Tumor Microenvironment, Clinical Features, and Advances in Therapy for Bone Metastasis in Gastric Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Bone and Bone Microenvironment
2.1. Composition of Bone
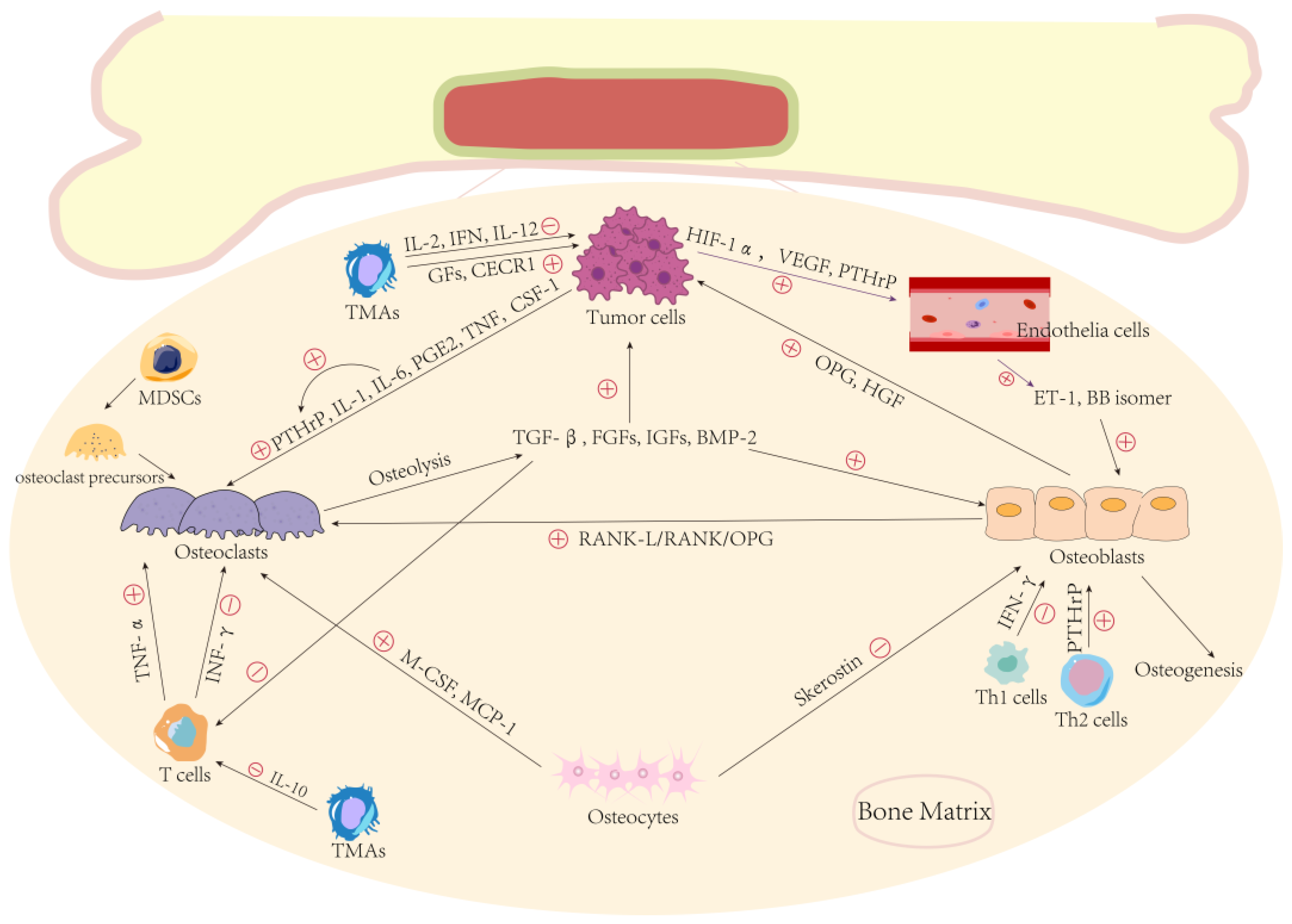
2.2. Bone Microenvironment and Metastasis
3. Clinical Bone Metastasis of Gastric Cancer
3.1. Clinical Features
3.2. Diagnosis Methods
3.3. Treatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Guadagni, S.; Catarci, M.; Kinoshitá, T.; Valenti, M.; De Bernardinis, G.; Carboni, M. Causes of death and recurrence after surgery for early gastric cancer. World J. Surg. 1997, 21, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef] [PubMed]
- Turkoz, F.P.; Solak, M.; Kilickap, S.; Ulas, A.; Esbah, O.; Oksuzoglu, B.; Yalcin, S. Bone metastasis from gastric cancer: The incidence, clinicopathological features, and influence on survival. J. Gastric Cancer 2014, 14, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, S.H.; Kim, J.W.; Lee, J.O.; Kim, J.H.; Bang, S.M.; Lee, J.S.; Lee, K.W. Gastric cancer with initial bone metastasis: A distinct group of diseases with poor prognosis. Eur. J. Cancer 2014, 50, 2810–2821. [Google Scholar] [CrossRef]
- Choi, C.W.; Lee, D.S.; Chung, J.K.; Lee, M.C.; Kim, N.K.; Choi, K.W.; Koh, C.S. Evaluation of bone metastases by Tc-99m MDP imaging in patients with stomach cancer. Clin. Nucl. Med. 1995, 20, 310–314. [Google Scholar] [CrossRef]
- Nishidoi, H.; Koga, S. Clinicopathological study of gastric cancer with bone metastasis. Gan Kagaku Ryoho 1987, 14, 1717–1722. [Google Scholar]
- Lim, S.M.; Kim, Y.N.; Park, K.H.; Kang, B.; Chon, H.J.; Kim, C.; Kim, J.H.; Rha, S.Y. Bone alkaline phosphatase as a surrogate marker of bone metastasis in gastric cancer patients. BMC Cancer 2016, 16, 385. [Google Scholar] [CrossRef]
- Imura, Y.; Tateiwa, D.; Sugimoto, N.; Inoue, A.; Wakamatsu, T.; Outani, H.; Tanaka, T.; Tamiya, H.; Yagi, T.; Naka, N.; et al. Prognostic factors and skeletal-related events in patients with bone metastasis from gastric cancer. Mol. Clin. Oncol. 2020, 13, 31. [Google Scholar] [CrossRef]
- Petrillo, A.; Giunta, E.F.; Pappalardo, A.; Bosso, D.; Attademo, L.; Cardalesi, C.; Diana, A.; Fabbrocini, A.; Fabozzi, T.; Giordano, P.; et al. Bone Metastases from Gastric Cancer: What We Know and How to Deal with Them. J. Clin. Med. 2021, 10, 1777. [Google Scholar] [CrossRef] [PubMed]
- Fornetti, J.; Welm, A.L.; Stewart, S.A. Understanding the Bone in Cancer Metastasis. J. Bone Miner. Res. 2018, 33, 2099–2113. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhu, Z.; Shi, Y. Metastasis mechanism and gene/protein expression in gastric cancer with distant organs metastasis. Bull Cancer 2014, 101, E1–E12. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, V.; Fleitas, T.; Tarazona, N.; Papaccio, F.; Huerta, M.; Roselló, S.; Gimeno-Valiente, F.; Roda, D.; Cervantes, A. Precision Medicine to Treat Advanced Gastroesophageal Adenocarcinoma: A Work in Progress. J. Clin. Med. 2020, 9, 3049. [Google Scholar] [CrossRef]
- Shitara, K.; Van Cutsem, E.; Bang, Y.J.; Fuchs, C.; Wyrwicz, L.; Lee, K.W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Lee, J.; et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy alone for Patients with First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1571–1580. [Google Scholar] [CrossRef]
- Lipton, A.; Fizazi, K.; Stopeck, A.T.; Henry, D.H.; Brown, J.E.; Yardley, D.A.; Richardson, G.E.; Siena, S.; Maroto, P.; Clemens, M.; et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: A combined analysis of 3 pivotal, randomised, phase 3 trials. Eur. J. Cancer 2012, 48, 3082–3092. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, H.; Dunstan, C.R.; Sutherland, R.L.; Seibel, M.J. The role of the bone microenvironment in skeletal metastasis. J. Bone Oncol. 2013, 2, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Capulli, M.; Paone, R.; Rucci, N. Osteoblast and osteocyte: Games without frontiers. Arch. Biochem. Biophys. 2014, 561, 3–12. [Google Scholar] [CrossRef]
