Gastrointestinal Adenocarcinoma Incidence and Survival Trends in South Australia, 1990–2017
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Selection of Cases
2.1.1. Inclusion Criteria
2.1.2. Statistical Analysis
3. Results
3.1. Demographic Data and Time Trend of Reported Cases
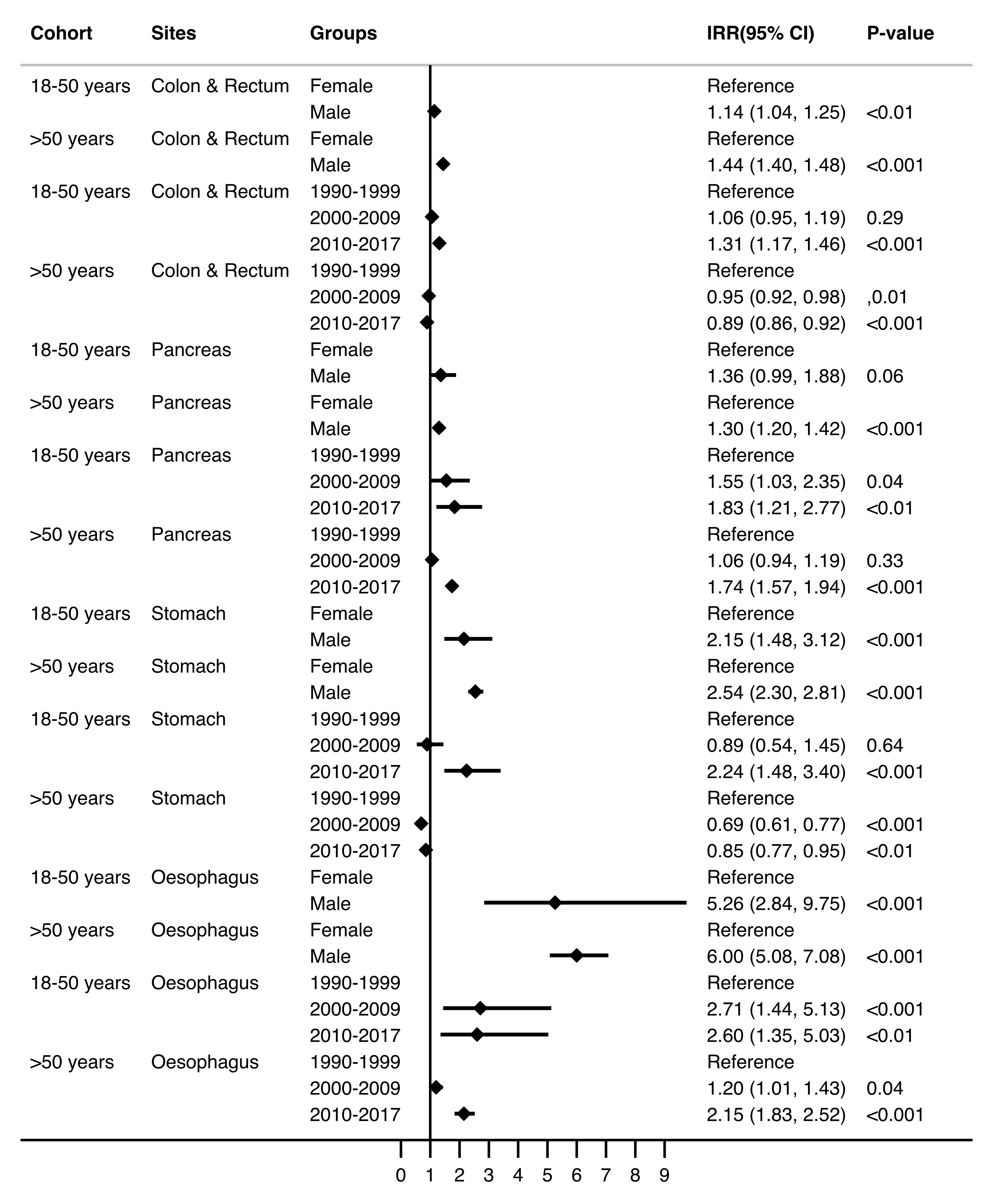
3.2. Trends in the Incidence of Gastrointestinal Adenocarcinomas
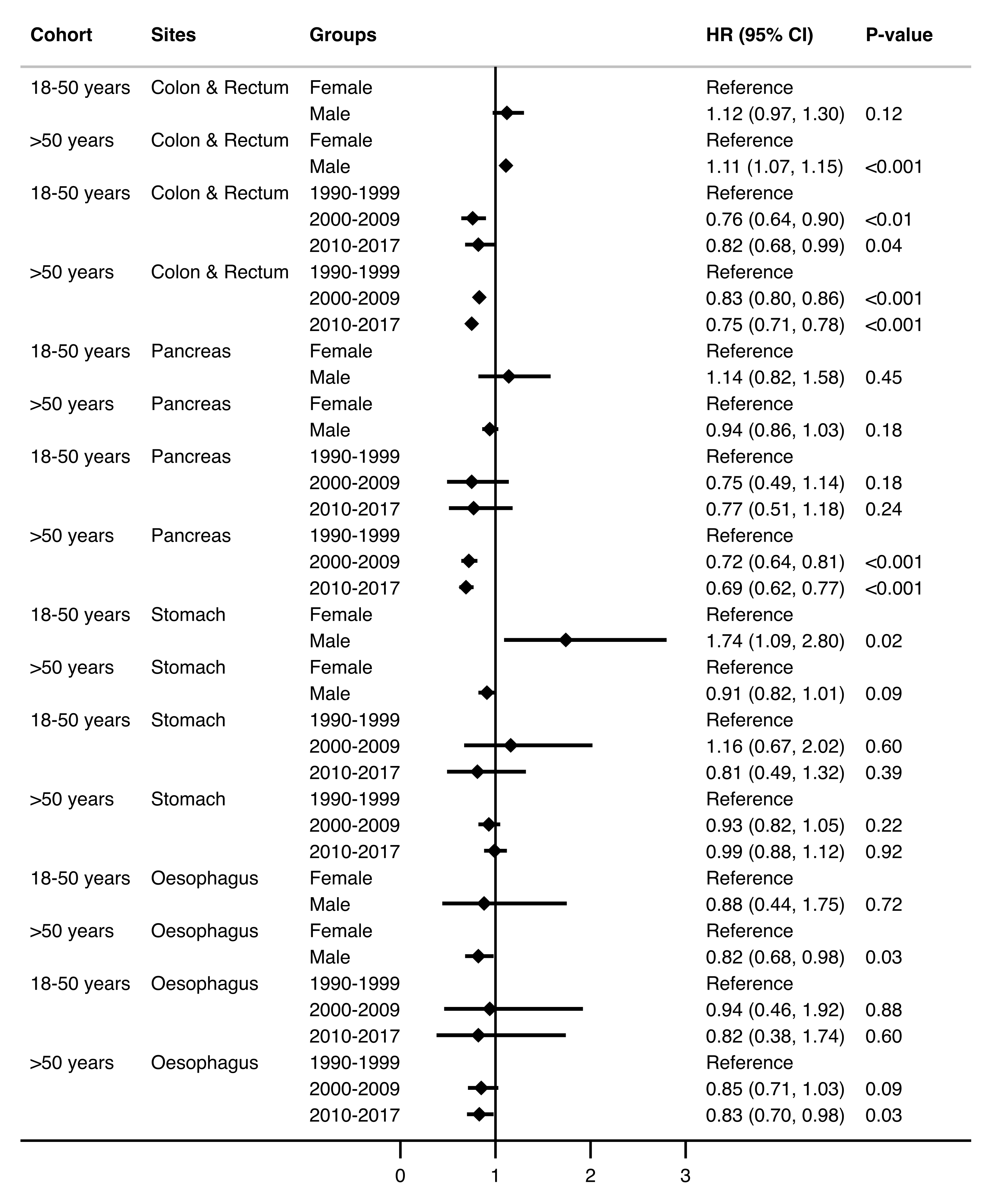
3.3. Survival by Time Trends and Site
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barreto, S.G. We Asked the Experts: Providing the Road Map to Uncovering the Pathophysiology of Young-Onset Cancer to Guide Treatment and Preventive Strategies. World J. Surg. 2020, 44, 3212–3213. [Google Scholar] [CrossRef]
- McWilliams, R.R.; Maisonneuve, P.; Bamlet, W.R.; Petersen, G.M.; Li, D.; Risch, H.A.; Yu, H.; Fontham, E.T.; Luckett, B.; Bosetti, C.; et al. Risk Factors for Early-Onset and Very-Early-Onset Pancreatic Adenocarcinoma: A Pancreatic Cancer Case-Control Consortium (Panc4) Analysis. Pancreas 2016, 45, 311–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chelmow, D.; Pearlman, M.D.; Young, A.; Bozzuto, L.; Dayaratna, S.; Jeudy, M.; Kremer, M.E.; Scott, D.M.; O’Hara, J.S. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obs. Gynecol. 2020, 135, 1457–1478. [Google Scholar] [CrossRef]
- Bernards, S.S.; Norquist, B.M.; Harrell, M.I.; Agnew, K.J.; Lee, M.K.; Walsh, T.; Swisher, E.M. Genetic Characterization of Early Onset Ovarian Carcinoma. Gynecol. Oncol. 