PGC-1β and ERRα Promote Glutamine Metabolism and Colorectal Cancer Survival via Transcriptional Upregulation of PCK2
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Lentiviral Transduction
2.3. Epitope-Tagging of Endogenous PGC-1β
2.4. Immunoprecipitation of PGC-1β
2.5. Inducible PGC-1β Vector and Cell Line Generation
2.6. siRNA Transfections
2.7. RNA Sequencing and Analysis
2.8. Western Blot Analyses
2.9. L-Glutamine Utilization Assays
2.10. Intracellular Metabolite Analysis
2.11. PCK2-ALFA Plasmid for Stable Expression
2.12. Confocal Microscopy
2.13. Statistical Analysis
3. Results
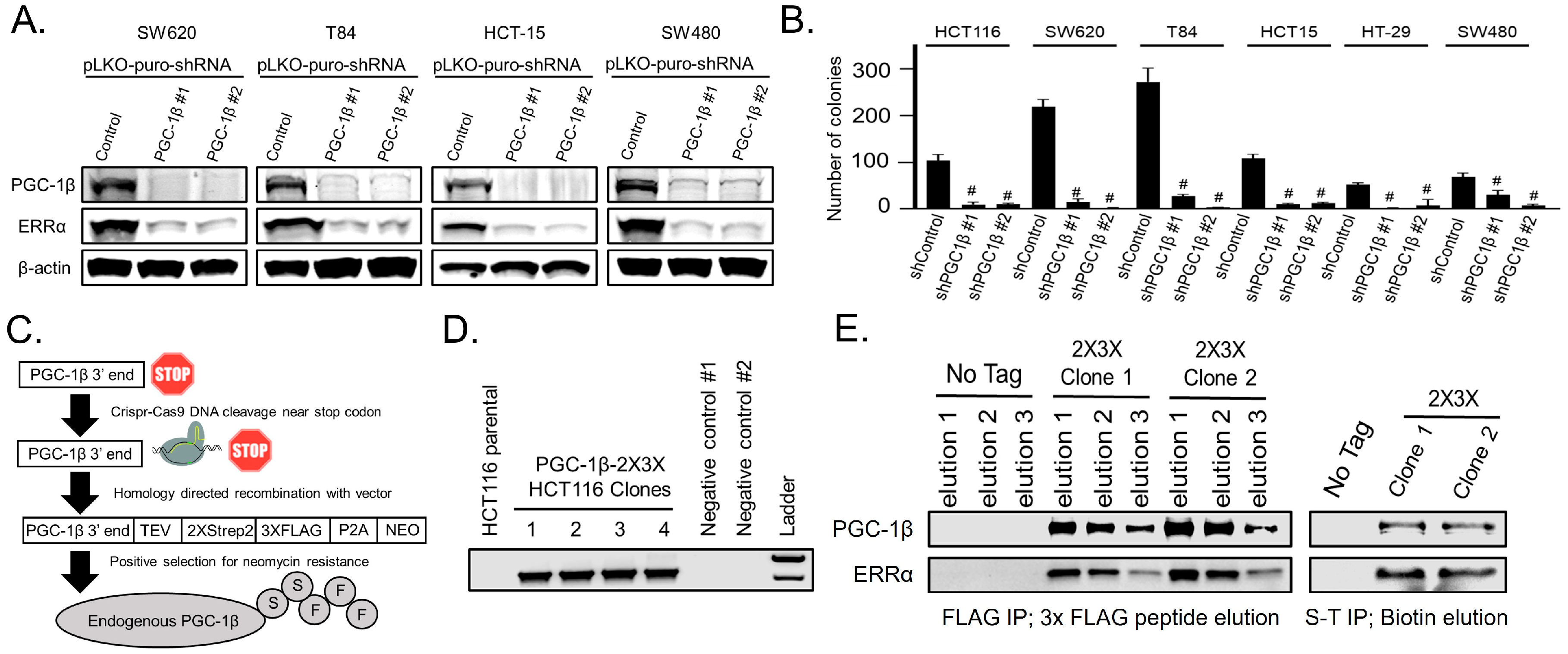
3.1. Endogenous PGC-1β Interacts with ERRα, Promotes ERRα Expression, and Anchorage-Independent Growth
3.2. PGC-1β Requires Its LRELL Motif at Amino Acids 343–347 to Interact with ERRα
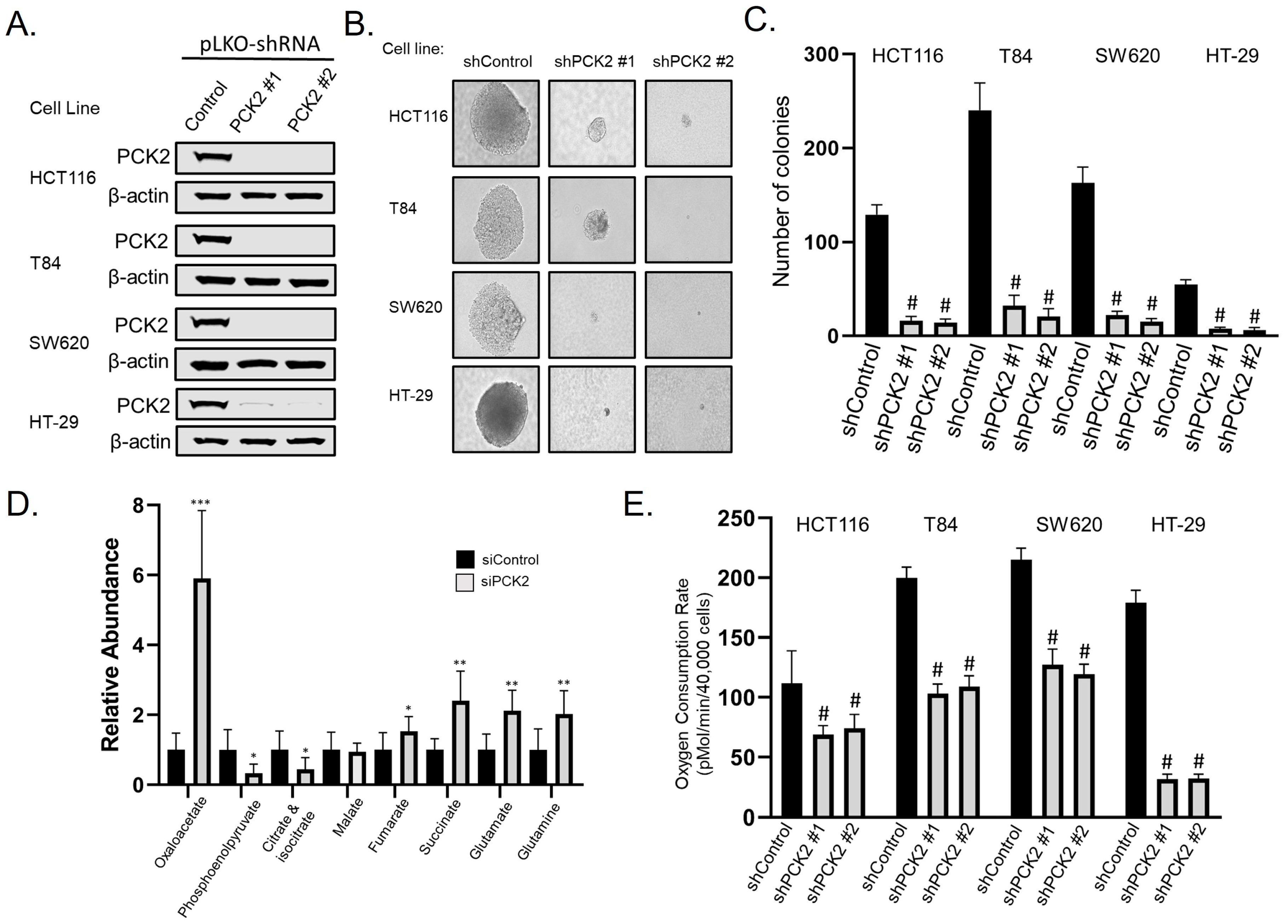
3.3. PGC-1β and ERRα Promote PCK2 Expression
3.4. PCK2 Promotes Anchorage-Independent Growth and Glutamine Utilization
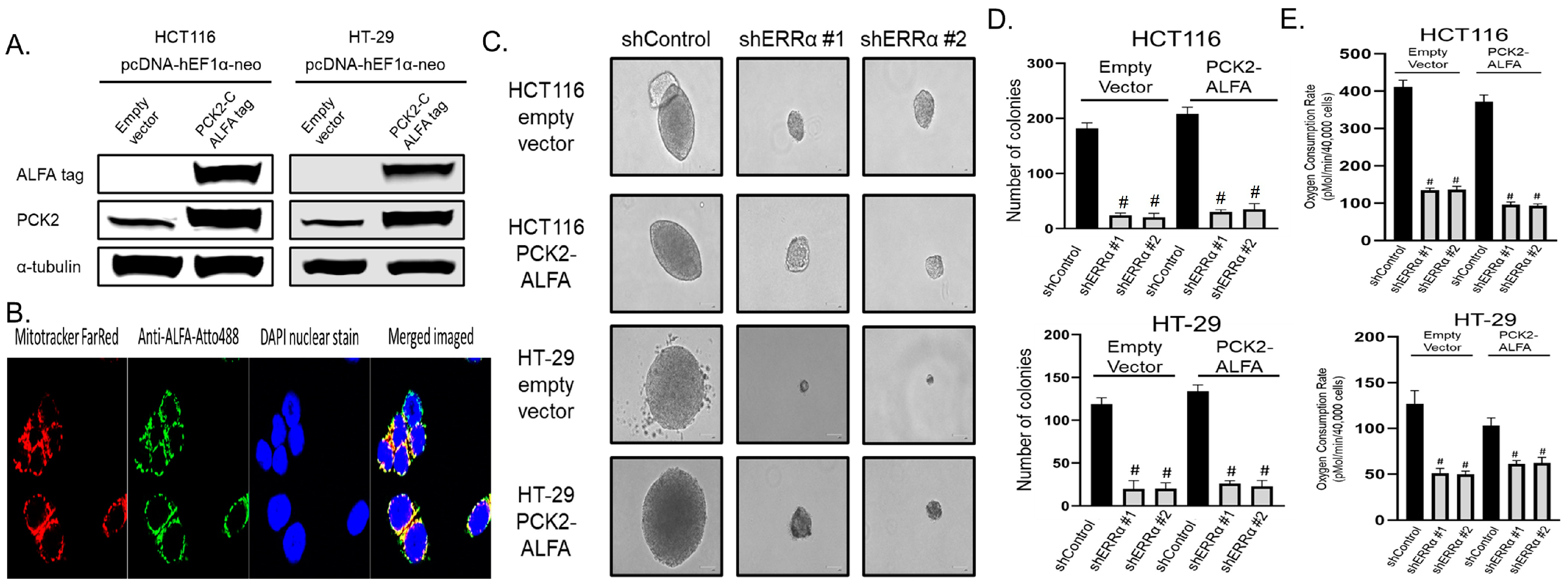
3.5. ERRα Depletion Causes Loss of Anchorage-Independent Growth and Glutamine Utilization That Is Not Rescued by Over-Expression of PCK2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hock, M.B.; Kralli, A. Transcriptional Control of Mitochondrial Biogenesis and Function. Annu. Rev. Physiol. 2009, 71, 177–203. [Google Scholar] [CrossRef]
- Bellafante, E.; Morgano, A.; Salvatore, L.; Murzilli, S.; Di Tullio, G.; D’Orazio, A.; Latorre, D.; Villani, G.; Moschetta, A. PGC-1β Promotes Enterocyte Lifespan and Tumorigenesis in the Intestine. Proc. Natl. Acad. Sci. USA 2014, 111, E4523–E4531. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.W.; Das, B.; Kim, H.S.; Clymer, B.K.; Gehring, D.; Smith, D.R.; Costanzo-Garvey, D.L.; Fernandez, M.R.; Brattain, M.G.; Kelly, D.L.; et al. AMPK Promotes Aberrant PGC1beta Expression To Support Human Colon Tumor Cell Survival. Mol. Cell Biol. 2015, 35, 3866–3879. [Google Scholar] [CrossRef]