- Takahashi, N.; Udagawa, N.; Takami, M.; Suda, T. Chapter 7—Cells of Bone: Osteoclast Generation. In Principles of Bone Biology, 2nd ed.; Bilezikian, J.P., Raisz, L.G., Rodan, G.A., Eds.; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Ono, T.; Nakashima, T. Recent advances in osteoclast biology. Histochem. Cell Biol. 2018, 149, 325–341. [Google Scholar] [CrossRef]
- Eriksen, E.F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef]
- Paic, F.; Igwe, J.C.; Nori, R.; Kronenberg, M.S.; Franceschetti, T.; Harrington, P.; Kuo, L.; Shin, D.G.; Rowe, D.W.; Harris, S.E.; et al. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone 2009, 45, 682–692. [Google Scholar] [CrossRef]
- Tamasi, J.A.; Vasilov, A.; Shimizu, E.; Benton, N.; Johnson, J.; Bitel, C.L.; Morrison, N.; Partridge, N.C. Monocyte chemoattractant protein-1 is a mediator of the anabolic action of parathyroid hormone on bone. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2013, 28, 1975–1986. [Google Scholar] [CrossRef]
- Demontiero, O.; Vidal, C.; Duque, G. Aging and bone loss: New insights for the clinician. Ther. Adv. Musculoskelet. Dis. 2012, 4, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Roodman, G.D. Mechanisms of bone metastasis. New Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Ell, B.; Mercatali, L.; Ibrahim, T.; Campbell, N.; Schwarzenbach, H.; Pantel, K.; Amadori, D.; Kang, Y. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell 2013, 24, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Thümmler, K.; Mirandola, L.; Garavelli, S.; Todoerti, K.; Apicella, L.; Lazzari, E.; Lancellotti, M.; Platonova, N.; Akbar, M.; et al. Notch signaling drives multiple myeloma induced osteoclastogenesis. Oncotarget 2014, 5, 10393–10406. [Google Scholar] [CrossRef]
- Soki, F.N.; Park, S.I.; McCauley, L.K. The multifaceted actions of PTHrP in skeletal metastasis. Future Oncol. 2012, 8, 803–817. [Google Scholar] [CrossRef]
- Andrade, K.; Fornetti, J.; Zhao, L.; Miller, S.C.; Randall, R.L.; Anderson, N.; Waltz, S.E.; McHale, M.; Welm, A.L. RON kinase: A target for treatment of cancer-induced bone destruction and osteoporosis. Sci. Transl. Med. 2017, 9, eaai9338. [Google Scholar] [CrossRef]
- Powell, G.J.; Southby, J.; Danks, J.A.; Stillwell, R.G.; Hayman, J.A.; Henderson, M.A.; Bennett, R.C.; Martin, T.J. Localization of parathyroid hormone-related protein in breast cancer metastases: Increased incidence in bone compared with other sites. Cancer Res. 1991, 51, 3059–3061. [Google Scholar]
- de la Mata, J.; Uy, H.L.; Guise, T.A.; Story, B.; Boyce, B.F.; Mundy, G.R.; Roodman, G.D. Interleukin-6 enhances hypercalcemia and bone resorption mediated by parathyroid hormone-related protein in vivo. J. Clin. Investig. 1995, 95, 2846–2852. [Google Scholar] [CrossRef]
- Yin, J.J.; Pollock, C.B.; Kelly, K. Mechanisms of cancer metastasis to the bone. Cell Res. 2005, 15, 57–62. [Google Scholar] [CrossRef]
- Khosla, S. Minireview: The OPG/RANKL/RANK system. Endocrinology 2001, 142, 5050–5055. [Google Scholar] [CrossRef]
- Vallet, S.; Bashari, M.H.; Fan, F.J.; Malvestiti, S.; Schneeweiss, A.; Wuchter, P.; Jäger, D.; Podar, K. Pre-Osteoblasts Stimulate Migration of Breast Cancer Cells via the HGF/MET Pathway. PLoS ONE 2016, 11, e0150507. [Google Scholar] [CrossRef]
- Mohammad, K.S.; Guise, T.A. Mechanisms of osteoblastic metastases: Role of endothelin-1. Clin. Orthop. Relat. Res. 2003, 415, S67–S74. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.B.; Chan-Tack, K.; Hedican, S.P.; Magnuson, S.R.; Opgenorth, T.J.; Bova, G.S.; Simons, J.W. Endothelin-1 production and decreased endothelin B receptor expression in advanced prostate cancer. Cancer Res. 1996, 56, 663–668. [Google Scholar] [PubMed]
- Brubaker, K.D.; Corey, E.; Brown, L.G.; Vessella, R.L. Bone morphogenetic protein signaling in prostate cancer cell lines. J. Cell. Biochem. 2004, 91, 151–160. [Google Scholar] [CrossRef]
- Achbarou, A.; Kaiser, S.; Tremblay, G.; Ste-Marie, L.G.; Brodt, P.; Goltzman, D.; Rabbani, S.A. Urokinase overproduction results in increased skeletal metastasis by prostate cancer cells in vivo. Cancer Res. 1994, 54, 2372–2377. [Google Scholar]