2016, 140, 221–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Nistelrooij, A.M.; van Marion, R.; Group, P.; Biermann, K.; Spaander, M.C.; van Lanschot, J.J.; Wijnhoven, B.P.; Dinjens, W.N. Early Onset Esophageal Adenocarcinoma: A Distinct Molecular Entity? Oncoscience 2016, 3, 42–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setia, N.; Wang, C.X.; Lager, A.; Maron, S.; Shroff, S.; Arndt, N.; Peterson, B.; Kupfer, S.S.; Ma, C.; Misdraji, J.; et al. Morphologic and Molecular Analysis of Early-Onset Gastric Cancer. Cancer 2020, 127, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.C.; Singal, A.G.; Baron, J.A.; Sandler, R.S. Decrease in Incidence of Young-Onset Colorectal Cancer before Recent Increase. Gastroenterology 2018, 155, 1716–1719.e4. [Google Scholar] [CrossRef] [PubMed]
- Rea, D.; Coppola, G.; Palma, G.; Barbieri, A.; Luciano, A.; del Prete, P.; Rossetti, S.; Berretta, M.; Facchini, G.; Perdona, S.; et al. Microbiota Effects on Cancer: From Risks to Therapies. Oncotarget 2018, 9, 17915–17927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrington, S.M.; McKinley, A.J.; Carothers, A.D.; Cunningham, C.; Bubb, V.J.; Sharp, L.; Wyllie, A.H.; Dunlop, M.G. Evidence for an Age-Related Influence of Microsatellite Instability on Colorectal Cancer Survival. Int. J. Cancer 2002, 98, 844–850. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J. Natl. Cancer Inst. 2017, 109, djw322. [Google Scholar] [CrossRef] [Green Version]
- French, S.A.; Story, M.; Jeffery, R.W. Environmental Influences on Eating and Physical Activity. Annu. Rev. Public Health 2001, 22, 309–335. [Google Scholar] [CrossRef] [Green Version]
