The Role of MARCKS in Metastasis and Treatment Resistance of Solid Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Protein Structure and Cellular Localization of MARCKS
3. Biological Functions of MARCKS
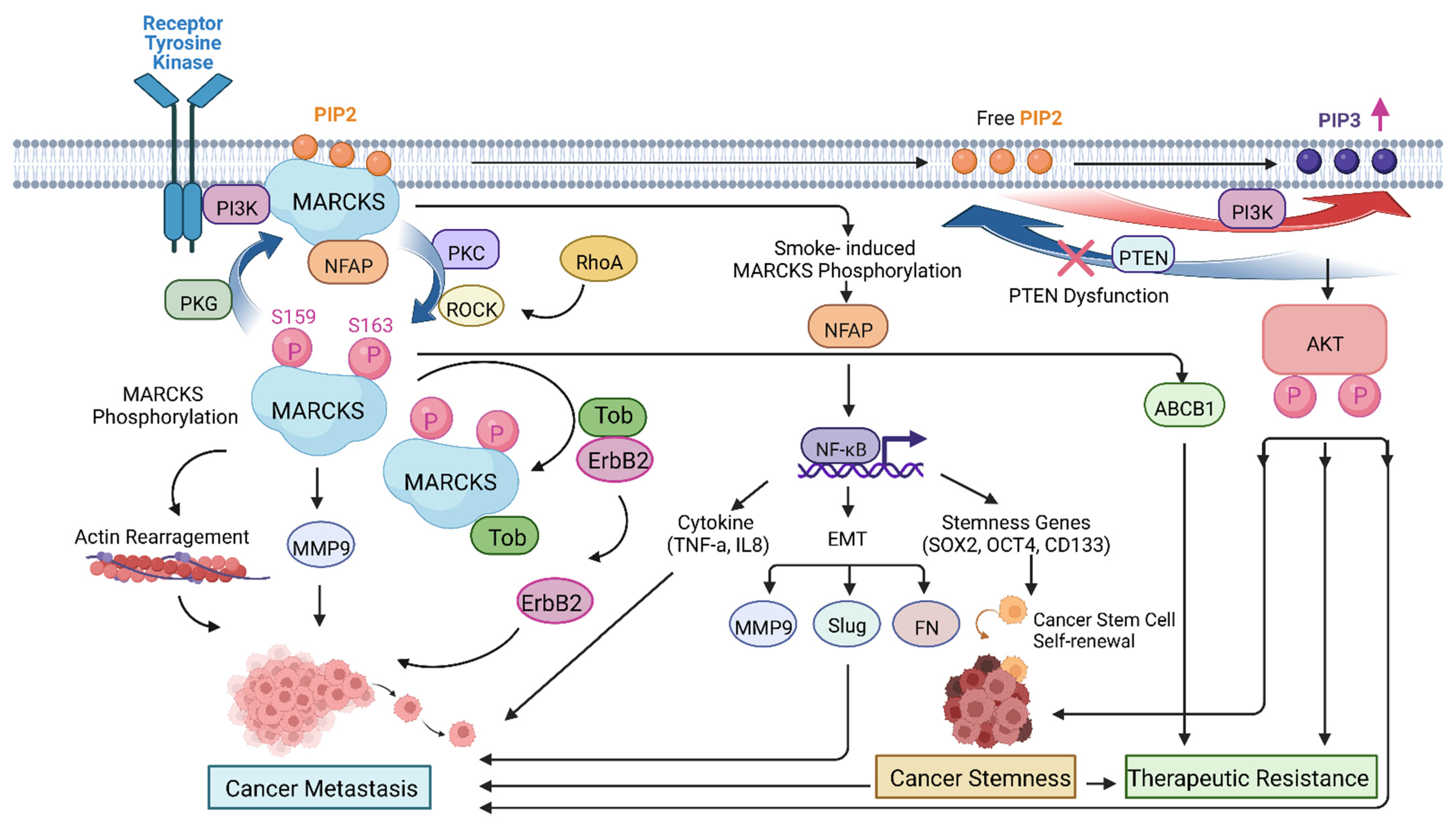
4. MARCKS in Cancer Metastasis
5. MARCKS in Cancer Stemness
6. MARCKS in Cancer Therapeutic Resistance
7. Targeting MARCKS as a New Therapeutic Strategy
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Deyell, M.; Garris, C.S.; Laughney, A.M. Cancer metastasis as a non-healing wound. Br. J. Cancer 2021, 124, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.G.; Croucher, P.I. The dormant cancer cell life cycle. Nat. Rev. Cancer 2020, 20, 398–411. [Google Scholar] [CrossRef]
- Majidpoor, J.; Mortezaee, K. Steps in metastasis: An updated review. Med. Oncol. 2021, 38, 3. [Google Scholar] [CrossRef]
- Dorris, E.; O’Neill, A.; Hanrahan, K.; Treacy, A.; Watson, R.W. MARCKS promotes invasion and is associated with biochemical recurrence in prostate cancer. Oncotarget 2017, 8, 72021–72030. [Google Scholar] [CrossRef]
- Li, C.; Xia, R.; Xue, H.; Hu, Y.; Sun, M.; Fang, D.; Yang, W.; Xiao, F.; Hou, J. Overexpression of MARCKS indicates a poor prognosis of oral squamous cell carcinoma. Oncol. Lett. 2018, 16, 5498–5504. [Google Scholar] [CrossRef]
- Mohapatra, P.; Yadav, V.; Toftdahl, M.; Andersson, T. WNT5A-Induced Activation of the Protein Kinase C Substrate MARCKS Is Required for Melanoma Cell Invasion. Cancers 2020, 12, 346. [Google Scholar] [CrossRef]
- Rao, G.; Pierobon, M.; Kim, I.-K.; Hsu, W.-H.; Deng, J.; Moon, Y.-W.; Petricoin, E.F.; Zhang, Y.-W.; Wang, Y.; Giaccone, G. Inhibition of AKT1 signaling promotes invasion and metastasis of non-small cell lung cancer cells with K-RAS or EGFR mutations. Sci. Rep. 2017, 7, 7066. [Google Scholar] [CrossRef]
- Sheats, M.K.; Yin, Q.; Fang, S.; Park, J.; Crews, A.L.; Parikh, I.; Dickson, B.; Adler, K.B. MARCKS and Lung Disease. Am. J. Respir. Cell Mol. Biol. 2019, 60, 16–27. [Google Scholar] [CrossRef]
- Liang, W.; Gao, R.; Yang, M.; Wang, X.; Cheng, K.; Shi, X.; He, C.; Li, Y.; Wu, Y.; Shi, L.; et al. MARCKSL1 promotes the proliferation, migration and invasion of lung adenocarcinoma cells. Oncol. Lett. 2020, 19, 2272–2280. [Google Scholar] [CrossRef]
- Aderem, A. The Marcks brothers: A family of protein kinase C substrates. Cell 1992, 71, 713–716. [Google Scholar] [CrossRef]
- Björkblom, B.; Padzik, A.; Mohammad, H.; Westerlund, N.; Komulainen, E.; Hollos, P.; Parviainen, L.; Papageorgiou, A.C.; Iljin, K.; Kallioniemi, O.; et al. c-Jun N-Terminal Kinase Phosphorylation of MARCKSL1 Determines Actin Stability and Migration in Neurons and in Cancer Cells. Mol. Cell. Biol. 2012, 32, 3513–3526. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, W.; Selmi, C.; Ridgway, W.M.; Leung, P.S.C.; Zhang, F.; Gershwin, M.E. The myristoylated alanine-rich C-kinase substrates (MARCKS): A membrane-anchored mediator of the cell function. Autoimmun. Rev. 2021, 20, 102942. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.W.R.; Yang, D.C.; Chen, C.-H. Myristoylated alanine-rich C kinase substrate (MARCKS): A multirole signaling protein in cancers. Cancer Metastasis Rev. 2017, 36, 737–747. [Google Scholar] [CrossRef]
