The Antitumor Effect of Caffeic Acid Phenethyl Ester by Downregulating Mucosa-Associated Lymphoid Tissue 1 via AR/p53/NF-κB Signaling in Prostate Carcinoma Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Cell Proliferation Assays
2.3. Expression Vector Constructs and Stable Transfection
2.4. Nuclear and Cytoplasmic Extraction Assay
2.5. Immunoblot Assay
2.6. Real-Time Reverse Transcriptase-Polymerase Chain Reaction
2.7. Enzyme-Linked Immunosorbent Assay
2.8. Reporter Vector Constructs
2.9. Transient Transfection and Reporter Assay
2.10. Xenograft Animal Model
2.11. Matrigel Invasion Assay
2.12. NF-κB (p65) Transcription Factor Binding Assay
2.13. Statistical Analysis
3. Results
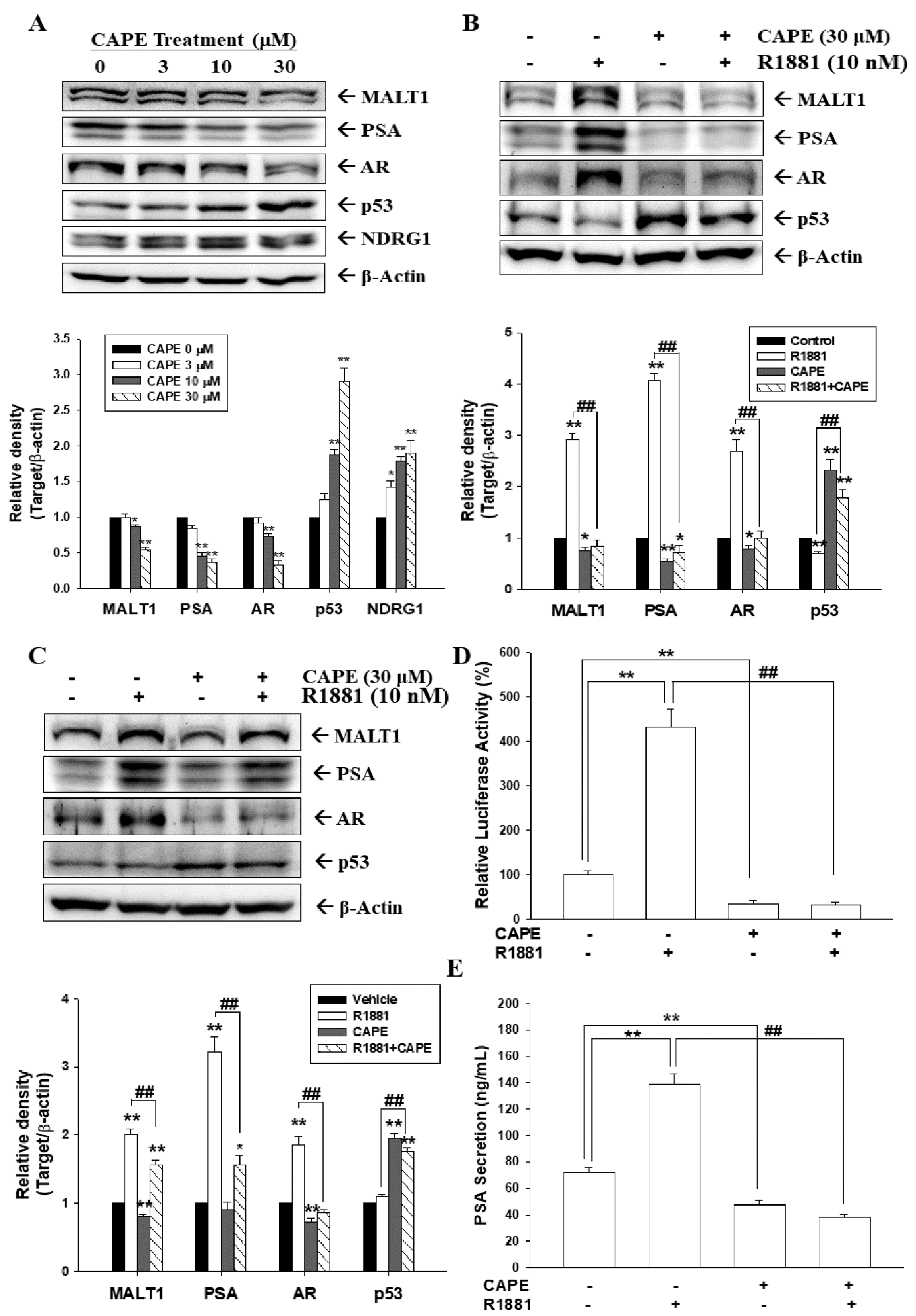
3.1. CAPE Attenuates Activation of Androgen in MALT1 Expression in Androgen-Positive Prostate Carcinoma Cells
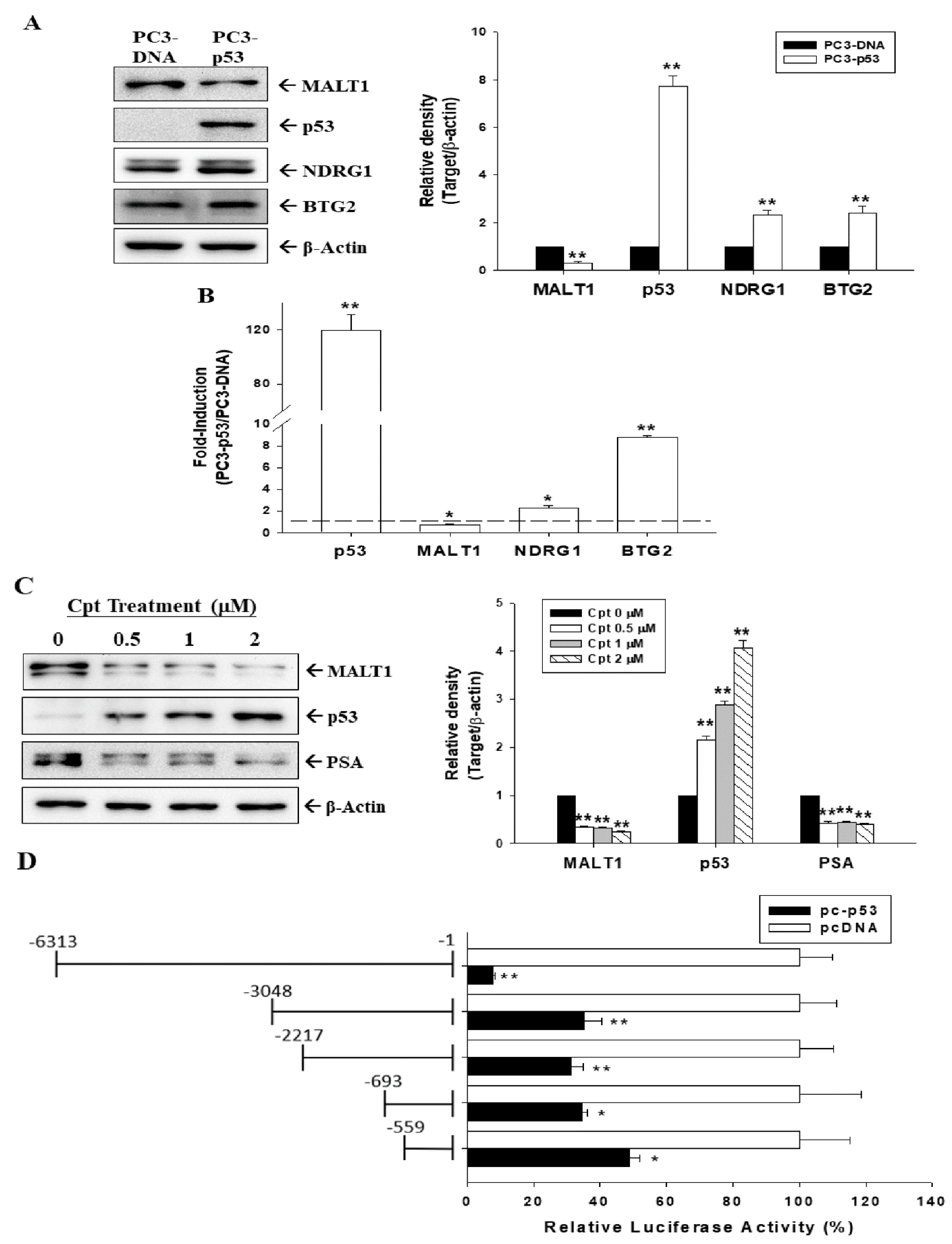
3.2. Tumor Suppressor p53 Downregulated MALT1 Expression in Prostate Carcinoma Cells
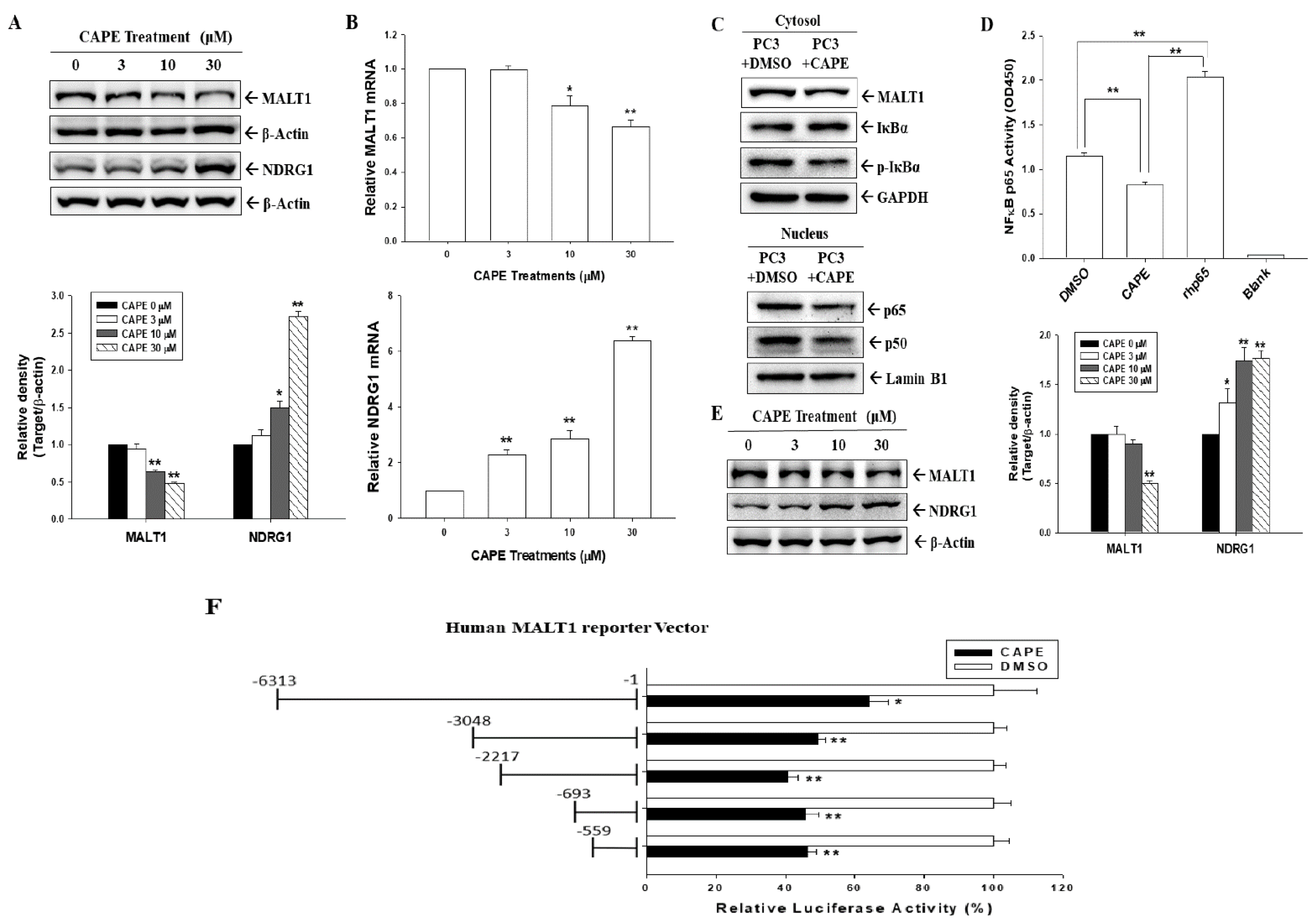
3.3. CAPE Blocks MALT1 Gene Expression to Downregulate NF-κB Activation in Androgen-Negative Prostate Carcinoma Cells
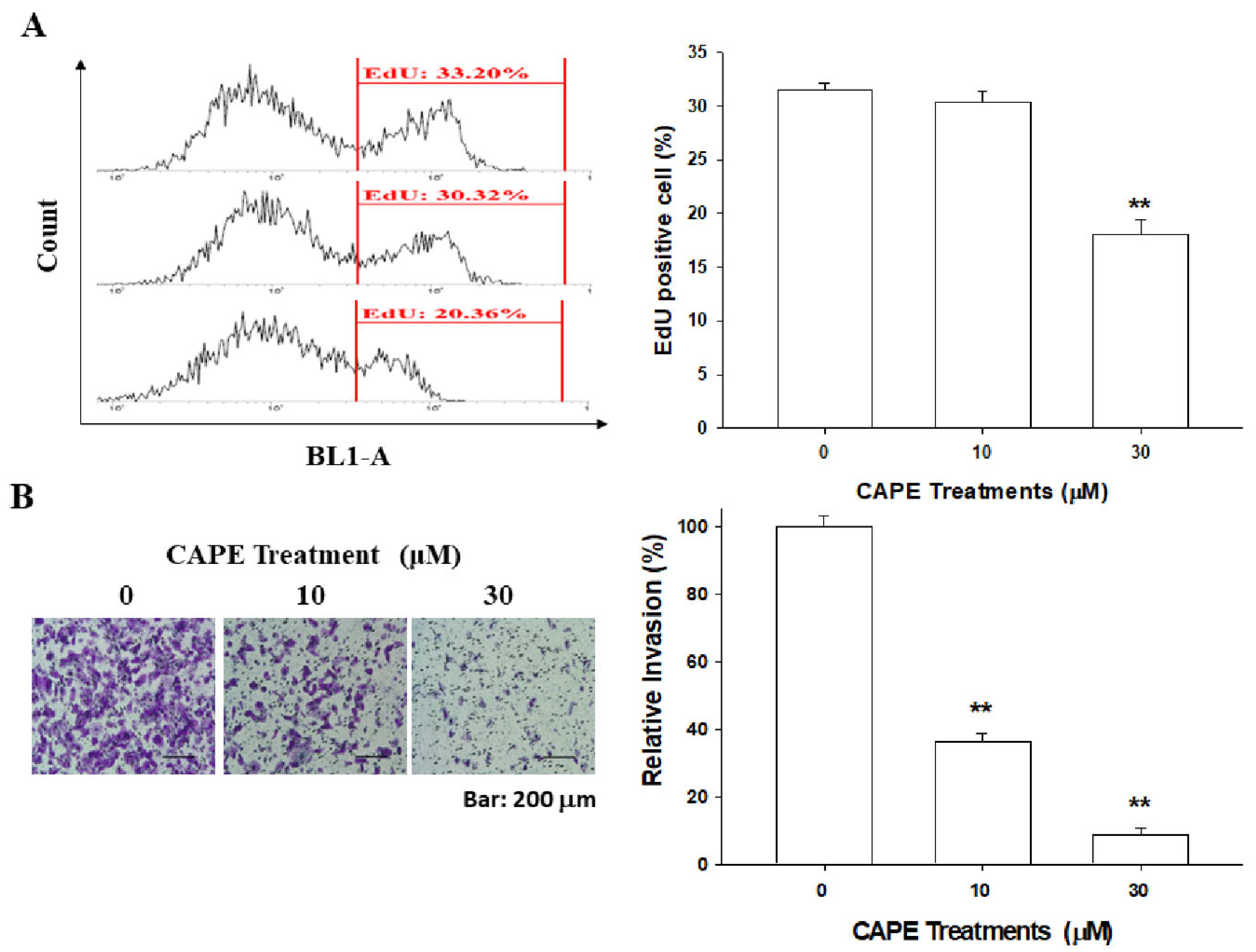
3.4. CAPE Downregulates PC-3 Cell Proliferation and Invasion
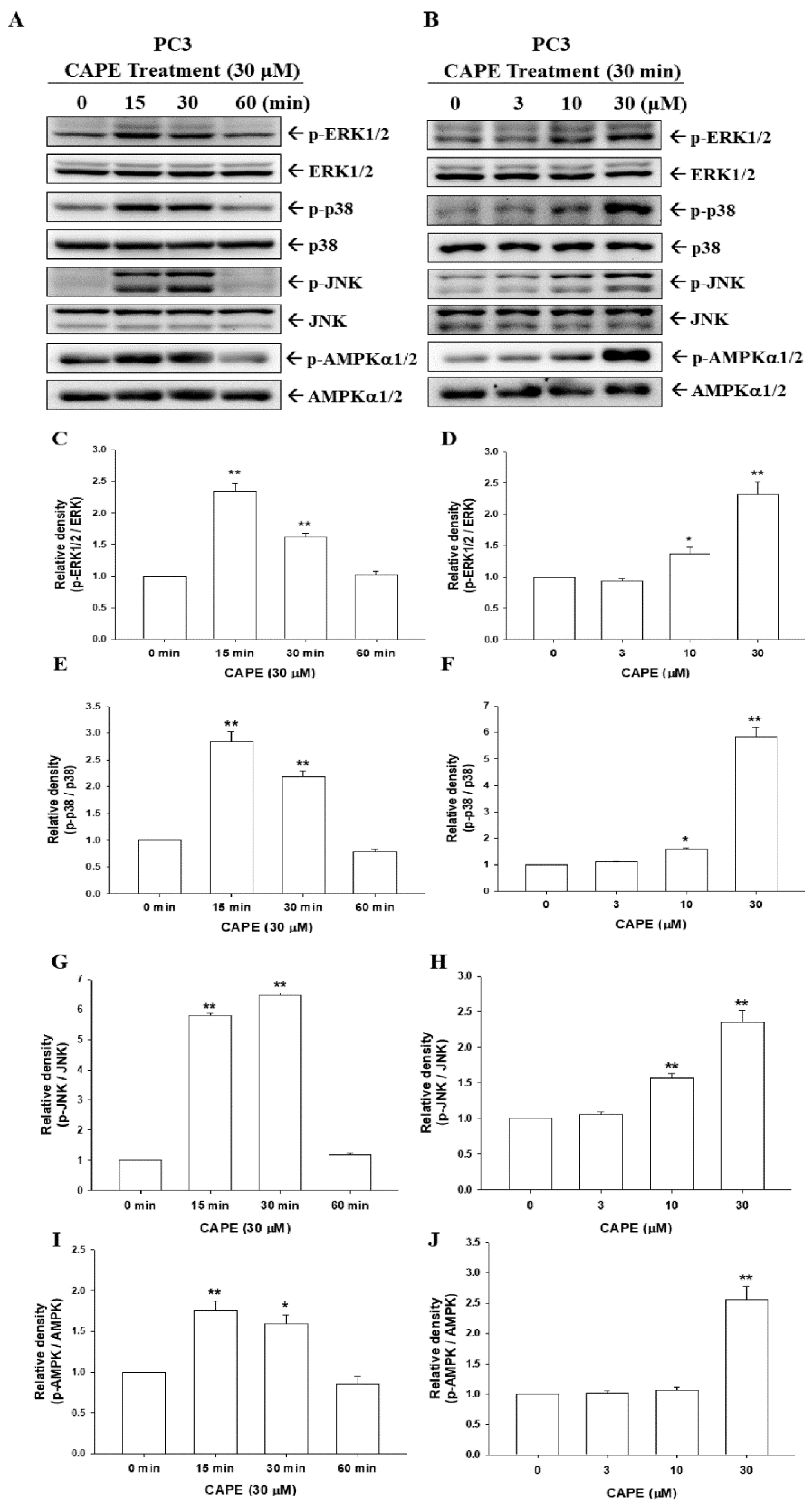
3.5. CAPE Induces Phosphorylation of ERK, p38, JNK, and AMPKα1/2 in PC-3 Cells
3.6. CAPE Induced Expression of MALT1, NDRG1, and Maspin via Different Signaling Pathways
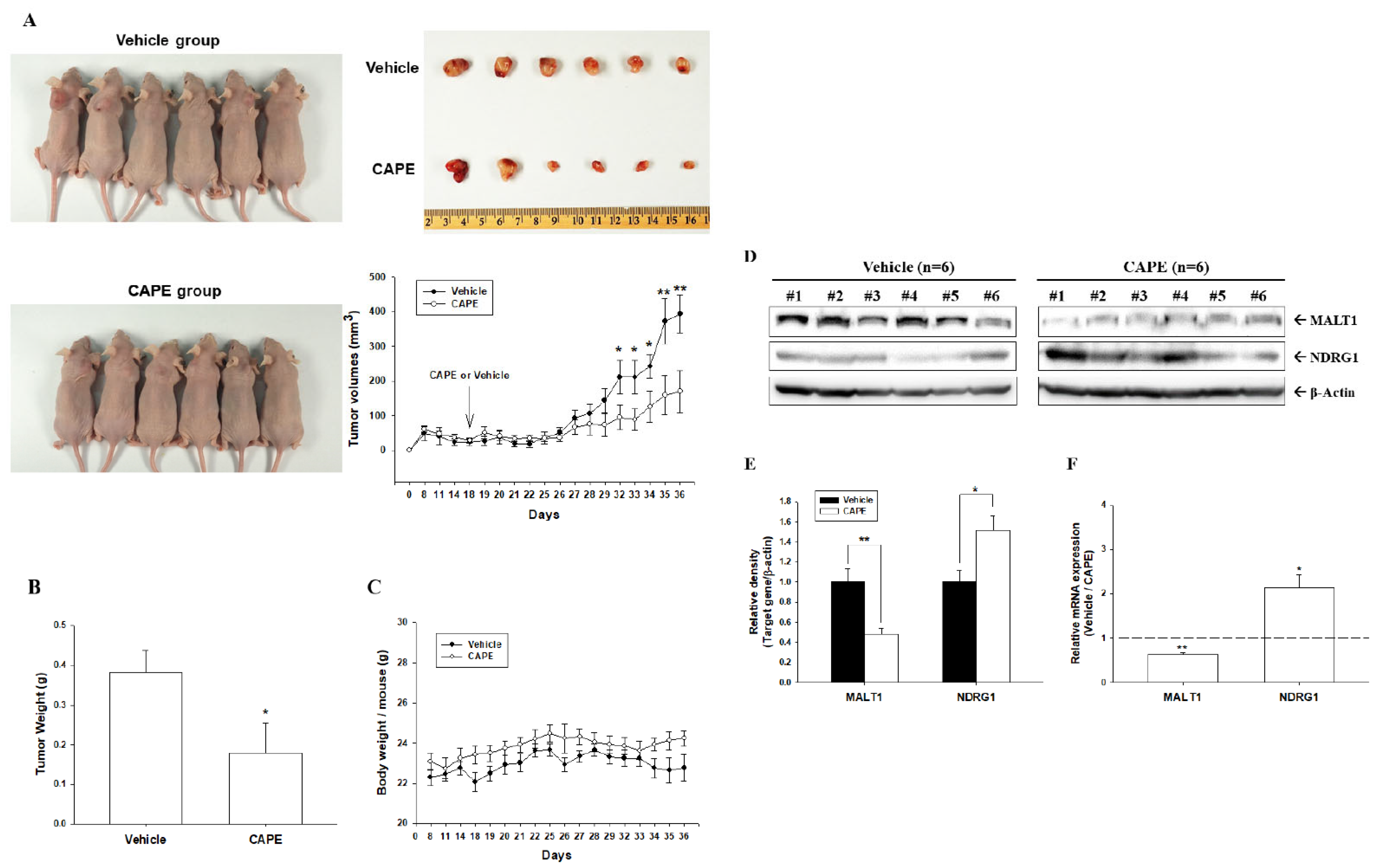
3.7. CAPE Inhibits Tumorigenesis of PC-3 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sawicka, D.; Car, H.; Borawska, M.H.; Niklinski, J. The anticancer activity of propolis. Folia Histochem. Cytobiol. 2012, 50, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Budisan, L.; Gulei, D.; Zanoaga, O.M.; Irimie, A.I.; Sergiu, C.; Braicu, C.; Gherman, C.D.; Berindan-Neagoe, I. Dietary intervention by phytochemicals and their role in modulating coding and non-coding genes in cancer. Int. J. Mol. Sci. 2017, 18, 1178. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Lewis, B.A. Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: Structure-activity relationship. J. Agric. Food Chem. 2002, 50, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.Y.; Jim, W.T.; Su, L.C.; Chung, C.J.; Lin, C.Y.; Huo, C.; Tseng, J.C.; Huang, S.H.; Lai, C.J.; Chen, B.C.; et al. Caffeic Acid phenethyl ester is a potential therapeutic agent for oral cancer. Int. J. Mol. Sci. 2015, 16, 10748–10766. [Google Scholar] [CrossRef]
- Liao, H.F.; Chen, Y.Y.; Liu, J.J.; Hsu, M.L.; Shieh, H.J.; Liao, H.J.; Shieh, C.J.; Shiao, M.S.; Chen, Y.J. Inhibitory effect of caffeic acid phenethyl ester on angiogenesis, tumor invasion, and metastasis. J. Agric. Food Chem. 2003, 51, 7907–7912. [Google Scholar] [CrossRef]
- Natarajan, K.; Singh, S.; Burke, T.R., Jr.; Grunberger, D.; Aggarwal, B.B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc. Natl. Acad. Sci. USA 1996, 93, 9090–9095. [Google Scholar] [CrossRef]
- Huang, Q.; Li, S.; Zhang, L.; Qiao, X.; Zhang, Y.; Zhao, X.; Xiao, G.; Li, Z. CAPE-pNO2 inhibited the growth and metastasis of triple-negative breast cancer via the EGFR/STAT3/Akt/E-Cadherin signaling pathway. Front. Oncol. 2019, 9, 461. [Google Scholar] [CrossRef]
- Chen, M.F.; Wu, C.T.; Chen, Y.J.; Keng, P.C.; Chen, W.C. Cell killing and radiosensitization by caffeic acid phenethyl ester (CAPE) in lung cancer cells. J. Radiat. Res. 2004, 45, 253–260. [Google Scholar] [CrossRef]
- Budisan, L.; Gulei, D.; Jurj, A.; Braicu, C.; Zanoaga, O.; Cojocneanu, R.; Pop, L.; Raduly, L.; Barbat, A.; Moldovan, A.; et al. Inhibitory effect of CAPE and kaempferol in colon cancer cell lines-possible implications in new therapeutic strategies. Int. J. Mol. Sci. 2019, 20, 1199. [Google Scholar] [CrossRef]
- Chuu, C.P.; Lin, H.P.; Ciaccio, M.F.; Kokontis, J.M.; Hause, R.J., Jr.; Hiipakka, R.A.; Liao, S.; Jones, R.B. Caffeic acid phenethyl ester suppresses the proliferation of human prostate cancer cells through inhibition of p70S6K and Akt signaling networks. Cancer Prev. Res. 2012, 5, 788–797. [Google Scholar] [CrossRef]