- Lui, R.N.; Tsoi, K.K.F.; Ho, J.M.W.; Lo, C.M.; Chan, F.C.H.; Kyaw, M.H.; Sung, J.J.Y. Global Increasing Incidence of Young-Onset Colorectal Cancer across 5 Continents: A Joinpoint Regression Analysis of 1,922,167 Cases. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1275–1282. [Google Scholar] [CrossRef] [Green Version]
- Barreto, S.G.; Pandol, S.J. Young-Onset Carcinogenesis—The Potential Impact of Perinatal and Early Life Metabolic Influences on the Epigenome. Front. Oncol. 2021, 11, 653289. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The Origins of the Developmental Origins Theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Knudson, A.G., Jr. Mutation and Cancer: Statistical Study of Retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahouel, K.; Younes, L.; Danilova, L.; Giardiello, F.M.; Hruban, R.H.; Groopman, J.; Kinzler, K.W.; Vogelstein, B.; Geman, D.; Tomasetti, C. Revisiting the Tumorigenesis Timeline with a Data-Driven Generative Model. Proc. Natl. Acad. Sci. USA 2020, 117, 857–864. [Google Scholar] [CrossRef] [Green Version]
- Koczwara, B.; Deckx, L.; Ullah, S.; van den Akker, M. Impact of Comorbidities on Physical Function and Survival of Middle-Aged, as Compared to Older, Individuals with Cancer. Support. Care Cancer 2021, 30, 1625–1632. [Google Scholar] [CrossRef]
- Schell, D.; Pandol, S.; Barreto, S.G. Is This the Evidence for Perinatal and Early Life Events Impacting on Cancer Outcomes? Support. Care Cancer 2021. [Google Scholar] [CrossRef]
- South Australian Legislation. Health Care Regulations 2008 under the Health Care Act 2008. Available online: https://www.legislation.sa.gov.au/LZ/C/R/Health%20Care%20Regulations%202008.aspx (accessed on 23 November 2021).