- McCall, J.L.; Gehring, D.; Clymer, B.K.; Fisher, K.W.; Das, B.; Kelly, D.L.; Kim, H.; White, M.A.; Lewis, R.E. KSR1 and EPHB4 Regulate Myc and PGC1beta To Promote Survival of Human Colon Tumors. Mol. Cell Biol. 2016, 36, 2246–2261. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.A.; Belyeu, J.R.; Morgan, J.T.; Ouyang, Y.; Bott, A.J.; Quinlan, A.R.; Gertz, J.; Rutter, J. XPRESSyourself: Enhancing, Standardizing, and Automating Ribosome Profiling Computational Analyses Yields Improved Insight into Data. PLOS Comput. Biol. 2020, 16, e1007625. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential Gene and Transcript Expression Analysis of RNA-Seq Experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative Genomics Viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef]
- Rha, G.B.; Wu, G.; Shoelson, S.E.; Chi, Y.-I. Multiple Binding Modes between HNF4alpha and the LXXLL Motifs of PGC-1alpha Lead to Full Activation. J. Biol. Chem. 2009, 284, 35165–35176. [Google Scholar] [CrossRef] [PubMed]
- Delerive, P.; Wu, Y.; Burris, T.P.; Chin, W.W.; Suen, C.S. PGC-1 Functions as a Transcriptional Coactivator for the Retinoid X Receptors. J. Biol. Chem. 2002, 277, 3913–3917. [Google Scholar] [CrossRef] [PubMed]
- Takacs, M.; Petoukhov, M.V.; Atkinson, R.A.; Roblin, P.; Ogi, F.-X.; Demeler, B.; Potier, N.; Chebaro, Y.; Dejaegere, A.; Svergun, D.I.; et al. The Asymmetric Binding of PGC-1α to the ERRα and ERRγ Nuclear Receptor Homodimers Involves a Similar Recognition Mechanism. PLoS ONE 2013, 8, e67810. [Google Scholar] [CrossRef]
- Greschik, H.; Althage, M.; Flaig, R.; Sato, Y.; Chavant, V.; Peluso-Iltis, C.; Choulier, L.; Cronet, P.; Rochel, N.; Schüle, R.; et al. Communication between the ERRalpha Homodimer Interface and the PGC-1alpha Binding Surface via the Helix 8–9 Loop. J. Biol. Chem. 2008, 283, 20220–20230. [Google Scholar] [CrossRef] [PubMed]
- Kallen, J.; Schlaeppi, J.-M.; Bitsch, F.; Filipuzzi, I.; Schilb, A.; Riou, V.; Graham, A.; Strauss, A.; Geiser, M.; Fournier, B. Evidence for Ligand-Independent Transcriptional Activation of the Human Estrogen-Related Receptor α (ERRα): Crystal structure of errα ligand binding domain in complex with peroxisome proliferator-activated receptor coactivator-1α. J. Biol. Chem. 2004, 279, 49330–49337. [Google Scholar] [CrossRef]
