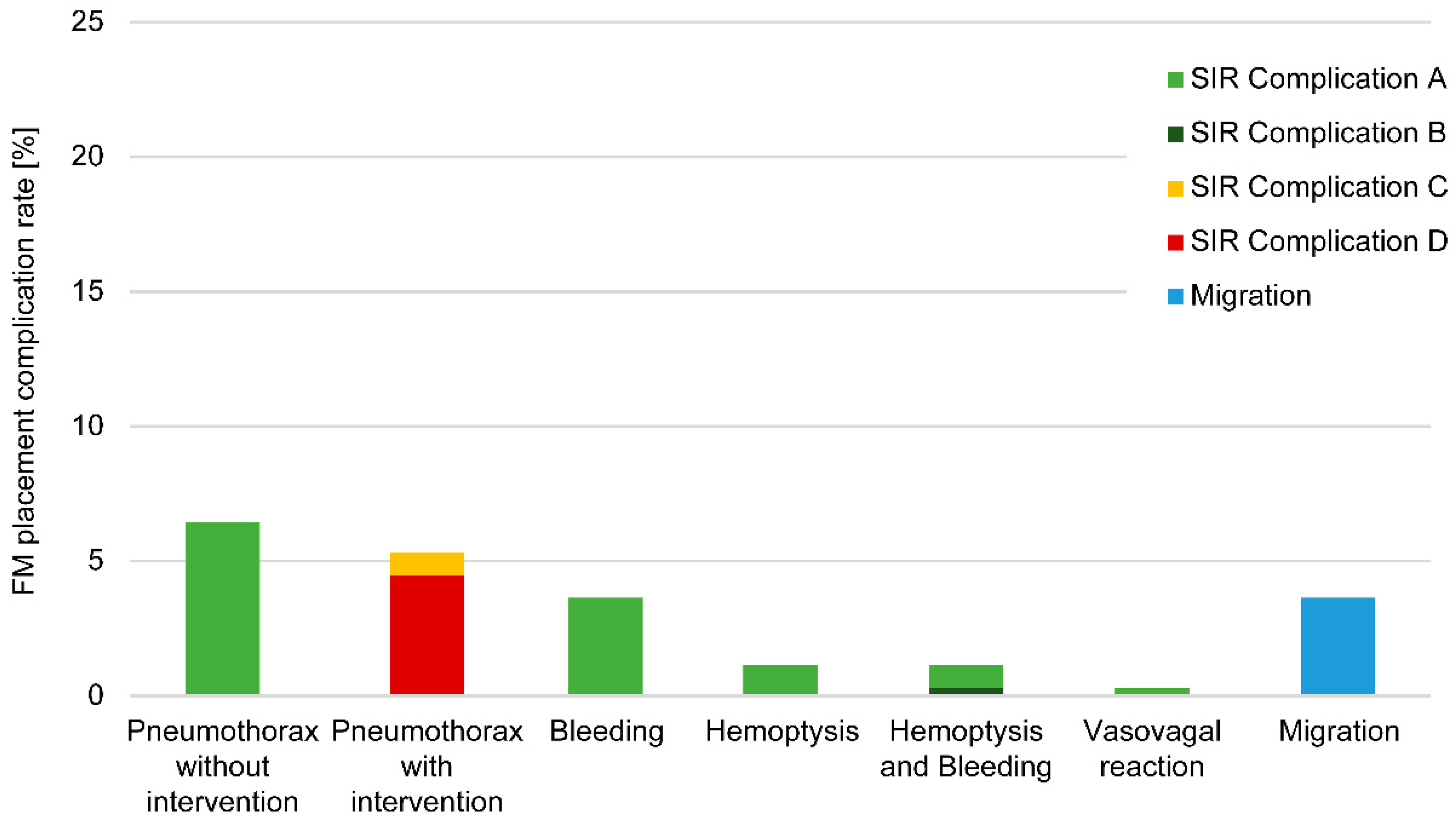
3.2. Marker Complications
3.2.1. Analysis of Modality-Associated Complications
3.2.2. Locations of Complications
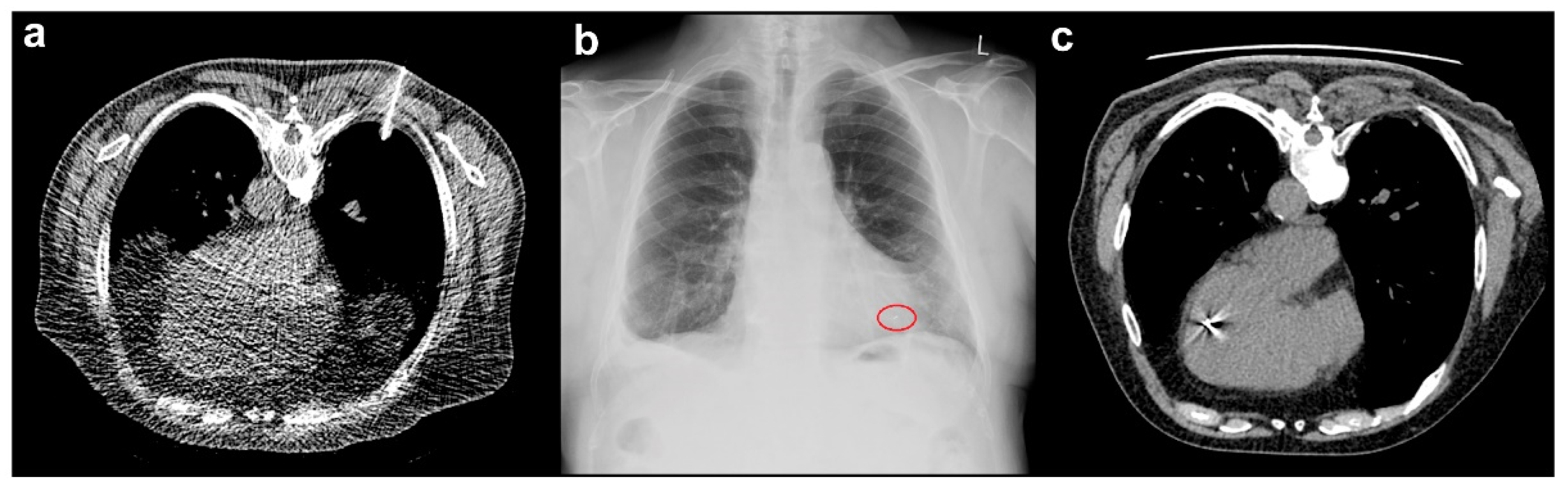
3.3. Marker Migration
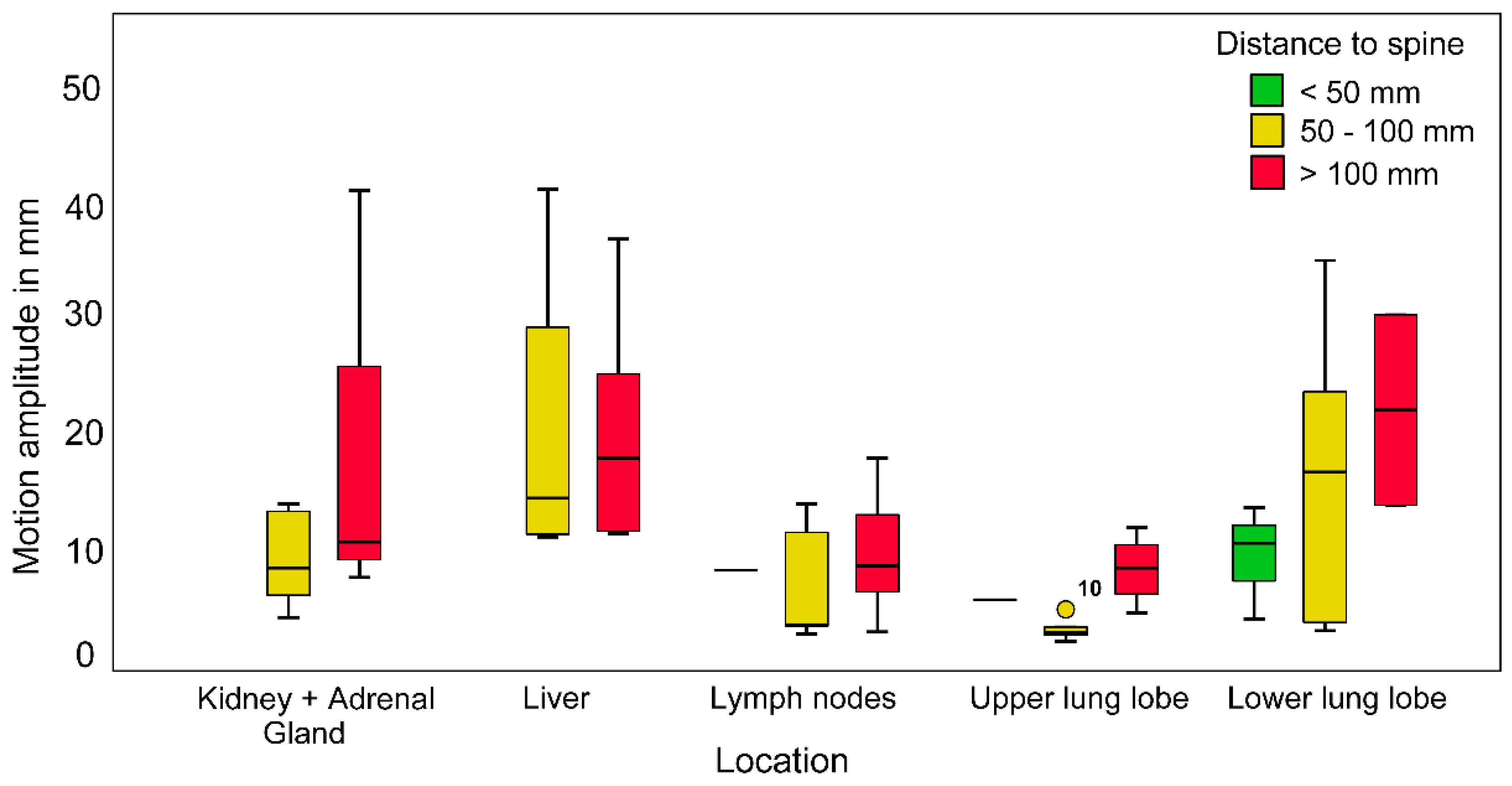
3.4. Maximum Radial Motion Amplitude
4. Discussion
4.1. Thoracic Complications
4.2. Abdominal and Pelvic Complications
4.3. Marker Migration
4.4. Motion Amplitude
4.5. Alternative Tracking Modalities
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calcerrada Díaz-Santos, N.; Blasco Amaro, J.A.; Cardiel, G.A.; Andradas Aragonés, E. The safety and efficacy of robotic image-guided radiosurgery system treatment for intra- and extracranial lesions: A systematic review of the literature. Radiother. Oncol. 2008, 89, 245–253. [Google Scholar] [CrossRef]
- Adler, J.R., Jr.; Chang, S.D.; Murphy, M.J.; Doty, J.; Geis, P.; Hancock, S.L. The Cyberknife: A frameless robotic system for radiosurgery. Stereotact. Funct. Neurosurg. 1997, 69, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Adler, J.R., Jr.; Murphy, M.J.; Chang, S.D.; Hancock, S.L. Image-guided robotic radiosurgery. Neurosurgery 1999, 44, 1299–1306. [Google Scholar]
- Chang, S.D.; Main, W.; Martin, D.P.; Gibbs, I.C.; Heilbrun, M.P. An analysis of the accuracy of the CyberKnife: A robotic frameless stereotactic radiosurgical system. Neurosurgery 2003, 52, 140–146. [Google Scholar] [CrossRef]
- Keall, P.J.; Mageras, G.S.; Balter, J.M.; Emery, R.S.; Forster, K.M.; Jiang, S.B.; Kapatoes, J.M.; Low, D.A.; Murphy, M.J.; Murray, B.R.; et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med. Phys. 2006, 33, 3874–3900. [Google Scholar] [CrossRef]
- Shirato, H.; Seppenwoolde, Y.; Kitamura, K.; Onimura, R.; Shimizu, S. Intrafractional tumor motion: Lung and liver. Semin. Radiat. Oncol. 2004, 14, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Shirato, H.; Seppenwoolde, Y.; Onimaru, R.; Oda, M.; Fujita, K.; Shimizu, S.; Shinohara, N.; Harabayashi, T.; Miyasaka, K. Three-dimensional intrafractional movement of prostate measured during real-time tumor-tracking radiotherapy in supine and prone treatment positions. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 1117–1123. [Google Scholar] [CrossRef]