- South Australian Cancer Registry. Cancer Indicence and Mortality Statistics; Prevention and Population Health Directorate, Wellbeing SA, Government of South Australia: Adelaide, Australia, 2021. [Google Scholar]
- Meng, R.; Chen, J.; D’Onise, K.; Barreto, S.G. Pancreatic Ductal Adenocarcinoma Survival in South Australia: Time Trends and Impact of Tumour Location. ANZ J. Surg. 2021, 91, 921–926. [Google Scholar] [CrossRef]
- Saad El Din, K.; Loree, J.M.; Sayre, E.C.; Gill, S.; Brown, C.J.; Dau, H.; de Vera, M.A. Trends in the Epidemiology of Young-Onset Colorectal Cancer: A Worldwide Systematic Review. BMC Cancer 2020, 20, 288. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, D.E.; Hilsden, R.J.; Ruan, Y.; Forbes, N.; Heitman, S.J.; Brenner, D.R. The Incidence of Young-Onset Colorectal Cancer in Canada Continues to Increase. Cancer Epidemiol. 2020, 69, 101828. [Google Scholar] [CrossRef]
- Feletto, E.; Yu, X.Q.; Lew, J.B.; John, D.J.B.S.; Jenkins, M.A.; Macrae, F.A.; Mahady, S.E.; Canfell, K. Trends in Colon and Rectal Cancer Incidence in Australia from 1982 to 2014: Analysis of Data on over 375,000 Cases. Cancer Epidemiol. Biomark. Prev. 2019, 28, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Young, J.P.; Win, A.K.; Rosty, C.; Flight, I.; Roder, D.; Young, G.P.; Frank, O.; Suthers, G.K.; Hewett, P.J.; Ruszkiewicz, A.; et al. Rising Incidence of Early-Onset Colorectal Cancer in Australia over Two Decades: Report and Review. J. Gastroenterol. Hepatol. 2015, 30, 6–13. [Google Scholar] [CrossRef]
- Codipilly, D.C.; Sawas, T.; Dhaliwal, L.; Johnson, M.L.; Lansing, R.; Wang, K.K.; Leggett, C.L.; Katzka, D.A.; Iyer, P.G. Epidemiology and Outcomes of Young-Onset Esophageal Adenocarcinoma: An Analysis from a Population-Based Database. Cancer Epidemiol. Biomark. Prev. 2021, 30, 142–149. [Google Scholar] [CrossRef]
- Sung, J.J.Y.; Chiu, H.M.; Jung, K.W.; Jun, J.K.; Sekiguchi, M.; Matsuda, T.; Kyaw, M.H. Increasing Trend in Young-Onset Colorectal Cancer in Asia: More Cancers in Men and More Rectal Cancers. Am. J. Gastroenterol. 2019, 114, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Q.; Li, Y.; Wang, W.; Feng, W.T.; Shi, O.M.; Wang, Q. International Incidence Trends in Early- and Late-Onset Colorectal Cancer: A Population-Based Study. Int. J. Colorectal. Dis. 2020, 35, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Mikaeel, R.R.; Symonds, E.L.; Kimber, J.; Smith, E.; Horsnell, M.; Uylaki, W.; Rico, G.T.; Hewett, P.J.; Yong, J.; Tonkin, D.; et al. Young-Onset Colorectal Cancer Is Associated with a Personal History of Type 2 Diabetes. Asia Pac. J. Clin. Oncol. 