- Killian, C.S.; Corral, D.A.; Kawinski, E.; Constantine, R.I. Mitogenic response of osteoblast cells to prostate-specific antigen suggests an activation of latent TGF-beta and a proteolytic modulation of cell adhesion receptors. Biochem. Biophys. Res. Commun. 1993, 192, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Kang, Y. Targeting tumor-stromal interactions in bone metastasis. Pharmacol. Ther. 2014, 141, 222–233. [Google Scholar] [CrossRef]
- Mine, S.; Fujisaki, T.; Kawahara, C.; Tabata, T.; Iida, T.; Yasuda, M.; Yoneda, T.; Tanaka, Y. Hepatocyte growth factor enhances adhesion of breast cancer cells to endothelial cells in vitro through up-regulation of CD44. Exp. Cell Res. 2003, 288, 189–197. [Google Scholar] [CrossRef]
- Schneider, J.G.; Amend, S.R.; Weilbaecher, K.N. Integrins and bone metastasis: Integrating tumor cell and stromal cell interactions. Bone 2011, 48, 54–65. [Google Scholar] [CrossRef]
- Isowa, S.; Shimo, T.; Ibaragi, S.; Kurio, N.; Okui, T.; Matsubara, K.; Hassan, N.M.; Kishimoto, K.; Sasaki, A. PTHrP regulates angiogenesis and bone resorption via VEGF expression. Anticancer Res. 2010, 30, 2755–2767. [Google Scholar]
- Baschuk, N.; Rautela, J.; Parker, B.S. Bone specific immunity and its impact on metastasis. BoneKEy Rep. 2015, 4, 665. [Google Scholar] [CrossRef]
- Kawai, T.; Matsuyama, T.; Hosokawa, Y.; Makihira, S.; Seki, M.; Karimbux, N.Y.; Goncalves, R.B.; Valverde, P.; Dibart, S.; Li, Y.P.; et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am. J. Pathol. 2006, 169, 987–998. [Google Scholar] [CrossRef]
- Cenci, S.; Weitzmann, M.N.; Roggia, C.; Namba, N.; Novack, D.; Woodring, J.; Pacifici, R. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J. Clin. Investig. 2000, 106, 1229–1237. [Google Scholar] [CrossRef]
- Takayanagi, H.; Ogasawara, K.; Hida, S.; Chiba, T.; Murata, S.; Sato, K.; Takaoka, A.; Yokochi, T.; Oda, H.; Tanaka, K.; et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature 2000, 408, 600–605. [Google Scholar] [CrossRef]
- Fournier, P.G.; Chirgwin, J.M.; Guise, T.A. New insights into the role of T cells in the vicious cycle of bone metastases. Curr. Opin. Rheumatol. 2006, 18, 396–404. [Google Scholar] [CrossRef]
- Young, N.; Mikhalkevich, N.; Yan, Y.; Chen, D.; Zheng, W.P. Differential regulation of osteoblast activity by Th cell subsets mediated by parathyroid hormone and IFN-gamma. J. Immunol. 2005, 175, 8287–8295. [Google Scholar] [CrossRef] [PubMed]
- Sawant, A.; Deshane, J.; Jules, J.; Lee, C.M.; Harris, B.A.; Feng, X.; Ponnazhagan, S. Myeloid-derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Cancer Res. 2013, 73, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, Z.S.; Kiesel, J.R.; DiPaolo, R.; Pagadala, M.S.; Aurora, R. Osteoclast activated FoxP3+ CD8+ T-cells suppress bone resorption in vitro. PLoS ONE 2012, 7, e38199. [Google Scholar] [CrossRef] [PubMed]
- Brigati, C.; Noonan, D.M.; Albini, A.; Benelli, R. Tumors and inflammatory infiltrates: Friends or foes? Clin. Exp. Metastasis 2002, 19, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, Z.Z.; Pamarthy, S.; Sagar, V.; Costa, R.; Abdulkadir, S.A.; Giles, F.J.; Carneiro, B.A. Overcoming immunosuppression in bone metastases. Crit. Rev. Oncol. Hematol. 2017, 117, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Théry, C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011, 3, 15. [Google Scholar] [CrossRef]
- Li, W.; Gao, Y.Q. MiR-217 is involved in the carcinogenesis of gastric cancer by down-regulating CDH1 expression. Kaohsiung J. Med. Sci. 2018, 34, 377–384. [Google Scholar] [CrossRef]
- Yang, H.; Fu, H.; Wang, B.; Zhang, X.; Mao, J.; Li, X.; Wang, M.; Sun, Z.; Qian, H.; Xu, W. Exosomal miR-423-5p targets SUFU to promote cancer growth and metastasis and serves as a novel marker for gastric cancer. Mol. Carcinog. 2018, 57, 1223–1236. [Google Scholar] [CrossRef]
- Steinbichler, T.B.; Dudás, J.; Riechelmann, H.; Skvortsova, I.I. The role of exosomes in cancer metastasis. Semin. Cancer Biol. 2017, 44, 170–181. [Google Scholar] [CrossRef]