- Iyer, D.N.; Faruq, O.; Zhang, L.; Rastgoo, N.; Liu, A.; Chang, H. Pathophysiological roles of myristoylated alanine-rich C-kinase substrate (MARCKS) in hematological malignancies. Biomark. Res. 2021, 9, 34. [Google Scholar] [CrossRef]
- Wu, W.C.; Walaas, S.I.; Nairn, A.C.; Greengard, P. Calcium/phospholipid regulates phosphorylation of a Mr “87k” substrate protein in brain synaptosomes. Proc. Natl. Acad. Sci. USA 1982, 79, 5249–5253. [Google Scholar] [CrossRef]
- Hartwig, J.H.; Thelen, M.; Resen, A.; Janmey, P.A.; Nairn, A.C.; Aderem, A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium–calmodulin. Nature 1992, 356, 618–622. [Google Scholar] [CrossRef]
- Green, T.D.; Crews, A.L.; Park, J.; Fang, S.; Adler, K.B. Regulation of mucin secretion and inflammation in asthma: A role for MARCKS protein? Biochim. Biophys. Acta 2011, 1810, 1110–1113. [Google Scholar] [CrossRef]
- Vergères, G.; Manenti, S.; Weber, T.; Stürzinger, C. The Myristoyl Moiety of Myristoylated Alanine-rich C Kinase Substrate (MARCKS) and MARCKS-related Protein Is Embedded in the Membrane. J. Biol. Chem. 1995, 270, 19879–19887. [Google Scholar] [CrossRef]
- Czech, M.P. PIP2 and PIP3: Complex Roles at the Cell Surface. Cell 2000, 100, 603–606. [Google Scholar] [CrossRef]
- Tanabe, A.; Kamisuki, Y.; Hidaka, H.; Suzuki, M.; Negishi, M.; Takuwa, Y. PKC phosphorylates MARCKS Ser159 not only directly but also through RhoA/ROCK. Biochem. Biophys. Res. Commun. 2006, 345, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Kalwa, H.; Michel, T. The MARCKS Protein Plays a Critical Role in Phosphatidylinositol 4,5-Bisphosphate Metabolism and Directed Cell Movement in Vascular Endothelial Cells. J. Biol. Chem. 2011, 286, 2320–2330. [Google Scholar] [CrossRef] [PubMed]
- Laux, T.; Fukami, K.; Thelen, M.; Golub, T.; Frey, D.; Caroni, P. Gap43, Marcks, and Cap23 Modulate Pi(4,5)p2 at Plasmalemmal Rafts, and Regulate Cell Cortex Actin Dynamics through a Common Mechanism. J. Cell Biol. 2000, 149, 1455–1472. [Google Scholar] [CrossRef]
- Chen, X.; Rotenberg, S.A. PhosphoMARCKS drives motility of mouse melanoma cells. Cell. Signal. 2010, 22, 1097–1103. [Google Scholar] [CrossRef]
- Li, Y.; Martin, L.D.; Spizz, G.; Adler, K.B. MARCKS Protein Is a Key Molecule Regulating Mucin Secretion by Human Airway Epithelial Cells in Vitro. J. Biol. Chem. 2001, 276, 40982–40990. [Google Scholar] [CrossRef]
- Sheats, M.K.; Sung, E.J.; Adler, K.B.; Jones, S.L. In Vitro Neutrophil Migration Requires Protein Kinase C-Delta (δ-PKC)-Mediated Myristoylated Alanine-Rich C-Kinase Substrate (MARCKS) Phosphorylation. Inflammation 2015, 38, 1126–1141. [Google Scholar] [CrossRef]