- Kitamura, K.; Shirato, H.; Seppenwoolde, Y.; Shimizu, T.; Kodama, Y.; Endo, H.; Onimaru, R.; Oda, M.; Fujita, K.; Shimizu, S.; et al. Tumor location, cirrhosis, and surgical history contribute to tumor movement in the liver, as measured during stereotactic irradiation using a real-time tumor-tracking radiotherapy system. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 221–228. [Google Scholar] [CrossRef]
- Seppenwoolde, Y.; Shirato, H.; Kitamura, K.; Shimizu, S.; van Herk, M.; Lebesque, J.V.; Miyasaka, K. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 822–834. [Google Scholar] [CrossRef]
- Park, S.H.; Won, H.J.; Kim, S.Y.; Shin, Y.M.; Kim, P.N.; Yoon, S.M.; Park, J.H.; Kim, J.H. Efficacy and safety of ultrasound-guided implantation of fiducial markers in the liver for stereotactic body radiation therapy. PLoS ONE 2017, 12, e0179676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldrini, G.; Taste-George, H.; Renard-Oldrini, S.; Baumann, A.S.; Marchesi, V.; Troufléau, P.; Peiffert, D.; Didot-Moisei, A.; Boyer, B.; Grignon, B.; et al. Implantation of fiducial markers in the liver for stereotactic body radiation therapy: Feasibility and results. Diagn. Interv. Imaging 2015, 96, 589–592. [Google Scholar] [CrossRef] [Green Version]
- Scher, N.; Bollet, M.; Bouilhol, G.; Tannouri, R.; Khemiri, I.; Vouillaume, A.; Sellami, N.; Von Eyben, R.; Vannetzel, J.M.; Darmon, I.; et al. Safety and efficacy of fiducial marker implantation for robotic stereotactic body radiation therapy with fiducial tracking. Radiat. Oncol. 2019, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Kothary, N.; Heit, J.J.; Louie, J.D.; Kuo, W.T.; Loo, B.W., Jr.; Koong, A.; Chang, D.T.; Hovsepian, D.; Sze, D.Y.; Hofmann, L.V. Safety and efficacy of percutaneous fiducial marker implantation for image-guided radiation therapy. J. Vasc. Interv. Radiol. 2009, 20, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Pepin, E.W.; Wu, H.; Zhang, Y.; Lord, B. Correlation and prediction uncertainties in the cyberknife synchrony respiratory tracking system. Med. Phys. 2011, 38, 4036–4044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweikard, A.; Shiomi, H.; Adler, J. Respiration tracking in radiosurgery. Med. Phys. 2004, 31, 2738–2741. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Okawa, K.; Taguchi, J.; Hirota, Y.; Yanagiya, Y.; Kikuchi, C.; Iwabuchi, M.; Murai, T.; Iwata, H.; Shiomi, H.; et al. Factors affecting the accuracy of respiratory tracking of the image-guided robotic radiosurgery system. Jpn. J. Radiol. 2019, 37, 727–734. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, H.; Xue, J.; Hu, B.; Zhu, B.; Li, Q.; Zhang, S.; Wu, G. Evaluation of the intra- and interfractional tumor motion and variability by fiducial-based real-time tracking in liver stereotactic body radiation therapy. J. Appl. Clin. Med. Phys. 2018, 19, 94–100. [Google Scholar] [CrossRef]
- Seppenwoolde, Y.; Berbeco, R.I.; Nishioka, S.; Shirato, H.; Heijmen, B. Accuracy of tumor motion compensation algorithm from a robotic respiratory tracking system: A simulation study. Med. Phys. 2007, 34, 2774–2784. [Google Scholar] [CrossRef]
- Hoogeman, M.; Prevost, J.B.; Nuyttens, J.; Poll, J.; Levendag, P.; Heijmen, B. Clinical accuracy of the respiratory tumor tracking system of the cyberknife: Assessment by analysis of log files. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 297–303. [Google Scholar] [CrossRef]
- Ohta, K.; Shimohira, M.; Murai, T.; Nishimura, J.; Iwata, H.; Ogino, H.; Hashizume, T.; Shibamoto, Y. Percutaneous fiducial marker placement prior to stereotactic body radiotherapy for malignant liver tumors: An initial experience. J. Radiat. Res. 2016, 57, 174–177. [Google Scholar] [CrossRef] [Green Version]
- Trumm, C.G.; Häussler, S.M.; Muacevic, A.; Stahl, R.; Stintzing, S.; Paprottka, P.M.; Strobl, F.; Jakobs, T.F.; Reiser, M.F.; Hoffmann, R.T. CT fluoroscopy-guided percutaneous fiducial marker placement for CyberKnife stereotactic radiosurgery: Technical results and complications in 222 consecutive procedures. J. Vasc. Interv. Radiol. 2014, 25, 760–768. [Google Scholar] [CrossRef]
- Patel, A.; Khalsa, B.; Lord, B.; Sandrasegaran, K.; Lall, C. Planting the seeds of success: CT-guided gold seed fiducial marker placement to guide robotic radiosurgery. J. Med. Imaging Radiat. Oncol. 2013, 57, 207–211. [Google Scholar] [CrossRef]