- Wang, J.; Guan, X.; Zhang, Y.; Ge, S.; Zhang, L.; Li, H.; Wang, X.; Liu, R.; Ning, T.; Deng, T.; et al. Exosomal miR-27a Derived from Gastric Cancer Cells Regulates the Transformation of Fibroblasts into Cancer-Associated Fibroblasts. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 49, 869–883. [Google Scholar] [CrossRef]
- Zheng, P.; Luo, Q.; Wang, W.; Li, J.; Wang, T.; Wang, P.; Chen, L.; Zhang, P.; Chen, H.; Liu, Y.; et al. Tumor-associated macrophages-derived exosomes promote the migration of gastric cancer cells by transfer of functional Apolipoprotein E. Cell Death Dis. 2018, 9, 434. [Google Scholar] [CrossRef]
- Wang, F.; Li, B.; Wei, Y.; Zhao, Y.; Wang, L.; Zhang, P.; Yang, J.; He, W.; Chen, H.; Jiao, Z.; et al. Tumor-derived exosomes induce PD1(+) macrophage population in human gastric cancer that promotes disease progression. Oncogenesis 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Chen, S.; Liu, Z.; Xie, H.; Deng, H.; Shang, S.; Wang, X.; Xia, M.; Zuo, C. miRNA-221 of exosomes originating from bone marrow mesenchymal stem cells promotes oncogenic activity in gastric cancer. OncoTargets Ther. 2017, 10, 4161–4171. [Google Scholar] [CrossRef]
- Huang, L.; Wu, R.L.; Xu, A.M. Epithelial-mesenchymal transition in gastric cancer. Am. J. Transl Res. 2015, 7, 2141–2158. [Google Scholar]
- Fattahi, S.; Amjadi-Moheb, F.; Tabaripour, R.; Ashrafi, G.H.; Akhavan-Niaki, H. PI3K/AKT/mTOR signaling in gastric cancer: Epigenetics and beyond. Life Sci. 2020, 262, 118513. [Google Scholar] [CrossRef]
- Brzozowa, M.; Mielańczyk, L.; Michalski, M.; Malinowski, L.; Kowalczyk-Ziomek, G.; Helewski, K.; Harabin-Słowińska, M.; Wojnicz, R. Role of Notch signaling pathway in gastric cancer pathogenesis. Contemp. Oncol. 2013, 17, 1–5. [Google Scholar] [CrossRef]
- Kobayashi, K.; Takahashi, N.; Jimi, E.; Udagawa, N.; Takami, M.; Kotake, S.; Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J. Exp. Med. 2000, 191, 275–286. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Neubauer, A.; Heufelder, A.E. Receptor activator of nuclear factor-kappaB ligand and osteoprotegerin: Potential implications for the pathogenesis and treatment of malignant bone diseases. Cancer 2001, 92, 460–470. [Google Scholar] [CrossRef]
- Choi, H.S.; Ha, S.Y.; Kim, H.M.; Ahn, S.M.; Kang, M.S.; Kim, K.M.; Choi, M.G.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; et al. The prognostic effects of tumor infiltrating regulatory T cells and myeloid derived suppressor cells assessed by multicolor flow cytometry in gastric cancer patients. Oncotarget 2016, 7, 7940–7951. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, Y.; Che, X.; Zhang, L.; Wang, Q.; Zhang, X.; Qu, J.; Li, Z.; Xu, L.; Zhang, Y.; et al. Caveolin-1 enhances RANKL-induced gastric cancer cell migration. Oncol. Rep. 2018, 40, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, L.; Satolli, M.A.; Mecca, C.; Castiglione, A.; Ceccarelli, M.; D’Amelio, P.; Garino, M.; De Giuli, M.; Sandrucci, S.; Ferracini, R.; et al. Bone metastases in gastric cancer follow a RANKL-independent mechanism. Oncol. Rep. 2013, 29, 1453–1458. [Google Scholar] [CrossRef]
- Ahn, J.B.; Ha, T.K.; Kwon, S.J. Bone Metastasis in Gastric Cancer Patients. J. Gastric Cancer 2011, 11, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Song, K.Y.; O, J.H.; Kim, W.C.; Choi, M.G.; Park, C.H. Bone recurrence after curative resection of gastric cancer. Gastric Cancer 2013, 16, 362–369. [Google Scholar] [CrossRef]
- Qiu, M.Z.; Shi, S.M.; Chen, Z.H.; Yu, H.E.; Sheng, H.; Jin, Y.; Wang, D.S.; Wang, F.H.; Li, Y.H.; Xie, D.; et al. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: A SEER-based study. Cancer Med. 2018, 7, 3662–3672. [Google Scholar] [CrossRef]
- Silvestris, N.; Pantano, F.; Ibrahim, T.; Gamucci, T.; De Vita, F.; Di Palma, T.; Pedrazzoli, P.; Barni, S.; Bernardo, A.; Febbraro, A.; et al. Natural history of malignant bone disease in gastric cancer: Final results of a multicenter bone metastasis survey. PLoS ONE 2013, 8, e74402. [Google Scholar] [CrossRef]