- Kudugunti, S.K.; Vad, N.M.; Ekogbo, E.; Moridani, M.Y. Efficacy of caffeic acid phenethyl ester (CAPE) in skin B16-F0 melanoma tumor bearing C57BL/6 mice. Investig. New Drugs 2011, 29, 52–62. [Google Scholar] [CrossRef]
- Onori, P.; DeMorrow, S.; Gaudio, E.; Franchitto, A.; Mancinelli, R.; Venter, J.; Kopriva, S.; Ueno, Y.; Alvaro, D.; Savage, J.; et al. Caffeic acid phenethyl ester decreases cholangiocarcinoma growth by inhibition of NF-kappaB and induction of apoptosis. Int. J. Cancer 2009, 125, 565–576. [Google Scholar] [CrossRef]
- Chen, M.J.; Chang, W.H.; Lin, C.C.; Liu, C.Y.; Wang, T.E.; Chu, C.H.; Shih, S.C.; Chen, Y.J. Caffeic acid phenethyl ester induces apoptosis of human pancreatic cancer cells involving caspase and mitochondrial dysfunction. Pancreatology 2008, 8, 566–576. [Google Scholar] [CrossRef]
- Chung, L.C.; Chiang, K.C.; Feng, T.H.; Chang, K.S.; Chuang, S.T.; Chen, Y.J.; Tsui, K.H.; Lee, J.C.; Juang, H.H. Caffeic acid phenethyl ester upregulates N-myc downstream regulated gene 1 via ERK pathway to inhibit human oral cancer cell growth in vitro and in vivo. Mol. Nutr. Food Res. 2017, 61, 1600842. [Google Scholar] [CrossRef]
- Chiang, K.C.; Yang, S.W.; Chang, K.P.; Feng, T.H.; Chang, K.S.; Tsui, K.H.; Shin, Y.S.; Chen, C.C.; Chao, M.; Juang, H.H. Caffeic acid phenethyl ester induces N-myc downstream regulated gene 1 to inhibit cell proliferation and invasion of human nasopharyngeal cancer cells. Int. J. Mol. Sci. 2018, 19, 1397. [Google Scholar] [CrossRef]
- Ho, C.P.; Tsui, K.H.; Chang, K.S.; Sung, H.C.; Hsu, S.Y.; Lin, Y.H.; Yang, P.S.; Chen, C.L.; Feng, T.H.; Juang, H.H. Caffeic acid phenethyl ester inhibits the growth of bladder carcinoma cells by upregulating growth differentiation factor 15. Biomed. J. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Young, R.M.; Staudt, L.M. A new “brew” of MALT1 inhibitors. Cancer Cell 2012, 22, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Afonina, I.S.; Elton, L.; Carpentier, I.; Beyaert, R. MALT1—A universal soldier: Multiple strategies to ensure NF-kappaB activation and target gene expression. FEBS J. 2015, 282, 3286–3297. [Google Scholar] [CrossRef]
- Rosebeck, S.; Rehman, A.O.; Lucas, P.C.; McAllister-Lucas, L.M. From MALT lymphoma to the CBM signalosome: Three decades of discovery. Cell Cycle 2011, 10, 2485–2496. [Google Scholar] [CrossRef] [PubMed]
- Thome, M. Multifunctional roles for MALT1 in T-cell activation. Nat. Rev. Immunol. 2008, 8, 495–500. [Google Scholar] [CrossRef]
- Sun, L.; Deng, L.; Ea, C.K.; Xia, Z.P.; Chen, Z.J. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell 2004, 14, 289–301. [Google Scholar] [CrossRef]
- Du, M.Q. MALT lymphoma: A paradigm of NF-kappaB dysregulation. Semin. Cancer Biol. 2016, 39, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, P.; Lee, J.L.; Hubel, N.E.; Hu, D.; Yerneni, S.; Campbell, P.G.; Pollock, N.; Klei, L.R.; Concel, V.J.; Delekta, P.C.; et al. The CARMA3-Bcl10-MALT1 signalosome drives NFkappaB activation and promotes aggressiveness in angiotensin II receptor-positive breast cancer. Cancer Res. 2018, 78, 1225–1240. [Google Scholar] [CrossRef]
- Pan, D.; Jiang, C.; Ma, Z.; Blonska, M.; You, M.J.; Lin, X. MALT1 is required for EGFR-induced NF-kappaB activation and contributes to EGFR-driven lung cancer progression. Oncogene 2016, 35, 919–928. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Jin, J.; Degan, S.; Tameze, Y.; Zhang, J.Y. MALT1 promotes melanoma progression through JNK/c-Jun signaling. Oncogenesis 2017, 6, e365. [Google Scholar] [CrossRef]
- Yeh, C.N.; Chang, Y.C.; Su, Y.; Hsu, D.S.S.; Cheng, C.T.; Wu, R.C.; Chung, Y.H.; Chiang, K.C.; Yeh, T.S.; Lu, M.L.; et al. Identification of MALT1 as both a prognostic factor and a potential therapeutic target of regorafenib in cholangiocarcinoma patients. Oncotarget 2017, 8, 113444–113459. [Google Scholar] [CrossRef]