2021, 17, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Haines, C.; Watson, A.J.M.; Hart, A.R.; Platt, M.J.; Pardoll, D.M.; Cosgrove, S.E.; Gebo, K.A.; Sears, C.L. Oral Antibiotic Use and Risk of Colorectal Cancer in the United Kingdom, 1989–2012: A Matched Case-Control Study. Gut 2019, 68, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Lew, J.B.; John, D.J.B.S.; Xu, X.M.; Greuter, M.J.E.; Caruana, M.; Cenin, D.R.; He, E.; Saville, M.; Grogan, P.; Coupe, V.M.H.; et al. Long-Term Evaluation of Benefits, Harms, and Cost-Effectiveness of the National Bowel Cancer Screening Program in Australia: A Modelling Study. Lancet Public Health 2017, 2, e331–e340. [Google Scholar] [CrossRef] [Green Version]
- Gausman, V.; Dornblaser, D.; Anand, S.; Hayes, R.B.; O’Connell, K.; Du, M.; Liang, P.S. Risk Factors Associated with Early-Onset Colorectal Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 2752–2759.e2. [Google Scholar] [CrossRef]
- Moore, A.; Hikri, E.; Goshen-Lago, T.; Barkan, T.; Morgenstern, S.; Brook, E.; Maderer, A.; Roth, W.; Gordon, N.; Kashtan, H.; et al. Young-Onset Gastric Cancer and Epstein-Barr Virus (Ebv)—A Major Player in the Pathogenesis? BMC Cancer 2020, 20, 34. [Google Scholar] [CrossRef]
- Xie, S.H.; Lagergren, J. The Male Predominance in Esophageal Adenocarcinoma. Clin. Gastroenterol. Hepatol. 2016, 14, 338–347.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neufeld, D.; Shpitz, B.; Bugaev, N.; Grankin, M.; Bernheim, J.; Klein, E.; Ziv, Y. Young-Age Onset of Colorectal Cancer in Israel. Tech. Coloproctol. 2009, 13, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Maisonneuve, P.; Lohr, J.M.; Lowenfels, A.B. Early Onset Pancreatic Cancer: Evidence of a Major Role for Smoking and Genetic Factors. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1894–1897. [Google Scholar] [CrossRef] [Green Version]
- Health Policy Centre. Key Smoking Statistics for SA—2019 Adelaide, Australia; South Australian Health and Medical Research Institute (SAHMRI): Adelaide, Australia, 2020; Available online: https://sahmri.blob.core.windows.net/communications/sahmri.org/Key_Smoking_Statistics_for_SA_2019_-March_2020.pdf (accessed on 23 November 2021).