- Park, H.S.; Rha, S.Y.; Kim, H.S.; Hyung, W.J.; Park, J.S.; Chung, H.C.; Noh, S.H.; Jeung, H.C. A prognostic model to predict clinical outcome in gastric cancer patients with bone metastasis. Oncology 2011, 80, 142–150. [Google Scholar] [CrossRef]
- Wen, L.; Li, Y.Z.; Zhang, J.; Zhou, C.; Yang, H.N.; Chen, X.Z.; Xu, L.W.; Kong, S.N.; Wang, X.W.; Zhang, H.M. Clinical analysis of bone metastasis of gastric cancer: Incidence, clinicopathological features and survival. Future Oncol. 2019, 15, 2241–2249. [Google Scholar] [CrossRef]
- Nakamura, K.; Tomioku, M.; Nabeshima, K.; Yasuda, S. Clinicopathologic features and clinical outcomes of gastric cancer patients with bone metastasis. Tokai J. Exp. Clin. Med. 2014, 39, 193–198. [Google Scholar]
- Liang, C.; Chen, H.; Yang, Z.; Han, C.; Ren, C. Risk factors and prognosis of bone metastases in newly diagnosed gastric cancer. Future Oncol. 2020, 16, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. Metastatic spread in patients with gastric cancer. Oncotarget 2016, 7, 52307–52316. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhao, Y.; Shi, Y.; Hu, W.; Zhang, J. Bone Metastasis From Gastric Adenocarcinoma-What Are the Risk Factors and Associated Survival? A Large Comprehensive Population-Based Cohort Study. Front. Oncol. 2022, 12, 743873. [Google Scholar] [CrossRef] [PubMed]
- Ubukata, H.; Motohashi, G.; Tabuchi, T.; Nagata, H.; Konishi, S.; Tabuchi, T. Overt bone metastasis and bone marrow micrometastasis of early gastric cancer. Surg. Today 2011, 41, 169–174. [Google Scholar] [CrossRef]
- Kan, C.; Vargas, G.; Pape, F.L.; Clézardin, P. Cancer Cell Colonisation in the Bone Microenvironment. Int. J. Mol. Sci. 2016, 17, 1674. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2022, 20, 167–192. [Google Scholar] [CrossRef]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27 (suppl. 5), v38–v49. [Google Scholar] [CrossRef] [PubMed]
- Grillo, A.; Capasso, R.; Petrillo, A.; De Vita, F.; Conforti, R. An intramedullary “flame” recognized as being an intramedullary spinal cord metastasis from esophageal cancer. J. Radiol. Case Rep. 2019, 13, 14–20. [Google Scholar] [PubMed]
- Kawamura, T.; Kusakabe, T.; Sugino, T.; Watanabe, K.; Fukuda, T.; Nashimoto, A.; Honma, K.; Suzuki, T. Expression of glucose transporter-1 in human gastric carcinoma: Association with tumor aggressiveness, metastasis, and patient survival. Cancer 2001, 92, 634–641. [Google Scholar] [CrossRef]
- Pollen, J.J.; Witztum, K.F.; Ashburn, W.L. The flare phenomenon on radionuclide bone scan in metastatic prostate cancer. AJR Am. J. Roentgenol. 1984, 142, 773–776. [Google Scholar] [CrossRef]
- Amoroso, V.; Pittiani, F.; Grisanti, S.; Valcamonico, F.; Simoncini, E.; Ferrari, V.D.; Marini, G. Osteoblastic flare in a patient with advanced gastric cancer after treatment with pemetrexed and oxaliplatin: Implications for response assessment with RECIST criteria. BMC Cancer 2007, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Tankó, L.B.; Karsdal, M.A.; Christiansen, C.; Leeming, D.J. Biochemical approach to the detection and monitoring of metastatic bone disease: What do we know and what questions need answers? Cancer Metastasis Rev. 2006, 25, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.E.; Cook, R.J.; Major, P.; Lipton, A.; Saad, F.; Smith, M.; Lee, K.A.; Zheng, M.; Hei, Y.J.; Coleman, R.E. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J. Natl. Cancer Inst. 2005, 97, 59–69. [Google Scholar] [CrossRef]
- Lara, P.N., Jr.; Ely, B.; Quinn, D.I.; Mack, P.C.; Tangen, C.; Gertz, E.; Twardowski, P.W.; Goldkorn, A.; Hussain, M.; Vogelzang, N.J.; et al. Serum biomarkers of bone metabolism in castration-resistant prostate cancer patients with skeletal metastases: Results from SWOG 0421. J. Natl. Cancer Inst. 2014, 106, dju013. [Google Scholar] [CrossRef]
- Coleman, R.E.; Major, P.; Lipton, A.; Brown, J.E.; Lee, K.A.; Smith, M.; Saad, F.; Zheng, M.; Hei, Y.J.; Seaman, J.; et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 4925–4935. [Google Scholar] [CrossRef] [PubMed]