- Tsui, K.H.; Chang, K.S.; Sung, H.C.; Hsu, S.Y.; Hou, C.P.; Yang, P.S.; Chen, C.L.; Feng, T.H.; Juang, H.H. Mucosa-associated lymphoid tissue 1 is an oncogene inducing cell proliferation, invasion, and tumor growth via the upregulation of NF-κB activity in human prostate carcinoma cells. Biomedicines 2021, 9, 250. [Google Scholar] [CrossRef]
- Chang, K.S.; Tsui, K.H.; Lin, Y.H.; Hou, C.P.; Feng, T.H.; Juang, H.H. Migration and invasion enhancer 1 is an NF-κB-inducing gene enhancing the cell proliferation and invasion ability of human prostate carcinoma cells in vitro and in vivo. Cancers 2019, 11, 1486. [Google Scholar] [CrossRef]
- Sramkoski, R.M.; Pretlow, T.G., II; Giaconia, J.M.; Pretlow, T.P.; Schwartz, S.; Sy, M.S.; Marengo, S.R.; Rhim, J.S.; Zhang, D.; Jacobberger, J.W. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell. Dev. Biol. Anim. 1999, 35, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Tsui, K.H.; Chang, K.S.; Hou, C.P.; Feng, T.H.; Juang, H.H. Maspin is a PTEN-upregulated and p53-upregulated tumor suppressor gene and acts as an HDAC1 inhibitor in Human Bladder Cancer. Cancers 2020, 12, 10. [Google Scholar] [CrossRef]
- Tsui, K.H.; Chiang, K.C.; Lin, Y.H.; Chang, K.S.; Feng, T.H.; Juang, H.H. BTG2 is a tumor suppressor gene upregulated by p53 and PTEN in human bladder carcinoma cells. Cancer Med. 2018, 7, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Tsui, K.H.; Lin, Y.H.; Hou, C.P.; Chang, K.S.; Tsai, H.H.; Shin, Y.S.; Chen, C.C.; Feng, T.H.; Juang, H.H. Antioxidation and antiapoptosis characteristics of heme oxygenase-1 enhance tumorigenesis of human prostate carcinoma cells. Transl. Oncol. 2020, 13, 102–112. [Google Scholar] [CrossRef]