- Mitchell, H.; Katelaris, P. Epidemiology, Clinical Impacts and Current Clinical Management of Helicobacter Pylori Infection. Med. J. Aust. 2016, 204, 376–380. [Google Scholar] [CrossRef]
- Moujaber, T.; MacIntyre, C.R.; Backhouse, J.; Gidding, H.; Quinn, H.; Gilbert, G.L. The Seroepidemiology of Helicobacter Pylori Infection in Australia. Int. J. Infect. Dis. 2008, 12, 500–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connell, J.B.; Maggard, M.A.; Livingston, E.H.; Yo, C.K. Colorectal Cancer in the Young. Am. J. Surg. 2004, 187, 343–348. [Google Scholar] [CrossRef]
- Dozois, E.J.; Boardman, L.A.; Suwanthanma, W.; Limburg, P.J.; Cima, R.R.; Bakken, J.L.; Vierkant, R.A.; Aakre, J.A.; Larson, D.W. Young-Onset Colorectal Cancer in Patients with No Known Genetic Predisposition: Can We Increase Early Recognition and Improve Outcome? Medicine 2008, 87, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kim, I.H.; Kim, J.S.; Kim, S.W.; Kim, J.G.; Oh, S.T.; Kang, W.K.; Lee, M.A. Different Clinical Characteristics in Sporadic Young-Age Onset Colorectal Cancer. Medicine 2016, 95, e4840. [Google Scholar] [CrossRef]
- Saluja, S.S.; Manipadam, J.M.; Mishra, P.K.; Sachdeva, S.; Solanki, N.; Shah, H. Young Onset Colorectal Cancer: How Does It Differ from Its Older Counterpart? Indian J. Cancer 2014, 51, 565–569. [Google Scholar]
- Bergquist, J.R.; Leiting, J.L.; Habermann, E.B.; Cleary, S.P.; Kendrick, M.L.; Smoot, R.L.; Nagorney, D.M.; Truty, M.J.; Grotz, T.E. Early-Onset Gastric Cancer Is a Distinct Disease with Worrisome Trends and Oncogenic Features. Surgery 2019, 166, 547–555. [Google Scholar] [CrossRef]
- Piciucchi, M.; Capurso, G.; Valente, R.; Larghi, A.; Archibugi, L.; Signoretti, M.; Stigliano, S.; Zerboni, G.; Barucca, V.; la Torre, M.; et al. Early Onset Pancreatic Cancer: Risk Factors, Presentation and Outcome. Pancreatology 2015, 15, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Ntala, C.; Debernardi, S.; Feakins, R.M.; Crnogorac-Jurcevic, T. Demographic, Clinical, and Pathological Features of Early Onset Pancreatic Cancer Patients. BMC Gastroenterol. 2018, 18, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendall, B.J.; Macdonald, G.A.; Hayward, N.K.; Prins, J.B.; Brown, I.; Walker, N.; Pandeya, N.; Green, A.C.; Webb, P.M.; Whiteman, D.C.; et al. Leptin and the Risk of Barrett’s Oesophagus. Gut 2008, 57, 448–454. [Google Scholar] [CrossRef]
- Roder, D.; Karapetis, C.S.; Wattchow, D.; Moore, J.; Singhal, N.; Joshi, R.; Keefe, D.; Fusco, K.; Powell, K.; Eckert, M.; et al. Colorectal Cancer Treatment and Survival: The Experience of Major Public Hospitals in South Australia over Three Decades. Asia Pac. J. Cancer Prev. 2015, 16, 2431–2440. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.N.; Zhang, Q.W.; Pan, Y.B.; Wang, Q.W.; Zhang, X.T.; Li, X.B. Young-Onset Early Colorectal Cancer Had Similar Relative Survival to but Better Overall Survival Than Conventional Early Colorectal Cancer: A Large Population-Based Study. Front. Oncol. 2020, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Barreto, S.G.; Chaubal, G.N.; Talole, S.; Desouza, A.; Suradkar, K.; Gaikwad, V.; Goel, M.; Shrikhande, S.V. Rectal Cancer in Young Indians-Are These Cancers Different Compared to Their Older Counterparts? Indian J. Gastroenterol. 