- Chang, C.; Kazmin, D.; Jasper, J.S.; Kunder, R.; Zuercher, W.J.; McDonnell, D.P. The Metabolic Regulator ERRα, a Downstream Target of HER2/IGF-1R, as a Therapeutic Target in Breast Cancer. Cancer Cell 2011, 20, 500–510. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kressler, D.; Schreiber, S.N.; Knutti, D.; Kralli, A. The PGC-1-Related Protein PERC Is a Selective Coactivator of Estrogen Receptor Alpha. J. Biol. Chem. 2002, 277, 13918–13925. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Chang, C.; Safi, R.; Liu, X.; Baldi, R.; Jasper, J.S.; Anderson, G.R.; Liu, T.; Rathmell, J.C.; Dewhirst, M.W.; et al. ERRα Regulated Lactate Metabolism Contributes to Resistance to Targeted Therapies in Breast Cancer. Cell Rep. 2016, 15, 323–335. [Google Scholar] [CrossRef]
- Lin, J.; Tarr, P.T.; Yang, R.; Rhee, J.; Puigserver, P.; Newgard, C.B.; Spiegelman, B.M. PGC-1beta in the Regulation of Hepatic Glucose and Energy Metabolism. J. Biol. Chem. 2003, 278, 30843–30848. [Google Scholar] [CrossRef] [PubMed]
- Deblois, G.; Hall, J.A.; Perry, M.-C.; Laganière, J.; Ghahremani, M.; Park, M.; Hallett, M.; Giguère, V. Genome-Wide Identification of Direct Target Genes Implicates Estrogen-Related Receptor Alpha as a Determinant of Breast Cancer Heterogeneity. Cancer Res. 2009, 69, 6149–6157. [Google Scholar] [CrossRef]
- Deblois, G.; Chahrour, G.; Perry, M.-C.; Sylvain-Drolet, G.; Muller, W.J.; Giguère, V. Transcriptional Control of the ERBB2 Amplicon by ERRalpha and PGC-1beta Promotes Mammary Gland Tumorigenesis. Cancer Res. 2010, 70, 10277–10287. [Google Scholar] [CrossRef]
- Laganière, J.; Tremblay, G.B.; Dufour, C.R.; Giroux, S.; Rousseau, F.; Giguère, V. A Polymorphic Autoregulatory Hormone Response Element in the Human Estrogen-Related Receptor Alpha (ERRalpha) Promoter Dictates Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1alpha Control of ERRalpha Expression. J. Biol. Chem. 2004, 279, 18504–18510. [Google Scholar] [CrossRef]