- Eckert, R.E.; Neuder, L.E.; Park, J.; Adler, K.B.; Jones, S.L. Myristoylated Alanine-Rich C-Kinase Substrate (MARCKS) Protein Regulation of Human Neutrophil Migration. Am. J. Respir. Cell Mol. Biol. 2010, 42, 586–594. [Google Scholar] [CrossRef]
- Green, T.D.; Park, J.; Yin, Q.; Fang, S.; Crews, A.L.; Jones, S.L.; Adler, K.B. Directed migration of mouse macrophages in vitro involves myristoylated alanine-rich C-kinase substrate (MARCKS) protein. J. Leukoc. Biol. 2012, 92, 633–639. [Google Scholar] [CrossRef]
- Ott, L.E.; Sung, E.J.; Melvin, A.T.; Sheats, M.K.; Haugh, J.M.; Adler, K.B.; Jones, S.L. Fibroblast Migration Is Regulated by Myristoylated Alanine-Rich C-Kinase Substrate (MARCKS) Protein. PLoS ONE 2013, 8, e66512. [Google Scholar] [CrossRef]
- Rombouts, K.; Mello, T.; Liotta, F.; Galli, A.; Caligiuri, A.; Annunziato, F.; Pinzani, M. MARCKS actin-binding capacity mediates actin filament assembly during mitosis in human hepatic stellate cells. Am. J. Physiol. Cell Physiol. 2012, 303, C357–C367. [Google Scholar] [CrossRef] [PubMed]
- Schulman, H. Chapter 4—Intracellular Signaling. In From Molecules to Networks, 3rd ed.; Byrne, J.H., Heidelberger, R., Waxham, M.N., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 119–148. [Google Scholar]
- Toker, A. Phosphatidylinositol Bisphosphate and Trisphosphate. In Encyclopedia of Biological Chemistry, 2nd ed.; Lennarz, W.J., Lane, M.D., Eds.; Academic Press: Waltham, MA, USA, 2013; pp. 451–455. [Google Scholar]
- Mandal, K. Review of PIP2 in Cellular Signaling, Functions and Diseases. Int. J. Mol. Sci. 2020, 21, 8342. [Google Scholar] [CrossRef] [PubMed]
- Damera, G.; Jester, W.F.; Jiang, M.; Zhao, H.; Fogle, H.W.; Mittelman, M.; Haczku, A.; Murphy, E.; Parikh, I.; Panettieri, R.A. Inhibition of myristoylated alanine-rich C kinase substrate (MARCKS) protein inhibits ozone-induced airway neutrophilia and inflammation. Exp. Lung Res. 2010, 36, 75–84. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takashi, S.; Park, J.; Fang, S.; Koyama, S.; Parikh, I.; Adler, K.B. A Peptide Against the N-Terminus of Myristoylated Alanine-Rich C Kinase Substrate Inhibits Degranulation of Human Leukocytes In Vitro. Am. J. Respir. Cell Mol. Biol. 2006, 34, 647–652. [Google Scholar] [CrossRef]
- Li, J.; D’Annibale-Tolhurst, M.A.; Adler, K.B.; Fang, S.; Yin, Q.; Birkenheuer, A.J.; Levy, M.G.; Jones, S.L.; Sung, E.J.; Hawkins, E.C.; et al. A Myristoylated Alanine-Rich C Kinase Substrate–Related Peptide Suppresses Cytokine mRNA and Protein Expression in LPS-Activated Canine Neutrophils. Am. J. Respir. Cell Mol. Biol. 2013, 48, 314–321. [Google Scholar] [CrossRef]
- Button, E.; Shapland, C.; Lawson, D. Actin, its associated proteins and metastasis. Cell Motil. Cytoskelet. 1995, 30, 247–251. [Google Scholar] [CrossRef]
- Reed, J.C.; Rapp, U.; Cuddy, M.P. Transformed 3T3 cells have reduced levels and altered subcellular distribution of the major PKC substrate protein marcks. Cell. Signal. 1991, 3, 569–576. [Google Scholar] [CrossRef]