- Bhagat, N.; Fidelman, N.; Durack, J.C.; Collins, J.; Gordon, R.L.; LaBerge, J.M.; Kerlan, R.K., Jr. Complications associated with the percutaneous insertion of fiducial markers in the thorax. Cardiovasc. Interv. Radiol. 2010, 33, 1186–1191. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.H.; Seo, D.W.; Park, D.H.; Lee, S.K.; Kim, M.H. Fiducial placement for stereotactic body radiation therapy under only endoscopic ultrasonography guidance in pancreatic and hepatic malignancy: Practical feasibility and safety. Gut Liver 2014, 8, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Mongeon, M.; Thibault, F.; Chartrand-Lefebvre, C.; Gorgos, A.B.; Soulez, G.; Filion, E.; Therasse, E. Safety and efficacy of endovascular fiducial marker insertion for Cyberknife stereotactic radiation therapy planning in early-stage lung Cancer. J. Vasc. Interv. Radiol. 2017, 28, 1090–1097. [Google Scholar] [CrossRef]
- Goldsmith, C.; Green, M.M.; Middleton, B.; Cowley, I.; Robinson, A.; Plowman, N.P.; Price, P.M. Evaluation of CyberKnife(R) fiducial tracking limitations to assist targeting accuracy: A phantom study with fiducial displacement. Cureus 2018, 10, e3523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, M.J. Fiducial-based targeting accuracy for external-beam radiotherapy. Med. Phys. 2002, 29, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Wallace, M.J.; Cardella, J.F.; Kundu, S.; Miller, D.L.; Rose, S.C. Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for percutaneous needle biopsy. J. Vasc. Interv. Radiol. 2010, 21, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Casutt, A.; Noirez, L.; Bernasconi, M.; Koutsokera, A.; Beigelman-Aubry, C.; Kinj, R.; Ozsahin, E.M.; Durham, A.D.; von Garnier, C.; Lovis, A. Endobronchial coil spring fiducial markers for CyberKnife(R) stereotactic body radiation therapy. Respirology 2021, 26, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Majid, A.; Palkar, A.; Kheir, F.; Alape, D.; Fernandez-Bussy, S.; Aronovitz, J.; Guerrero, J.; Gangadharan, S.; Kent, M.; Whyte, R.; et al. Convex probe EBUS-guided fiducial placement for malignant central lung lesions. J. Bronchol. Interv. Pulmonol. 2018, 25, 283–289. [Google Scholar] [CrossRef]