- Tremblay, A.M.; Wilson, B.J.; Yang, X.-J.; Giguère, V. Phosphorylation-Dependent Sumoylation Regulates Estrogen-Related Receptor-Alpha and -Gamma Transcriptional Activity through a Synergy Control Motif. Mol. Endocrinol. 2008, 22, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.Y.; Johnson, C.; Washburn, J.G.; Cruz-Correa, M.R.; Dang, D.T.; Dang, L.H. Oncogenic KRAS Modulates Mitochondrial Metabolism in Human Colon Cancer Cells by Inducing HIF-1α and HIF-2α Target Genes. Mol. Cancer 2010, 9, 293. [Google Scholar] [CrossRef]
- Götzke, H.; Kilisch, M.; Martínez-Carranza, M.; Sograte-Idrissi, S.; Rajavel, A.; Schlichthaerle, T.; Engels, N.; Jungmann, R.; Stenmark, P.; Opazo, F.; et al. The ALFA-Tag Is a Highly Versatile Tool for Nanobody-Based Bioscience Applications. Nat. Commun. 2019, 10, 4403. [Google Scholar] [CrossRef] [PubMed]
- Lelliott, C.J.; Medina-Gomez, G.; Petrovic, N.; Kis, A.; Feldmann, H.M.; Bjursell, M.; Parker, N.; Curtis, K.; Campbell, M.; Hu, P.; et al. Ablation of PGC-1beta Results in Defective Mitochondrial Activity, Thermogenesis, Hepatic Function, and Cardiac Performance. PLoS Biol. 2006, 4, e369. [Google Scholar] [CrossRef] [PubMed]
- Victorino, V.J.; Barroso, W.A.; Assunção, A.K.M.; Cury, V.; Jeremias, I.C.; Petroni, R.; Chausse, B.; Ariga, S.K.; Herrera, A.C.S.A.; Panis, C.; et al. PGC-1β Regulates HER2-Overexpressing Breast Cancer Cells Proliferation by Metabolic and Redox Pathways. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 6035–6044. [Google Scholar] [CrossRef]
- Hernandez, C.; Molusky, M.; Li, Y.; Li, S.; Lin, J.D. Regulation of Hepatic ApoC3 Expression by PGC-1β Mediates Hypolipidemic Effect of Nicotinic Acid. Cell Metab. 2010, 12, 411–419. [Google Scholar] [CrossRef]
- Bensard, C.L.; Wisidagama, D.R.; Olson, K.A.; Berg, J.A.; Krah, N.M.; Schell, J.C.; Nowinski, S.M.; Fogarty, S.; Bott, A.J.; Wei, P.; et al. Regulation of Tumor Initiation by the Mitochondrial Pyruvate Carrier. Cell Metab. 2020, 31, 284–300. [Google Scholar] [CrossRef]
- Schell, J.C.; Olson, K.A.; Jiang, L.; Hawkins, A.J.; Van Vranken, J.G.; Xie, J.; Egnatchik, R.A.; Earl, E.G.; Deberardinis, R.J.; Rutter, J. A Role for the Mitochondrial Pyruvate Carrier as a Repressor of the Warburg Effect and Colon Cancer Cell Growth. Mol. Cell 2014, 56, 400–413. [Google Scholar] [CrossRef]