- Hanada, S.; Kakehashi, A.; Nishiyama, N.; Wei, M.; Yamano, S.; Chung, K.; Komatsu, H.; Inoue, H.; Suehiro, S.; Wanibuchi, H. Myristoylated alanine-rich C-kinase substrate as a prognostic biomarker in human primary lung squamous cell carcinoma. Cancer Biomark. 2013, 13, 289–298. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chiu, C.-L.; Adler, K.B.; Wu, R. A Novel Predictor of Cancer Malignancy: Up-regulation of Myristoylated Alanine-Rich C Kinase Substrate Phosphorylation in Lung Cancer. Am. J. Respir. Crit. Care Med. 2014, 189, 1002–1004. [Google Scholar] [CrossRef]
- Chen, C.-H.; Thai, P.; Yoneda, K.; Adler, K.B.; Yang, P.-C.; Wu, R. A peptide that inhibits function of Myristoylated Alanine-Rich C Kinase Substrate (MARCKS) reduces lung cancer metastasis. Oncogene 2014, 33, 3696–3706. [Google Scholar] [CrossRef]
- Rohrbach, T.D.; Jarboe, J.S.; Anderson, J.C.; Trummell, H.Q.; Hicks, P.H.; Weaver, A.N.; Yang, E.S.; Oster, R.A.; Deshane, J.S.; Steele, C.; et al. Targeting the effector domain of the myristoylated alanine rich C-kinase substrate enhances lung cancer radiation sensitivity. Int. J. Oncol. 2014, 46, 1079–1088. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.-J.; Hsu, S.-W.; Zhang, J.; Li, J.-M.; Yang, D.C.; Gu, S.; Pinkerton, K.E.; Chen, C.-H. MARCKS cooperates with NKAP to activate NF-kB signaling in smoke-related lung cancer. Theranostics 2021, 11, 4122–4136. [Google Scholar] [CrossRef] [PubMed]
- Adler, K.B.; Dickson, B. An Inhaled MARCKS (Myristoylated Alanine-Rich C Kinase Substrate)—Inhibitory Drug, BIO-11006, Elicited a StatisticallySignificant Result (p = 0.02) in Overall Response Rate (ORR) After 3 Months Compared to Standard of Care Chemotherapy in a Phase 2 Trial of Late Stage Non Small Cell Lung Cancer (NSCLC). Am. J. Resp. Crit. Care 2020, 201, A6197. [Google Scholar]
- Browne, B.C.; Hochgräfe, F.; Wu, J.; Millar, E.K.A.; Barraclough, J.; Stone, A.; McCloy, R.A.; Lee, C.S.; Roberts, C.; Ali, N.A.; et al. Global characterization of signalling networks associated with tamoxifen resistance in breast cancer. FEBS J. 2013, 280, 5237–5257. [Google Scholar] [CrossRef]
- Manai, M.; Thomassin-Piana, J.; Gamoudi, A.; Finetti, P.; Lopez, M.; Eghozzi, R.; Ayadi, S.; Lamine, O.B.; Manai, M.; Rahal, K.; et al. MARCKS protein overexpression in inflammatory breast cancer. Oncotarget 2017, 8, 6246–6257. [Google Scholar] [CrossRef] [PubMed]
- Manai, M.; Abdeljaoued, S.; Goucha, A.; Adouni, O.; Bettaieb, I.; Bouzaien, H.; Rahal, K.; Birnbaum, D.; Bertucci, F.; Gamoudi, A. MARCKS protein overexpression is associated with poor prognosis in male breast cancer. Cancer Biomark. 2019, 26, 513–522. [Google Scholar] [CrossRef]
- Chen, C.-H.; Cheng, C.-T.; Yuan, Y.; Zhai, J.; Arif, M.; Fong, L.W.R.; Wu, R.; Ann, D.K. Elevated MARCKS phosphorylation contributes to unresponsiveness of breast cancer to paclitaxel treatment. Oncotarget 2015, 6, 15194–15208. [Google Scholar] [CrossRef]