- Brook, O.R.; Gourtsoyianni, S.; Mendiratta-Lala, M.; Mahadevan, A.; Siewert, B.; Sheiman, R.R. Safety profile and technical success of imaging-guided percutaneous fiducial seed placement with and without core biopsy in the abdomen and pelvis. AJR Am. J. Roentgenol. 2012, 198, 466–470. [Google Scholar] [CrossRef]
- Yousefi, S.; Collins, B.T.; Reichner, C.A.; Anderson, E.D.; Jamis-Dow, C.; Gagnon, G.; Malik, S.; Marshall, B.; Chang, T.; Banovac, F. Complications of thoracic computed tomography-guided fiducial placement for the purpose of stereotactic body radiation therapy. Clin. Lung Cancer 2007, 8, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Goldstein, J.; Lawrence, Y.R.; Weiss, I.; Saad, R.; Spieler, B.; Symon, Z. Transperineal implantation of gold fiducial markers (gold seeds) for prostate image-guided radiation therapy: A feasible technique associated with a low risk of complications. J. Med. Radiat. Sci. 2015, 62, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Sarudis, S.; Karlsson Hauer, A.; Nyman, J.; Back, A. Systematic evaluation of lung tumor motion using four-dimensional computed tomography. Acta Oncol. 2017, 56, 525–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, J.D.; Wong, R.; Swaminath, A.; Chow, T. Accuracy of robotic radiosurgical liver treatment throughout the respiratory cycle. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 916–924. [Google Scholar] [CrossRef]
- Wolthaus, J.W.; Sonke, J.J.; van Herk, M.; Belderbos, J.S.; Rossi, M.M.; Lebesque, J.V.; Damen, E.M. Comparison of different strategies to use four-dimensional computed tomography in treatment planning for lung cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1229–1238. [Google Scholar] [CrossRef]
- Guckenberger, M.; Krieger, T.; Richter, A.; Baier, K.; Wilbert, J.; Sweeney, R.A.; Flentje, M. Potential of image-guidance, gating and real-time tracking to improve accuracy in pulmonary stereotactic body radiotherapy. Radiother. Oncol. 2009, 91, 288–295. [Google Scholar] [CrossRef]
- Bibault, J.E.; Prevost, B.; Dansin, E.; Mirabel, X.; Lacornerie, T.; Lartigau, E. Image-guided robotic stereotactic radiation therapy with fiducial-free tumor tracking for lung cancer. Radiat. Oncol. 2012, 7, 102. [Google Scholar] [CrossRef] [Green Version]