- Barry, J.B.; Laganière, J.; Giguère, V. A Single Nucleotide in an Estrogen-Related Receptor Alpha Site Can Dictate Mode of Binding and Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1alpha Activation of Target Promoters. Mol. Endocrinol. 2006, 20, 302–310. [Google Scholar] [CrossRef][Green Version]
- Lin, J.; Puigserver, P.; Donovan, J.; Tarr, P.; Spiegelman, B.M. Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1beta (PGC-1beta ), a Novel PGC-1-Related Transcription Coactivator Associated with Host Cell Factor. J. Biol. Chem. 2002, 277, 1645–1648. [Google Scholar] [CrossRef]
- Sammarco, G.; Gallo, G.; Vescio, G.; Picciariello, A.; De Paola, G.; Trompetto, M.; Currò, G.; Ammendola, M. Mast Cells, MicroRNAs and Others: The Role of Translational Research on Colorectal Cancer in the Forthcoming Era of Precision Medicine. J. Clin. Med. 2020, 9, 2852. [Google Scholar] [CrossRef]
- Denbo, J.W.; Yamashita, S.; Passot, G.; Egger, M.; Chun, Y.S.; Kopetz, S.E.; Maru, D.; Brudvik, K.W.; Wei, S.H.; Conrad, C.; et al. RAS Mutation Is Associated with Decreased Survival in Patients Undergoing Repeat Hepatectomy for Colorectal Liver Metastases. J. Gastrointest. Surg. 2017, 21, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Shady, W.; Petre, E.N.; Vakiani, E.; Ziv, E.; Gonen, M.; Brown, K.T.; Kemeny, N.E.; Solomon, S.B.; Solit, D.B.; Sofocleous, C.T. Kras Mutation Is a Marker of Worse Oncologic Outcomes after Percutaneous Radiofrequency Ablation of Colorectal Liver Metastases. Oncotarget 2017, 8, 66117–66127. [Google Scholar] [CrossRef] [PubMed]
- Goffredo, P.; Utria, A.F.; Beck, A.C.; Chun, Y.S.; Howe, J.R.; Weigel, R.J.; Vauthey, J.-N.; Hassan, I. The Prognostic Impact of KRAS Mutation in Patients Having Curative Resection of Synchronous Colorectal Liver Metastases. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2019, 23, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, T.; Li, R.; Du, Y.; Lv, H.; Zhang, L.; Chen, H.; Shi, X.; Li, Q.; Shen, J. The Discovery of a Novel Series of Potential ERRα Inverse Agonists Based on P-Nitrobenzenesulfonamide Template for Triple-Negative Breast Cancer in Vivo. J. Enzym. Inhib. Med. Chem. 2022, 37, 125–134. [Google Scholar] [CrossRef]