- D’Oronzo, S.; Brown, J.; Coleman, R. The role of biomarkers in the management of bone-homing malignancies. J. Bone Oncol. 2017, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Lipton, A.; Costa, L.; Cook, R.J.; Lee, K.A.; Saad, F.; Brown, J.E.; Terpos, E.; Major, P.P.; Kohno, N.; et al. Possible survival benefits from zoledronic acid treatment in patients with bone metastases from solid tumours and poor prognostic features-An exploratory analysis of placebo-controlled trials. J. Bone Oncol. 2013, 2, 70–76. [Google Scholar] [CrossRef]
- Lipton, A.; Smith, M.R.; Fizazi, K.; Stopeck, A.T.; Henry, D.; Brown, J.E.; Shore, N.D.; Saad, F.; Spencer, A.; Zhu, L.; et al. Changes in Bone Turnover Marker Levels and Clinical Outcomes in Patients with Advanced Cancer and Bone Metastases Treated with Bone Antiresorptive Agents. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 5713–5721. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.L.; Boyle, W.J.; Simonet, W.S.; Kostenuik, P.J.; Dougall, W.C.; Sullivan, J.K.; San Martin, J.; Dansey, R. Bench to bedside: Elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat. Reviews. Drug Discov. 2012, 11, 401–419. [Google Scholar] [CrossRef]
- Grimaud, E.; Soubigou, L.; Couillaud, S.; Coipeau, P.; Moreau, A.; Passuti, N.; Gouin, F.; Redini, F.; Heymann, D. Receptor activator of nuclear factor kappaB ligand (RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysis. Am. J. Pathol. 2003, 163, 2021–2031. [Google Scholar] [CrossRef]
- Jung, K.; Lein, M. Bone turnover markers in serum and urine as diagnostic, prognostic and monitoring biomarkers of bone metastasis. Biochim. Et Biophys. Acta 2014, 1846, 425–438. [Google Scholar] [CrossRef]
- Du, W.X.; Duan, S.F.; Chen, J.J.; Huang, J.F.; Yin, L.M.; Tong, P.J. Serum bone-specific alkaline phosphatase as a biomarker for osseous metastases in patients with malignant carcinomas: A systematic review and meta-analysis. J. Cancer Res. Ther. 2014, 10, 140–143. [Google Scholar]
- Coleman, R.; Costa, L.; Saad, F.; Cook, R.; Hadji, P.; Terpos, E.; Garnero, P.; Brown, J.; Body, J.J.; Smith, M.; et al. Consensus on the utility of bone markers in the malignant bone disease setting. Crit. Rev. Oncol. /Hematol. 2011, 80, 411–432. [Google Scholar] [CrossRef]
- Ohtsu, A. Chemotherapy for metastatic gastric cancer: Past, present, and future. J. Gastroenterol. 2008, 43, 256–264. [Google Scholar] [CrossRef]
- Power, D.G.; Kelsen, D.P.; Shah, M.A. Advanced gastric cancer--slow but steady progress. Cancer Treat. Rev. 2010, 36, 384–392. [Google Scholar] [CrossRef]
- Koizumi, W.; Kurihara, M.; Nakano, S.; Hasegawa, K. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology 2000, 58, 191–197. [Google Scholar] [CrossRef]
- Koizumi, W.; Narahara, H.; Hara, T.; Takagane, A.; Akiya, T.; Takagi, M.; Miyashita, K.; Nishizaki, T.; Kobayashi, O.; Takiyama, W.; et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol. 2008, 9, 215–221. [Google Scholar] [CrossRef]
- Kang, H.; Kauh, J.S. Chemotherapy in the treatment of metastatic gastric cancer: Is there a global standard? Curr. Treat. Options Oncol. 2011, 12, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2021, 24, 1–21. [Google Scholar]
- Wang, F.H.; Zhang, X.T.; Li, Y.F.; Tang, L.; Qu, X.J.; Ying, J.E.; Zhang, J.; Sun, L.Y.; Lin, R.B.; Qiu, H.; et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. 2021, 41, 747–795. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Hartmann, J.T.; Probst, S.; Schmalenberg, H.; Hollerbach, S.; Hofheinz, R.; Rethwisch, V.; Seipelt, G.; Homann, N.; Wilhelm, G.; et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft Internistische Onkologie. J. Clin. Oncol. 2008, 26, 1435–1442. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Kang, J.H.; Lee, S.I.; Lim, D.H.; Park, K.W.; Oh, S.Y.; Kwon, H.C.; Hwang, I.G.; Lee, S.C.; Nam, E.; Shin, D.B.; et al. Salvage chemotherapy for pretreated gastric cancer: A randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J. Clin. Oncol. 2012, 30, 1513–1518. [Google Scholar] [CrossRef]
- Kang, Y. Randomized phase III trial of capecitabine/cisplatin (XP) vs. continuous infusion of 5-FU/cisplatin (FP) as first-line therapy in patients (pts) with advanced gastric cancer (AGC): Efficacy and safety results. Asco 2006, 24, LBA4018. [Google Scholar] [CrossRef]