- Bahig, H.; Campeau, M.P.; Vu, T.; Doucet, R.; Beliveau Nadeau, D.; Fortin, B.; Roberge, D.; Lambert, L.; Carrier, J.F.; Filion, E. Predictive parameters of CyberKnife fiducial-less (XSight Lung) applicability for treatment of early non-small cell lung cancer: A single-center experience. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 583–589. [Google Scholar] [CrossRef]
- De Bruin, K.; Dahele, M.; Mostafavi, H.; Slotman, B.J.; Verbakel, W. Markerless Real-Time 3-Dimensional kV Tracking of Lung Tumors During Free Breathing Stereotactic Radiation Therapy. Adv. Radiat. Oncol. 2021, 6, 100705. [Google Scholar] [CrossRef]
- Papalazarou, C.; Klop, G.J.; Milder, M.T.W.; Marijnissen, J.P.A.; Gupta, V.; Heijmen, B.J.M.; Nuyttens, J.; Hoogeman, M.S. CyberKnife with integrated CT-on-rails: System description and first clinical application for pancreas SBRT. Med. Phys. 2017, 44, 4816–4827. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, S.A.; Henke, L.E.; Shaverdian, N.; Mittauer, K.; Wojcieszynski, A.P.; Hullett, C.R.; Kamrava, M.; Lamb, J.; Cao, M.; Green, O.L.; et al. A Multi-Institutional Experience of MR-Guided Liver Stereotactic Body Radiation Therapy. Adv. Radiat. Oncol. 2019, 4, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Robinson, D.; Liu, D.; Steciw, S.; Field, C.; Daly, H.; Saibishkumar, E.P.; Fallone, G.; Parliament, M.; Amanie, J. An evaluation of the Clarity 3D ultrasound system for prostate localization. Int. J. Radiat. Oncol. Biol. Phys. 2012, 13, 3753. [Google Scholar] [CrossRef] [PubMed]
- Western, C.; Hristov, D.; Schlosser, J. Ultrasound Imaging in Radiation Therapy: From Interfractional to Intrafractional Guidance. Cureus 2015, 7, e280. [Google Scholar] [CrossRef] [Green Version]
- Moningi, S.; Abi Jaoude, J.; Kouzy, R.; Lin, D.; Nguyen, N.D.; Garcia Garcia, C.J.; Phan, J.L.; Avila, S.; Smani, D.; Cazacu, I.M.; et al. Impact of Fiducial Marker Placement Before Stereotactic Body Radiation Therapy on Clinical Outcomes in Patients With Pancreatic Cancer. Adv. Radiat. Oncol. 2021, 6, 100621. [Google Scholar] [CrossRef] [PubMed]
- Rich, L.J.; Miller, A.; Singh, A.K.; Seshadri, M. Photoacoustic Imaging as an Early Biomarker of Radio Therapeutic Efficacy in Head and Neck Cancer. Theranostics 2018, 8, 2064–2078. [Google Scholar] [CrossRef]