- Cho, S.J.; La, M.-H.; Ahn, J.K.; Meadows, G.G.; Joe, C.O. Tob-Mediated Cross-Talk between MARCKS Phosphorylation and ErbB-2 Activation. Biochem. Biophys. Res. Commun. 2001, 283, 273–277. [Google Scholar] [CrossRef]
- Yu, D.; Hung, M.-C. Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene 2000, 19, 6115–6121. [Google Scholar] [CrossRef]
- Chen, C.-H.; Fong, L.W.R.; Yu, E.; Wu, R.; Trott, J.F.; Weiss, R.H. Upregulation of MARCKS in kidney cancer and its potential as a therapeutic target. Oncogene 2017, 36, 3588–3598. [Google Scholar] [CrossRef]
- Techasen, A.; Loilome, W.; Namwat, N.; Takahashi, E.; Sugihara, E.; Puapairoj, A.; Miwa, M.; Saya, H.; Yongvanit, P. Myristoylated alanine-rich C kinase substrate phosphorylation promotes cholangiocarcinoma cell migration and metastasis via the protein kinase C-dependent pathway. Cancer Sci. 2010, 101, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, Q.; Luo, Y.; Yuan, P.; Tang, C.; Hui, Y.; Wang, Z. miR-34c-3p inhibits cell proliferation, migration and invasion of hepatocellular carcinoma by targeting MARCKS. Int. J. Clin. Exp. Pathol. 2015, 8, 12728–12737. [Google Scholar]
- Rombouts, K.; Carloni, V.; Mello, T.; Omenetti, S.; Galastri, S.; Madiai, S.; Galli, A.; Pinzani, M. Myristoylated Alanine-Rich protein Kinase C Substrate (MARCKS) expression modulates the metastatic phenotype in human and murine colon carcinoma in vitro and in vivo. Cancer Lett. 2013, 333, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Bickeböller, M.; Tagscherer, K.E.; Kloor, M.; Jansen, L.; Chang-Claude, J.; Brenner, H.; Hoffmeister, M.; Toth, C.; Schirmacher, P.; Roth, W.; et al. Functional characterization of the tumor-suppressor MARCKS in colorectal cancer and its association with survival. Oncogene 2015, 34, 1150–1159. [Google Scholar] [CrossRef]
- Micallef, J.; Taccone, M.; Mukherjee, J.; Croul, S.; Busby, J.; Moran, M.F.; Guha, A. Epidermal Growth Factor Receptor Variant III–Induced Glioma Invasion Is Mediated through Myristoylated Alanine-Rich Protein Kinase C Substrate Overexpression. Cancer Res. 2009, 69, 7548–7556. [Google Scholar] [CrossRef] [PubMed]
- Jarboe, J.S.; Anderson, J.C.; Duarte, C.W.; Mehta, T.; Nowsheen, S.; Hicks, P.H.; Whitley, A.C.; Rohrbach, T.D.; McCubrey, R.O.; Chiu, S.; et al. MARCKS Regulates Growth and Radiation Sensitivity and Is a Novel Prognostic Factor for Glioma. Clin. Cancer Res. 2012, 18, 3030–3041. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, D.; Sha, J.; Sun, P.; Huang, Y. MicroRNA-21 directly targets MARCKS and promotes apoptosis resistance and invasion in prostate cancer cells. Biochem. Biophys. Res. Commun. 2009, 383, 280–285. [Google Scholar] [CrossRef]
- Finlayson, A.E.; Freeman, K.W. A Cell Motility Screen Reveals Role for MARCKS-Related Protein in Adherens Junction Formation and Tumorigenesis. PLoS ONE 2009, 4, e7833. [Google Scholar] [CrossRef]