- Tabernero, J.; Hoff, P.M.; Shen, L.; Ohtsu, A.; Shah, M.A.; Cheng, K.; Song, C.; Wu, H.; Eng-Wong, J.; Kim, K.; et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018, 19, 1372–1384. [Google Scholar] [CrossRef]
- Hecht, J.R.; Bang, Y.J.; Qin, S.K.; Chung, H.C.; Xu, J.M.M.; Park, J.O.; Jeziorski, K.; Shparyk, Y.; Hoff, P.M.; Sobrero, A.; et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC-A Randomized Phase III Trial. J. Clin. Oncol. 2016, 34, 443–451. [Google Scholar] [CrossRef]
- Qin, S.K.; Ji, J.F.; Xu, R.H.; Wang, W.; Tang, Y.; Bi, F.; Li, J.; Wang, K.; Xu, J.M.; Fan, Q.X.; et al. Treatment patterns and outcomes in Chinese gastric cancer by HER2 status: A non-interventional registry study (EVIDENCE). J. Clin. Oncol. 2019, 37, 1. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Xu, R.H.; Wang, Z.Q.; Shen, L.; Wang, W.; Lu, J.W.; Dai, G.H.; Xu, J.M.; Zhang, Y.Q.; Chen, X.B.; Deng, Y.H.; et al. S-1 plus oxaliplatin versus S-1 plus cisplatin as first-line treatment for advanced diffuse-type or mixed-type gastric/gastroesophageal junction adenocarcinoma: A randomized, phase 3 trial. J. Clin. Oncol. 2019, 37, 2. [Google Scholar] [CrossRef]
- Lu, Z.H.; Zhang, X.T.; Liu, W.; Liu, T.S.; Hu, B.; Li, W.; Fan, Q.X.; Xu, J.M.; Xu, N.; Bai, Y.X.; et al. A multicenter, randomized trial comparing efficacy and safety of paclitaxel/capecitabine and cisplatin/capecitabine in advanced gastric cancer. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2018, 21, 782–791. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Moiseyenko, V.M.; Tjulandin, S.; Majlis, A.; Constenla, M.; Boni, C.; Rodrigues, A.; Fodor, M.; Chao, Y.; Voznyi, E.; et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 study group. J. Clin. Oncol. 2006, 24, 4991–4997. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Xu, R.H.; Li, J.; Bai, Y.X.; Liu, T.S.; Jiao, S.C.; Dai, G.H.; Xu, J.M.; Liu, Y.P.; Fan, N.F.; et al. Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2016, 19, 234–244. [Google Scholar] [CrossRef]
- Hall, P.S.; Swinson, D.; Waters, J.S.; Wadsley, J.; Falk, S.; Roy, R.; Tillett, T.; Nicoll, J.; Cummings, S.; Grumett, S.A.; et al. Optimizing chemotherapy for frail and elderly patients (pts) with advanced gastroesophageal cancer (aGOAC): The GO2 phase III trial. J. Clin. Oncol. 2019, 37, 2. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.K.; Xu, J.M.; Xiong, J.P.; Wu, C.P.; Bai, Y.X.; Liu, W.; Tong, J.D.; Liu, Y.P.; Xu, R.H.; et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J. Clin. Oncol. 2016, 34, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.J.; Jalal, S.I.; Shah, M.A.; Metges, J.P.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018, 4, 8. [Google Scholar] [CrossRef]
- Rich, S.E.; Chow, R.; Raman, S.; Liang Zeng, K.; Lutz, S.; Lam, H.; Silva, M.F.; Chow, E. Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 126, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, A.J.; Thompson, K.; Ebetino, F.H.; Rogers, M.J.; Coxon, F.P. Bisphosphonates: Molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr. Pharm. Des. 2010, 16, 2950–2960. [Google Scholar] [CrossRef] [PubMed]
- Senaratne, S.G.; Colston, K.W. Direct effects of bisphosphonates on breast cancer cells. Breast Cancer Res. BCR 2002, 4, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Lipton, A.; Balakumaran, A. Denosumab for the treatment of cancer therapy-induced bone loss and prevention of skeletal-related events in patients with solid tumors. Expert Rev. Clin. Pharmacol. 2012, 5, 359–371. [Google Scholar] [CrossRef]
- Sakai, K.; Tomoda, Y.; Saito, H.; Tanaka, K. Hungry bone syndrome and osteoblastic bone metastasis from gastric cancer. QJM Mon. J. Assoc. Physicians 2020, 113, 903–904. [Google Scholar] [CrossRef] [PubMed]
| Markers | Signification of Increase | Application |
|---|---|---|