- Giza, O.M.; Sánchez-Parcerisa, D.; Sanchez-Tembleque, V.; Herraiz, J.; Camacho, J.; Avery, S.; Udías, J. Photoacoustic dose monitoring in clinical high-energy photon beams. Biomed. Phys. Eng. Express. 2019, 5, 035028. [Google Scholar] [CrossRef]
| Characteristic | Value |
|---|---|
| Number of patients | 288 |
| Age (years) | 63.1 ± 13.0 (16.0–87.0) |
| Women:men ratio | 120:168 |
| Number of lesions | 350 |
| Primary tumors | 179 (51.1) |
| Gynecological cancer | 64 (18.3) |
| Prostate cancer | 63 (18.0) |
| Lung cancer | 42 (12.0) |
| Renal cell cancer | 10 (2.9) |
| Metastases | 171 (48.9) |
| Lung | 66 (18.9) |
| Lymph node | 50 (14.3) |
| Liver | 26 (7.4) |
| Adrenal gland | 8 (2.3) |
| Bone | 8 (2.3) |
| Kidney | 7 (2.0) |
| Other | 6 (1.7) |
| Number of implantation procedures | 357 |
| Number of fiducial markers | 725 |
| Markers per lesion | 2.1 ± 1.4 (1.0–8.0) |
| Implantation Modality | Implantations | FM | Patients | Complications | FM Migration |
|---|---|---|---|---|---|
| CT-guided | 221 (63.1) | 262 (36.1) | 160 (55.6) | 63 (98.4) | 7 (53.8) |
| Clinical | 69 (19.3) | 222 (30.6) | 63 (21.9) | 0 (0) | 6 (46.2) |
| Ultrasound | 59 (16.5) | 230 (31.7) | 59 (20.5) | 1 (1.6) | 0 (0) |
| EBUS | 7 (2.0) | 9 (1.2) | 5 (1.7) | 0 (0) | 0 (0) |
| Perioperative | 1 (0.3) | 2 (0.3) | 1 (0.3) | 0 (0) | 0 (0) |
| Total | 357 | 725 | 288 | 64 | 13 |
| Localization | Lesions | FM | Implantations | Complications | FM Migration |
|---|---|---|---|---|---|
| Thorax | 137 (71.7) | 162 (71.7) | 140 (72.2) | 60 (96.8) | 6 (100) |
| Lung, upper lobe | 51 (26.7) | 60 (26.6) | 52 (26.8) | 27 (43.6) | 1 (16.7) |
| Lung, lower lobe | 49 (25.7) | 58 (25.7) | 51 (26.3) | 19 (30.7) | 4 (66.7) |
| Lung, middle lobe | 8 (4.2) | 8 (3.5) | 8 (4.1) | 6 (9.7) | 0 (0) |
| Lymph node | 20 (10.5) | 24 (10.6) | 20 (10.3) | 7 (11.3) | 0 (0) |
| Mediastinum | 3 (1.6) | 5 (2.2) | 3 (1.6) | 1 (1.6) | 0 (0) |
| Bone * | 6 (3.1) | 7 (3.1) | 6 (3.1) | 0 (0) | 1 (16.7) |
| Abdomen | 54 (28.3) | 64 (28.3) | 54 (27.8) | 2 (3.2) | 0 |
| Liver | 26 (13.6) | 34 (15.0) | 26 (13.4) | 1 (1.6) | 0 (0) |