- Chiang, K.C.; Tsui, K.H.; Chung, L.C.; Yeh, C.N.; Feng, T.H.; Chen, W.T.; Chang, P.L.; Chiang, H.Y.; Juang, H.H. Cisplatin modulates B-cell translocation gene 2 to attenuate cell proliferation of prostate carcinoma cells in both p53-dependent and p53-independent pathways. Sci. Rep. 2014, 4, 5511. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.H.; Chang, Y.L.; Feng, T.H.; Hou, C.P.; Lin, Y.H.; Yang, P.S.; Lee, B.W.; Juang, H.H. Capillarisin blocks prostate-specific antigen expression on activation of androgen receptor in prostate carcinoma cells. Prostate 2018, 78, 242–249. [Google Scholar] [CrossRef]
- Tsui, K.H.; Wu, L.; Chang, P.L.; Hsieh, M.L.; Juang, H.H. Identifying the combination of the transcriptional regulatory sequences on prostate specific antigen and human glandular kallikrein genes. J. Urol. 2004, 172, 2029–2034. [Google Scholar] [CrossRef]
- Celli, N.; Dragani, L.K.; Murzilli, S.; Paglani, T.; Poggi, A. In vitro and in vivo stability of caffeic acid phenethyl ester, a bioactive compound of propolis. J. Agric. Food Chem. 2007, 55, 3398–3407. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Zabolian, A.; Saleki, H.; Farahani, M.V.; Hamzehlou, S.; Far, F.B.; Sharifzadeh, S.O.; Samarghandian, S.; Khan, H.; et al. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: New hope in the fight against cancer. Pharmacol. Res. 2021, 171, 105759. [Google Scholar] [CrossRef]
- Wachtel, M.S.; Nelius, T.; Haynes, A.L.; Dahlbeck, S.; de Riese, W. PSA screening and deaths from prostate cancer after diagnosis—A population based analysis. Prostate 2013, 73, 1365–1369. [Google Scholar] [CrossRef]
- Kuo, Y.Y.; Huo, C.; Lin, C.Y.; Lin, H.P.; Liu, J.S.; Wang, W.C.; Chang, C.R.; Chuu, C.P. Caffeic acid phenethyl ester suppresses androgen receptor signaling and stability via inhibition of phosphorylation on Ser81 and Ser213. Cell. Commun. Signal. 2019, 17, 100. [Google Scholar] [CrossRef]
- Austin, D.C.; Strand, D.W.; Love, H.L.; Franco, O.E.; Jang, A.; Grabowska, M.M.; Miller, N.L.; Hameed, O.; Clark, P.E.; Fowke, J.H.; et al. NF-κB and androgen receptor variant expression correlate with human BPH progression. Prostate 2016, 76, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.W.S.; Yau, W.L.; Tam, C.W.; Yao, K.M.; Shiu, S.Y.W. Melatonin inhibits androgen receptor splice variant-7 (AR-V7)-induced nuclear factor-kappa B (NF-κB) activation and NF-κB activator-induced AR-V7 expression in prostate cancer cells: Potential implications for the use of melatonin in castration-resistant prostate cancer (CRPC) therapy. Int. J. Mol. Sci. 2017, 18, 1130. [Google Scholar] [CrossRef]
- Gurova, K.V.; Roklin, O.W.; Krivokrysenko, V.I.; Chumakov, P.M.; Cohen, M.B.; Feinstein, E.; Gudkov, A.V. Expression of prostate specific antigen (PSA) is negatively regulated by p53. Oncogene 2002, 21, 153–157. [Google Scholar] [CrossRef][Green Version]
- Tsui, K.H.; Chang, P.L.; Lin, H.T.; Juang, H.H. Down-regulation of the prostate specific antigen promoter by p53 in human prostate cancer cells. J. Urol. 2004, 172, 2035–2039. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.D.; Sawyers, C.L. NF-kappa B activates prostate-specific antigen expression and is upregulated in androgen-independent prostate cancer. Mol. Cell Biol. 2002, 22, 2862–2870. [Google Scholar] [CrossRef] [PubMed]
- Saletta, F.; Suryo, R.Y.; Noulsri, E.; Richardson, D.R. Iron chelator-mediated alterations in gene expression: Identification of novel iron-regulated molecules that are molecular targets of hypoxia-inducible factor-1α and p53. Mol. Pharmacol. 2010, 77, 443–458. [Google Scholar] [CrossRef] [PubMed]
- el-Deiry, W.S.; Kern, S.E.; Pietenpol, J.A.; Kinzler, K.W.; Vogelstein, B. Definition of a consensus binding site for p53. Nat. Genet. 1992, 1, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lagowski, J.P.; Vanderbeek, G.E.; Kulesz-Martin, M.F. Facilitated search for specific genomic targets by p53 C-terminal basic DNA binding domain. Cancer Biol. Ther. 2004, 3, 1102–1108. [Google Scholar] [CrossRef][Green Version]
- Gasparian, A.V.; Yao, Y.J.; Kowalczyk, D.; Lyakh, L.A.; Karseladze, A.; Slaga, T.J.; Budunova, I.V. The role of IKK in constitutive activation of NF-kappaB transcription factor in prostate carcinoma cells. J. Cell. Sci. 2002, 115, 141–151. [Google Scholar] [CrossRef]