- Tomiyama, E.; Matsuzaki, K.; Fujita, K.; Shiromizu, T.; Narumi, R.; Jingushi, K.; Koh, Y.; Matsushita, M.; Nakano, K.; Hayashi, Y.; et al. Proteomic analysis of urinary and tissue-exudative extracellular vesicles to discover novel bladder cancer biomarkers. Cancer Sci. 2021, 112, 2033–2045. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Ito, T.; Hanson, V.; Schwartz, G.K.; Aderem, A.A.; Holland, J.F.; Tamaya, T.; Ohnuma, T. PMA-induced reduction in invasiveness is associated with hyperphosphorylation of MARCKS and talin in invasive bladder cancer cells. Int. J. Cancer 1998, 75, 774–779. [Google Scholar] [CrossRef]
- Chen, C.-H.; Statt, S.; Chiu, C.-L.; Thai, P.; Arif, M.; Adler, K.B.; Wu, R. Targeting Myristoylated Alanine-Rich C Kinase Substrate Phosphorylation Site Domain in Lung Cancer. Mechanisms and Therapeutic Implications. Am. J. Respir. Crit. Care Med. 2014, 190, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Benedetti, L.G.; Abera, M.B.; Wang, H.; Abba, M.; Kazanietz, M.G. Protein kinase C and cancer: What we know and what we do not. Oncogene 2014, 33, 5225–5237. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.M.; Salama, M.F.; Hannun, Y.A. Protein Kinase C as a Therapeutic Target in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 5527. [Google Scholar] [CrossRef] [PubMed]
- Regala, R.P.; Davis, R.K.; Kunz, A.; Khoor, A.; Leitges, M.; Fields, A.P. Atypical Protein Kinase Cι Is Required for Bronchioalveolar Stem Cell Expansion and Lung Tumorigenesis. Cancer Res. 2009, 69, 7603–7611. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Justilien, V.; Jamieson, L.; Murray, N.R.; Fields, A.P. Protein Kinase Cι Drives a NOTCH3-dependent Stem-like Phenotype in Mutant KRAS Lung Adenocarcinoma. Cancer Cell 2016, 29, 367–378. [Google Scholar] [CrossRef]
- Tam, W.L.; Lu, H.; Buikhuisen, J.; Soh, B.S.; Lim, E.; Reinhardt, F.; Wu, Z.J.; Krall, J.A.; Bierie, B.; Guo, W.; et al. Protein Kinase C α Is a Central Signaling Node and Therapeutic Target for Breast Cancer Stem Cells. Cancer Cell 2013, 24, 347–364. [Google Scholar] [CrossRef]
- Affer, M.; Dao, S.; Liu, C.; Olshen, A.B.; Mo, Q.; Viale, A.; Lambek, C.L.; Marr, T.G.; Clarkson, B.D. Gene Expression Differences between Enriched Normal and Chronic Myelogenous Leukemia Quiescent Stem/Progenitor Cells and Correlations with Biological Abnormalities. J. Oncol. 2011, 2011, 798592. [Google Scholar] [CrossRef]
- Brandi, J.; Pozza, E.D.; Dando, I.; Biondani, G.; Robotti, E.; Jenkins, R.; Elliott, V.; Park, K.; Marengo, E.; Costello, E.; et al. Secretome protein signature of human pancreatic cancer stem-like cells. J. Proteom. 2016, 136, 1–12. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Yao, L.; Guo, S.; Gao, Y.; Zhu, P. The long noncoding RNA lncZic2 drives the self-renewal of liver tumor–initiating cells via the protein kinase C substrates MARCKS and MARCKSL1. J. Biol. Chem. 2018, 293, 7982–7992. [Google Scholar] [CrossRef]
- Chatterjee, N.; Bivona, T.G. Polytherapy and Targeted Cancer Drug Resistance. Trends Cancer 2019, 5, 170–182. [Google Scholar] [CrossRef]