| Kidney | 16 (8.4) | 17 (7.5) | 16 (8.3) | 1 (1.6) | 0 (0) |
| Adrenal gland | 8 (4.2) | 9 (4.0) | 8 (4.1) | 0 (0) | 0 (0) |
| Other ** | 4 (2.1) | 4 (1.8) | 4 (2.1) | 0 (0) | 0 (0) |
| Total | 191 | 226 | 194 | 62 | 6 |
| Localization | Lesions | FM | Implantations | Complications | FM Migration |
|---|---|---|---|---|---|
| Pelvis | 153 (96.2) | 488 (97.8) | 157 (96.3) | 2 (100) | 7 (100) |
| Gynecological | 64 (40.3) | 222 (44.5) | 68 (41.7) | 0 (0) | 6 (85.7) |
| Prostatic | 63 (39.6) | 229 (45.9) | 63 (38.7) | 1 (50.0) | 0 (0) |
| Lymph node | 26 (16.4) | 37 (7.4) | 26 (16.0) | 1 (50.0) | 1 (14.3) |
| Abdomen | 2 (1.3) | 2 (0.4) | 2 (1.2) | 0 (0) | 0 (0) |
| Lymph node | 2 (1.3) | 2 (0.4) | 2 (1.2) | 0 (0) | 0 (0) |
| Other * | 4 (2.5) | 9 (1.8) | 4 (2.5) | 0 (0) | 0 (0) |
| Total | 159 | 499 | 163 | 2 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kord, M.; Kluge, A.; Kufeld, M.; Kalinauskaite, G.; Loebel, F.; Stromberger, C.; Budach, V.; Gebauer, B.; Acker, G.; Senger, C. Risks and Benefits of Fiducial Marker Placement in Tumor Lesions for Robotic Radiosurgery: Technical Outcomes of 357 Implantations. Cancers 2021, 13, 4838. https://doi.org/10.3390/cancers13194838
Kord M, Kluge A, Kufeld M, Kalinauskaite G, Loebel F, Stromberger C, Budach V, Gebauer B, Acker G, Senger C. Risks and Benefits of Fiducial Marker Placement in Tumor Lesions for Robotic Radiosurgery: Technical Outcomes of 357 Implantations. Cancers. 2021; 13(19):4838. https://doi.org/10.3390/cancers13194838
Chicago/Turabian StyleKord, Melina, Anne Kluge, Markus Kufeld, Goda Kalinauskaite, Franziska Loebel, Carmen Stromberger, Volker Budach, Bernhard Gebauer, Gueliz Acker, and Carolin Senger. 2021. "Risks and Benefits of Fiducial Marker Placement in Tumor Lesions for Robotic Radiosurgery: Technical Outcomes of 357 Implantations" Cancers 13, no. 19: 4838. https://doi.org/10.3390/cancers13194838
APA StyleKord, M., Kluge, A., Kufeld, M., Kalinauskaite, G., Loebel, F., Stromberger, C., Budach, V., Gebauer, B., Acker, G., & Senger, C. (2021). Risks and Benefits of Fiducial Marker Placement in Tumor Lesions for Robotic Radiosurgery: Technical Outcomes of 357 Implantations. Cancers, 13(19), 4838. https://doi.org/10.3390/cancers13194838