- Suh, J.; Payvandi, F.; Edelstein, L.C.; Amenta, P.S.; Zong, W.X.; Gelinas, C.; Rabson, A.B. Mechanisms of constitutive NF-kappaB activation in human prostate cancer cells. Prostate 2002, 52, 183–200. [Google Scholar] [CrossRef]
- Xavier, D.S.; Filippos, K.; Sylvain, M.; Gerardo, F. ERKs in cancer: Friends or foes? Cancer Res. 2014, 74, 412–419. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.-S.; Tsui, K.-H.; Hsu, S.-Y.; Sung, H.-C.; Lin, Y.-H.; Hou, C.-P.; Yang, P.-S.; Chen, C.-L.; Feng, T.-H.; Juang, H.-H. The Antitumor Effect of Caffeic Acid Phenethyl Ester by Downregulating Mucosa-Associated Lymphoid Tissue 1 via AR/p53/NF-κB Signaling in Prostate Carcinoma Cells. Cancers 2022, 14, 274. https://doi.org/10.3390/cancers14020274
Chang K-S, Tsui K-H, Hsu S-Y, Sung H-C, Lin Y-H, Hou C-P, Yang P-S, Chen C-L, Feng T-H, Juang H-H. The Antitumor Effect of Caffeic Acid Phenethyl Ester by Downregulating Mucosa-Associated Lymphoid Tissue 1 via AR/p53/NF-κB Signaling in Prostate Carcinoma Cells. Cancers. 2022; 14(2):274. https://doi.org/10.3390/cancers14020274
Chicago/Turabian StyleChang, Kang-Shuo, Ke-Hung Tsui, Shu-Yuan Hsu, Hsin-Ching Sung, Yu-Hsiang Lin, Chen-Pang Hou, Pei-Shan Yang, Chien-Lun Chen, Tsui-Hsia Feng, and Horng-Heng Juang. 2022. "The Antitumor Effect of Caffeic Acid Phenethyl Ester by Downregulating Mucosa-Associated Lymphoid Tissue 1 via AR/p53/NF-κB Signaling in Prostate Carcinoma Cells" Cancers 14, no. 2: 274. https://doi.org/10.3390/cancers14020274
APA StyleChang, K.-S., Tsui, K.-H., Hsu, S.-Y., Sung, H.-C., Lin, Y.-H., Hou, C.-P., Yang, P.-S., Chen, C.-L., Feng, T.-H., & Juang, H.-H. (2022). The Antitumor Effect of Caffeic Acid Phenethyl Ester by Downregulating Mucosa-Associated Lymphoid Tissue 1 via AR/p53/NF-κB Signaling in Prostate Carcinoma Cells. Cancers, 14(2), 274. https://doi.org/10.3390/cancers14020274