| Bone alkaline phosphatase (BALP) a [90,91,92,93] | A bone formation marker. High levels of it are associated with increased risks for all negative clinical outcomes, including a shorter time of a first SRE, disease progression, and death. Compared with high NTX, less increased risk associated with high bone-specific alkaline phosphatase levels. | Diagnosis and prognosis of BM from solid tumors. Prognosis skeletal-related events. Prognosis during antiresorptive therapy. Prediction of response to treatment. |
| N-telopeptide (NTX) b [92,93,94,95] | A bone resorption marker. High baseline levels are associated with a significantly increased risk of SREs, bone disease progression, and death. | Prognosis of BM from solid tumors. Prognosis skeletal-related events. Prognosis during antiresorptive therapy. Prediction of response to treatment. |
| Receptor activator of nuclear factor κB-ligand/osteoprotegerin (RANKL/OPG) [96,97,98] | Bone resorption markers. In severe osteolysis, RANKL Expression and RANKL/OPG mRNA Ratio are significantly increased, and the severity of osteolysis is correlated with the increase of serum RANKL and RANKL/OPG levels. | Diagnosis of bone metastasis in solid tumors |
| United States [83] | Japan [106] | |
|---|---|---|
| First Line | HER2(+): Trastuzumab+ Fluoropyrimidine+ Cisplatin/Oxaliplatin HER2(-): ① Nivolumab + Fluoropyrimidine +Oxaliplatin (PD-L1 CPS of ≥5) ② Fluoropyrimidine + Oxaliplatin/Cisplatin. ③ Capecitabine + Oxaliplatin ④ Irinotecan + Fluorouracil ⑤ mDCF regimens (Docetaxel + Oxaliplatin + Calcium Folinate + Tegafur) | HER2(+): trastuzumab+ cisplatin+ capecitabine/S-1 HER2(-): ① S-1+ Cisplatin/Oxaliplatin ② Capecitabine + Cisplatin/Oxaliplatin ③ 5-FU + Levofolinate calcium + Oxaliplatin |
| Second Line | ① Ramucirumab + Paclitaxel ② Fam-trastuzumab deruxtecan-nxki (for patients with HER2+ and had received prior trastuzumab-based therapy) ③ Monotherapy: Docetaxel, Paclitaxel, and Irinotecan ④ Irinotecan+Fluorouracil/Cisplatin/Ramucirumab/Docetaxel ⑤ Pembrolizumab (MSI-H/dMMR Tumors) ⑥ Ntrectinib/Larotrectinib (NTRK gene fusion-positive tumors) | Ramucirumab + Paclitaxel |
| Third Line | - | Nivolumab/Irinotecan |
| First Line a | Second Line b | Third and Above Linesc | |
|---|---|---|---|
| First choice | HER2(+):①Trastuzumab + Oxaliplatin + 5-FU/Capecitabine ②Trastuzumab +Cisplatin+ 5-FU/Capecitabine HER2(-):①Fluorouracil (5-FU/capecitabine/tegafur)+oxaliplatin/cisplatin ②Fluorouracil (5-FU/capecitabine/tegafur)+paclitaxel/docetaxel | Monotherapy: Paclitaxel/Docetaxel/Irinotecan | Anti-angiogenic targeted drugs: Apatinib, Bevacizumab |
| Second choice | HER2(+): Trastuzumab + Fluorouracil (5-FU/capecitabine/tegafur)+ Oxaliplatin/Cisplatin HER2(-): ①DCF regimens (Docetaxel + cisplatin + 5-FU) ②mDCF regimens (Docetaxel + Oxaliplatin + Calcium Folinate + tegafur) | ①Trastuzumab + paclitaxel ②Paclitaxel/Docetaxel + Fluorouracil ③Pembrolizumab (for patients with MSI-H, PD-1/PD-L1 positive) | Pembrolizumab (for patients with MSI-H, PD-1/PD-L1 CPS ≥ 1) |
| Third choice | ①Trastuzumab + first-line chemotherapy regimens ②pembrolizumab monotherapy (PD-L1 CPS≥1) | Trastuzumab + other lines chemotherapy regimens | Single drug chemotherapy (refer to the second-line recommendations) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, P.; Antwi, S.O.; Sartorius, K.; Zheng, X.; Li, X. Tumor Microenvironment, Clinical Features, and Advances in Therapy for Bone Metastasis in Gastric Cancer. Cancers 2022, 14, 4888. https://doi.org/10.3390/cancers14194888
Sun P, Antwi SO, Sartorius K, Zheng X, Li X. Tumor Microenvironment, Clinical Features, and Advances in Therapy for Bone Metastasis in Gastric Cancer. Cancers. 2022; 14(19):4888. https://doi.org/10.3390/cancers14194888
Chicago/Turabian StyleSun, Pengcheng, Samuel O. Antwi, Kurt Sartorius, Xiao Zheng, and Xiaodong Li. 2022. "Tumor Microenvironment, Clinical Features, and Advances in Therapy for Bone Metastasis in Gastric Cancer" Cancers 14, no. 19: 4888. https://doi.org/10.3390/cancers14194888
APA StyleSun, P., Antwi, S. O., Sartorius, K., Zheng, X., & Li, X. (2022). Tumor Microenvironment, Clinical Features, and Advances in Therapy for Bone Metastasis in Gastric Cancer. Cancers, 14(19), 4888. https://doi.org/10.3390/cancers14194888