- Lynch, C.; Zhao, J.; Sakamuru, S.; Zhang, L.; Huang, R.; Witt, K.L.; Merrick, B.A.; Teng, C.T.; Xia, M. Identification of Compounds That Inhibit Estrogen-Related Receptor Alpha Signaling Using High-Throughput Screening Assays. Molecules 2019, 24, 841. [Google Scholar] [CrossRef]
- Duellman, S.J.; Calaoagan, J.M.; Sato, B.G.; Fine, R.; Klebansky, B.; Chao, W.-R.; Hobbs, P.; Collins, N.; Sambucetti, L.; Laderoute, K.R. A Novel Steroidal Inhibitor of Estrogen-Related Receptor α (ERRα). Biochem. Pharmacol. 2010, 80, 819–826. [Google Scholar] [CrossRef]
- Du, Y.; Song, L.; Zhang, L.; Ling, H.; Zhang, Y.; Chen, H.; Qi, H.; Shi, X.; Li, Q. The Discovery of Novel, Potent ERR-Alpha Inverse Agonists for the Treatment of Triple Negative Breast Cancer. Eur. J. Med. Chem. 2017, 136, 457–467. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, P.; Chen, H.; Li, Q.; Chen, L.; Qi, H.; Shi, X.; Du, Y. Characterization of a Selective Inverse Agonist for Estrogen Related Receptor α as a Potential Agent for Breast Cancer. Eur. J. Pharmacol. 2016, 789, 439–448. [Google Scholar] [CrossRef]
- Willy, P.J.; Murray, I.R.; Qian, J.; Busch, B.B.; Stevens, W.C.; Martin, R.; Mohan, R.; Zhou, S.; Ordentlich, P.; Wei, P.; et al. Regulation of PPARγ Coactivator 1α (PGC-1α) Signaling by an Estrogen-Related Receptor α (ERRα) Ligand. Proc. Natl. Acad. Sci. USA 2004, 101, 8912–8917. [Google Scholar] [CrossRef]
- Patch, R.J.; Searle, L.L.; Kim, A.J.; De, D.; Zhu, X.; Askari, H.B.; O’Neill, J.C.; Abad, M.C.; Rentzeperis, D.; Liu, J.; et al. Identification of Diaryl Ether-Based Ligands for Estrogen-Related Receptor α as Potential Antidiabetic Agents. J. Med. Chem. 2011, 54, 788–808. [Google Scholar] [CrossRef] [PubMed]