2014, 33, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Kotapalli, V.; Adduri, R.; Gowrishankar, S.; Bashyam, L.; Chaudhary, A.; Vamsy, M.; Patnaik, S.; Srinivasulu, M.; Sastry, R.; et al. Evidence for Possible Non-Canonical Pathway(S) Driven Early-Onset Colorectal Cancer in India. Mol. Carcinog. 2012, 53, E181–E186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, A.; Lee, D.Y.; Cai, J.; Patel, S.S.; Bilchik, A.J.; Goldfarb, M.R. Patterns and Outcomes of Colorectal Cancer in Adolescents and Young Adults. J. Surg. Res. 2016, 205, 19–27. [Google Scholar] [CrossRef]
- Holowatyj, A.N.; Ruterbusch, J.J.; Rozek, L.S.; Cote, M.L.; Stoffel, E.M. Racial/Ethnic Disparities in Survival among Patients with Young-Onset Colorectal Cancer. J. Clin. Oncol. 2016, 34, 2148–2156. [Google Scholar] [CrossRef]
- Mayne, B.T.; Bianco-Miotto, T.; Buckberry, S.; Breen, J.; Clifton, V.; Shoubridge, C.; Roberts, C.T. Large Scale Gene Expression Meta-Analysis Reveals Tissue-Specific, Sex-Biased Gene Expression in Humans. Front. Genet. 2016, 7, 183. [Google Scholar] [CrossRef]
- Bampton, P.A.; Schloithe, A.; Bull, J.; Fraser, R.J.; Padbury, R.T.; Watson, D.I. Improving Surveillance for Barrett’s Oesophagus. BMJ 2006, 332, 1320–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vissapragada, R.; Bulamu, N.B.; Brumfitt, C.; Karnon, J.; Yazbeck, R.; Watson, D.I. Improving Cost-Effectiveness of Endoscopic Surveillance for Barrett’s Esophagus by Reducing Low-Value Care: A Review of Economic Evaluations. Surg. Endosc. 2021, 35, 5905–5917. [Google Scholar] [CrossRef]
- Shaukat, A.; Kahi, C.J.; Burke, C.A.; Rabeneck, L.; Sauer, B.G.; Rex, D.K. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am. J. Gastroenterol. 2021, 116, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of, Health, and Welfare. Cancer Survival and Prevalence in Australia: Period Estimates from 1982 to 2010. Asia Pac. J. Clin. Oncol. 2013, 9, 29–39. [Google Scholar] [CrossRef]
- Cocker, F.; Yee, K.C.; Palmer, A.J.; de Graaff, B. Increasing Incidence and Mortality Related to Liver Cancer in Australia: Time to Turn the Tide. Aust. N. Z. J. Public Health 2019, 43, 267–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Patient Characteristics | (18–50 Years) | (>50 Years) | |||
|---|---|---|---|---|---|
| n = 2129 (7.5%) | n = 26,437 (92.5%) | ||||
| n | % | n | % | p-Value | |
| Age (years): median ± IQR | 46 | (41–49) | 72 | (64–79) | <0.001 |
| Sex | 0.09 | ||||
| Female | 1190 | 55.9 | 15275 | 57.8 | |
| Male | 939 | 44.1 | 11162 | 42.2 | |
| Era | 0.49 | ||||
| 1990–1999 | 650 | 30.5 | 7994 | 30.2 | |
| 2000–2009 | 720 | 33.8 | 9270 | 35.1 | |
| 2010–2017 | 759 | 35.7 | 9173 | 34.7 | |
| Primary site | 0.058 | ||||
| Colon and Rectum | 1776 | 83.4 | 21422 | 81.0 | |
| Pancreas | 150 | 7.0 | 2163 | 8.2 | |
| Stomach | 127 | 6.0 | 1808 | 6.8 | |
| Oesophagus | 76 | 3.6 | 1044 | 3.9 | |
| Patient Characteristics | (18–50 Years) | (>50 Years) | ||||
|---|---|---|---|---|---|---|
| n = 2129 | n = 26,437 | |||||
| * IR (95% CI) | IRR (95% CI) | p-Value | * IR (95% CI) | IRR (95% CI) | p-Value | |
| Overall | 10.60 (10.16–11.06) | 196.56 (194.20–198.94) | ||||
| Age (years) | - | 1.17 (1.16–1.18) | <0.001 | - | 1.05 (1.05–1.05) | <0.001 |
| Sex | ||||||
| Female | 9.42 (8.82–10.04) | Reference | - | 156.19 (153.30–159.11) | Reference | - |