- Dianat-Moghadam, H.; Heidarifard, M.; Jahanban-Esfahlan, R.; Panahi, Y.; Hamishehkar, H.; Pouremamali, F.; Rahbarghazi, R.; Nouri, M. Cancer stem cells-emanated therapy resistance: Implications for liposomal drug delivery systems. J. Control. Release 2018, 288, 62–83. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Song, X.; Xu, D.; Tiek, D.; Goenka, A.; Wu, B.; Sastry, N.; Hu, B.; Cheng, S.-Y. Stem cell programs in cancer initiation, progression, and therapy resistance. Theranostics 2020, 10, 8721–8743. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, D.R.; Weinberg, R.A. Tackling the cancer stem cells—What challenges do they pose? Nat. Rev. Drug Discov. 2014, 13, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Kim, E.-K.; Song, M.-J.; Kim, T.-Y.; Jang, H.H.; Kang, D. SILAC-Based Quantitative Proteomic Analysis of Oxaliplatin-Resistant Pancreatic Cancer Cells. Cancers 2021, 13, 724. [Google Scholar] [CrossRef]
- Wenzel, T.; Büch, T.; Urban, N.; Weirauch, U.; Schierle, K.; Aigner, A.; Schaefer, M.; Kalwa, H. Restoration of MARCK enhances chemosensitivity in cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 843–858. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Xiao, H.; Zheng, Y.; Ma, L.; Tian, L.; Sun, Q. Clinically-Relevant ABC Transporter for Anti-Cancer Drug Resistance. Front. Pharmacol. 2021, 12, 648407. [Google Scholar] [CrossRef]
- Eustace, N.J.; Anderson, J.C.; Langford, C.P.; Trummell, H.Q.; Hicks, P.H.; Jarboe, J.S.; Mobley, J.A.; Hjelmeland, A.B.; Hackney, J.R.; Pedersen, R.T.; et al. Myristoylated alanine-rich C-kinase substrate effector domain phosphorylation regulates the growth and radiation sensitization of glioblastoma. Int. J. Oncol. 2019, 54, 2039–2053. [Google Scholar] [CrossRef]
- Agrawal, A.; Murphy, E.C., III; Park, J.; Adler, K.B.; Parikh, I. Aerosolized BIO-11006, a Novel MARCKS- Related Peptide, Improves Airway Obstruction in a Mouse Model of Mucus Hypersecretion. J. Epithel. Biol. Pharmacol. 2011, 4, 1–6. [Google Scholar] [CrossRef]
- Zhang, L.; Rastgoo, N.; Wu, J.; Zhang, M.; Pourabdollah, M.; Zacksenhaus, E.; Chen, Y.; Chang, H. MARCKS inhibition cooperates with autophagy antagonists to potentiate the effect of standard therapy against drug-resistant multiple myeloma. Cancer Lett. 2020, 480, 29–38. [Google Scholar] [CrossRef]
- Eustace, N.J.; Anderson, J.C.; Warram, J.M.; Widden, H.N.; Pedersen, R.T.; Alrefai, H.; Patel, Z.; Hicks, P.H.; Placzek, W.J.; Gillespie, G.Y.; et al. A cell-penetrating MARCKS mimetic selectively triggers cytolytic death in glioblastoma. Oncogene 2020, 39, 6961–6974. [Google Scholar] [CrossRef] [PubMed]
- Corradin, S.; Ransijn, A.; Corradin, G.; Bouvier, J.; Delgado, M.B.; Fernández-Carneado, J.; Mottram, J.C.; Vergères, G.; Mauël, J. Novel peptide inhibitors of Leishmania gp63 based on the cleavage site of MARCKS (myristoylated alanine-rich C kinase substrate)-related protein. Biochem. J. 2002, 367, 761–769. [Google Scholar] [CrossRef] [PubMed]
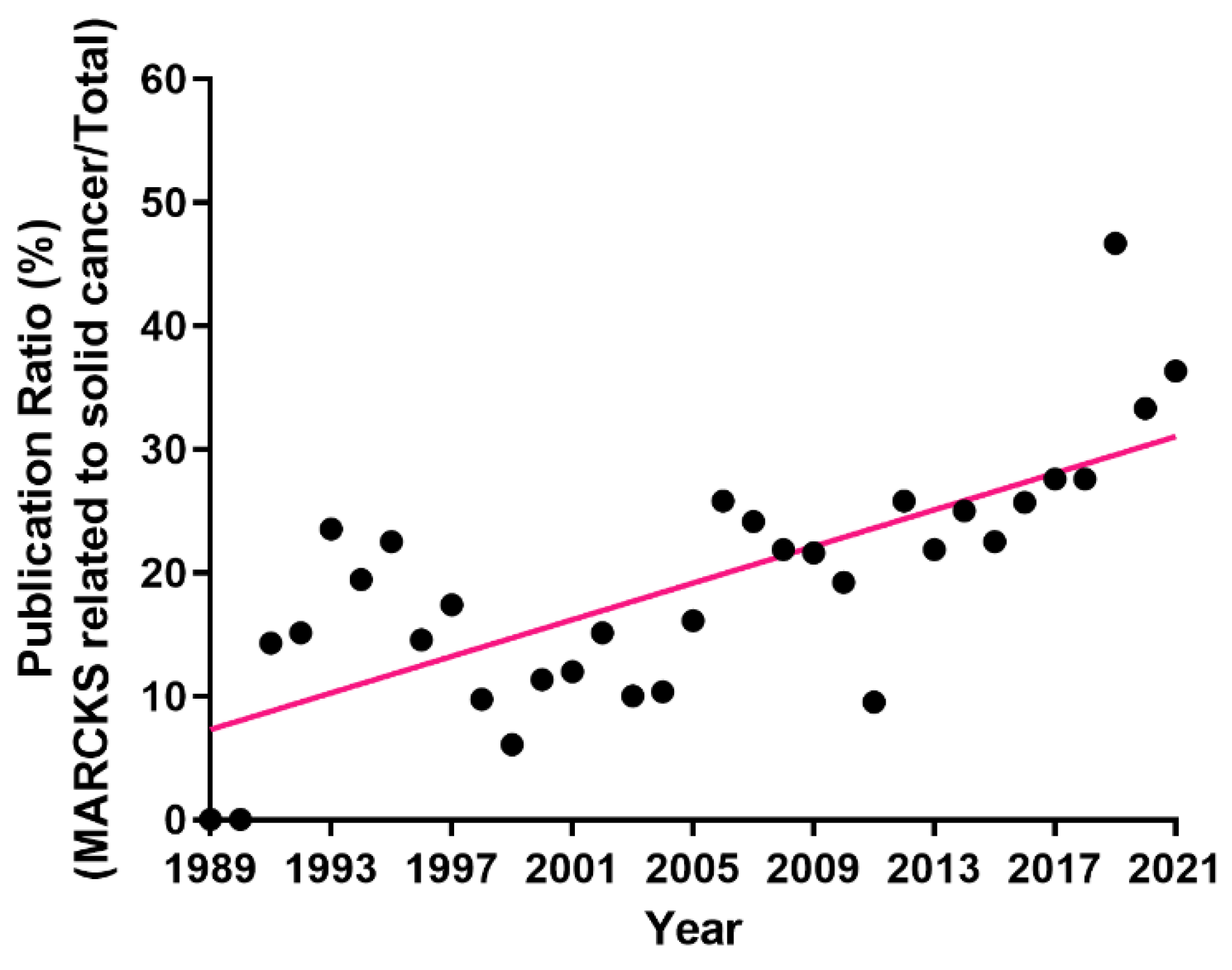