- Brearley, M.C.; Daniel, Z.C.T.R.; Loughna, P.T.; Parr, T.; Brameld, J.M. The Phosphoenolpyruvate Carboxykinase (PEPCK) Inhibitor, 3-Mercaptopicolinic Acid (3-MPA), Induces Myogenic Differentiation in C2C12 Cells. Sci. Rep. 2020, 10, 22177. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, J.; Latorre, P.; Carrodeguas, J.A.; Velázquez-Campoy, A.; Sancho, J.; López-Buesa, P. Inhibition of Pig Phosphoenolpyruvate Carboxykinase Isoenzymes by 3-Mercaptopicolinic Acid and Novel Inhibitors. PLoS ONE 2016, 11, e0159002. [Google Scholar] [CrossRef]
- Balan, M.D.; Mcleod, M.J.; Lotosky, W.R.; Ghaly, M.; Holyoak, T. Inhibition and Allosteric Regulation of Monomeric Phosphoenolpyruvate Carboxykinase by 3-Mercaptopicolinic Acid. Biochemistry 2015, 54, 5878–5887. [Google Scholar] [CrossRef]
- Mcleod, M.J.; Krismanich, A.P.; Assoud, A.; Dmitrienko, G.I.; Holyoak, T. Characterization of 3-[(Carboxymethyl)Thio]Picolinic Acid: A Novel Inhibitor of Phosphoenolpyruvate Carboxykinase. Biochemistry 2019, 58, 3918–3926. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frodyma, D.E.; Troia, T.C.; Rao, C.; Svoboda, R.A.; Berg, J.A.; Shinde, D.D.; Thomas, V.C.; Lewis, R.E.; Fisher, K.W. PGC-1β and ERRα Promote Glutamine Metabolism and Colorectal Cancer Survival via Transcriptional Upregulation of PCK2. Cancers 2022, 14, 4879. https://doi.org/10.3390/cancers14194879
Frodyma DE, Troia TC, Rao C, Svoboda RA, Berg JA, Shinde DD, Thomas VC, Lewis RE, Fisher KW. PGC-1β and ERRα Promote Glutamine Metabolism and Colorectal Cancer Survival via Transcriptional Upregulation of PCK2. Cancers. 2022; 14(19):4879. https://doi.org/10.3390/cancers14194879
Chicago/Turabian StyleFrodyma, Danielle E., Thomas C. Troia, Chaitra Rao, Robert A. Svoboda, Jordan A. Berg, Dhananjay D. Shinde, Vinai C. Thomas, Robert E. Lewis, and Kurt W. Fisher. 2022. "PGC-1β and ERRα Promote Glutamine Metabolism and Colorectal Cancer Survival via Transcriptional Upregulation of PCK2" Cancers 14, no. 19: 4879. https://doi.org/10.3390/cancers14194879
APA StyleFrodyma, D. E., Troia, T. C., Rao, C., Svoboda, R. A., Berg, J. A., Shinde, D. D., Thomas, V. C., Lewis, R. E., & Fisher, K. W. (2022). PGC-1β and ERRα Promote Glutamine Metabolism and Colorectal Cancer Survival via Transcriptional Upregulation of PCK2. Cancers, 14(19), 4879. https://doi.org/10.3390/cancers14194879