| Male | 11.78 (11.12–12.46) | 1.25 (1.15–1.36) | <0.001 | 242.33 (238.51–246.21) | 1.55 (1.51–1.59) | <0.001 |
| Era | ||||||
| 1990–1999 | 9.13 (8.44–9.86) | Reference | - | 203.04 (198.61–207.53) | Reference | - |
| 2000–2009 | 10.19 (9.46–10.96) | 1.12 (1.00–1.24) | 0.04 | 190.74 (186.87–194.66) | 0.94 (0.91–0.97) | <0.001 |
| 2010–2017 | 12.89 (11.98–13.83) | 1.41 (1.27–1.57) | <0.001 | 197.16 (193.15–201.24) | 0.97 (0.94–1.00) | 0.06 |
| Cancer site | ||||||
| Colon & Rectum | 8.85 (8.44–9.27) | - | - | 159.27 (157.15–161.42) | - | - |
| Pancreas | 0.75 (0.63–0.88) | - | - | 16.08 (15.41–16.77) | - | - |
| Stomach | 0.63 (0.53–0.75) | - | - | 13.44 (12.83–14.08) | - | - |
| Oesophagus | 0.38 (0.30–0.47) | - | - | 7.76 (7.30–8.25) | - | - |
| Cancer Sites | (18–50 Years) | (>50 Years) |
|---|---|---|
| n = 2107 (7.6%) | n = 25,748 (92.4%) | |
| Median (95% CI) | Median (95% CI) | |
| Overall | 12.67 (9.19–17.07) | 4.60 (4.44–4.77) |
| Colon and Rectum | 25.86 (19.90-NA) | 7.00 (6.81–7.24) |
| Pancreas | 0.70 (0.56–0.86) | 0.48 (0.44–0.52) |
| Stomach | 1.32 (1.02–1.91) | 0.94 (0.85–1.02) |
| Oesophagus | 1.36 (0.87–2.54) | 0.99 (0.88–1.05) |
| Variables | (18–50 Years) | (>50 Years) | ||
|---|---|---|---|---|
| n = 2107 | n = 25,748 | |||
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age (years) | 1.01 (1.00–1.02) | 0.03 | 1.04 (1.04–1.04) | <0.001 |
| Sex | ||||
| Female | Reference | - | Reference | - |
| Male | 1.24 (1.09–1.40) | <0.01 | 1.13 (1.10–1.16) | <0.001 |
| Era | ||||
| 1990–1999 | Reference | - | Reference | - |
| 2000–2009 | 0.86 (0.74–1.00) | 0.046 | 0.85 (0.82–0.88) | <0.001 |
| 2010–2017 | 0.92 (0.79–1.08) | 0.32 | 0.89 (0.86–0.93) | <0.001 |
| Cancer site | ||||
| Colon and Rectum | 0.28 (0.22–0.37) | <0.001 | 0.34 (0.31–0.36) | <0.001 |
| Pancreas | 2.48 (1.83–3.36) | <0.001 | 2.13 (1.97–2.31) | <0.001 |
| Stomach | 0.95 (0.69–1.32) | 0.77 | 0.94 (0.86–0.36) | 0.12 |
| Oesophagus | Reference | - | Reference | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schell, D.; Ullah, S.; Brooke-Smith, M.E.; Hollington, P.; Yeow, M.; Karapetis, C.S.; Watson, D.I.; Pandol, S.J.; Roberts, C.T.; Barreto, S.G. Gastrointestinal Adenocarcinoma Incidence and Survival Trends in South Australia, 1990–2017. Cancers 2022, 14, 275. https://doi.org/10.3390/cancers14020275
Schell D, Ullah S, Brooke-Smith ME, Hollington P, Yeow M, Karapetis CS, Watson DI, Pandol SJ, Roberts CT, Barreto SG. Gastrointestinal Adenocarcinoma Incidence and Survival Trends in South Australia, 1990–2017. Cancers. 2022; 14(2):275. https://doi.org/10.3390/cancers14020275
Chicago/Turabian StyleSchell, Dominique, Shahid Ullah, Mark E. Brooke-Smith, Paul Hollington, Marina Yeow, Christos S. Karapetis, David I. Watson, Stephen J. Pandol, Claire T. Roberts, and Savio G. Barreto. 2022. "Gastrointestinal Adenocarcinoma Incidence and Survival Trends in South Australia, 1990–2017" Cancers 14, no. 2: 275. https://doi.org/10.3390/cancers14020275
APA StyleSchell, D., Ullah, S., Brooke-Smith, M. E., Hollington, P., Yeow, M., Karapetis, C. S., Watson, D. I., Pandol, S. J., Roberts, C. T., & Barreto, S. G. (2022). Gastrointestinal Adenocarcinoma Incidence and Survival Trends in South Australia, 1990–2017. Cancers, 14(2), 275. https://doi.org/10.3390/cancers14020275