| Cancer Type [Reference] | Role | Models | Proliferation /Apoptosis | Migration /Invasion | Tumor Growth | Metastasis | Survival/Grade/Stage | Treatment Resistance | Target |
|---|---|---|---|---|---|---|---|---|---|
| NSCLC [42] | Pro | cell line, xenograft, and TMA | ↑ | ↑ | higher grade | E-cad, pAKT, pPI3K, and Slug | |||
| NSCLC [41] | Pro | TMA | ↑ | higher stage | |||||
| NSCLC [63] | Pro | cell line, xenograft, and TMA | proliferation ↑ /apoptosis ↓ | ↑ | ↑ | shorter survival | erlotinib | pAKT | |
| NSCLC [45] | Pro | clinical trial | BIO-11006 (MARCK inhibitor) plus carboplatin showed a less disease progression and a higher response rate compared to carboplatin alone. | ||||||
| LC [43] | Pro | cell line, TMA, and TCGA | proliferation ↑ /apoptosis ↓ | shorter survival | radiation | ||||
| LC [44] | Pro | cell line, xenograft, and TMA | proliferation ↑ | ↑ | shorter survival | NF-κB, EMT, and stemness | |||
| LSCC [40] | Pro | TMA | shorter survival | ||||||
| BC [46] | Pro | cell line and TMA | proliferation ↑ | ↑ | shorter survival | tamoxifen | |||
| BC [47,48] | Pro | TMA | shorter survival | ||||||
| BC [49] | Pro | cell line, xenograft, and TMA | proliferation ↑ /apoptosis ↑ | ↑ | ↑ | ↑ | shorter survival | paclitaxel | angiogenic factors |
| BC [50] | Pro | cell lines | proliferation ↑ | ErbB2 | |||||
| RCC [52] | Pro | cell line, xenograft, and TMA | proliferation ↑ | ↑ | ↑ | higher grade | regorafenib | AKT, mTOR, VEGF, and MM9 | |
| CCA [53] | Pro | cell line, xenograft, and human tissue | ↑ | ↑ | shorter survival | ||||
| HCC [54] | Pro | cell line | ↑ | ||||||
| CC [55] | Pro | cell line and xenograft | ↑ | ↑ | AURKB | ||||
| CC [56] | Sup | cell line and TMA | apoptosis ↑ | longer survival | TRAIL and AKT | ||||
| GBM [58] | Sup | cell line, xenograft, clinical trial, and TCGA | proliferation ↓ | ↓ | longer survival | ||||
| PCa [6] | Pro | cell line and TMA | ↑ | more recurrence | |||||
| PCa [59] | Sup | cell line | ↓ | ||||||
| BC, PCa [60] * | Sup | cell line and xenograft | ↓ | ↓ | E-cad, b-catenin, and APC | ||||
| PCa [13] * | Sup | cell line | ↓ | ||||||
| BlaC [62] | Sup | cell line | ↓ | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, C.-L.; Zhao, H.; Chen, C.-H.; Wu, R.; Brooks, J.D. The Role of MARCKS in Metastasis and Treatment Resistance of Solid Tumors. Cancers 2022, 14, 4925. https://doi.org/10.3390/cancers14194925
Chiu C-L, Zhao H, Chen C-H, Wu R, Brooks JD. The Role of MARCKS in Metastasis and Treatment Resistance of Solid Tumors. Cancers. 2022; 14(19):4925. https://doi.org/10.3390/cancers14194925
Chicago/Turabian StyleChiu, Chun-Lung, Hongjuan Zhao, Ching-Hsien Chen, Reen Wu, and James D. Brooks. 2022. "The Role of MARCKS in Metastasis and Treatment Resistance of Solid Tumors" Cancers 14, no. 19: 4925. https://doi.org/10.3390/cancers14194925
APA StyleChiu, C.-L., Zhao, H., Chen, C.-H., Wu, R., & Brooks, J. D. (2022). The Role of MARCKS in Metastasis and Treatment Resistance of Solid Tumors. Cancers, 14(19), 4925. https://doi.org/10.3390/cancers14194925






