Subpathway Analysis of Transcriptome Profiles Reveals New Molecular Mechanisms of Acquired Chemotherapy Resistance in Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Genomics Studies of Chemoresistance in Breast Cancer
1.2. Pathway Analysis of Genomics Profiles
2. Methods
2.1. Data Collection and Integration
2.2. Differential Gene Expression Analysis and Pathway Analysis
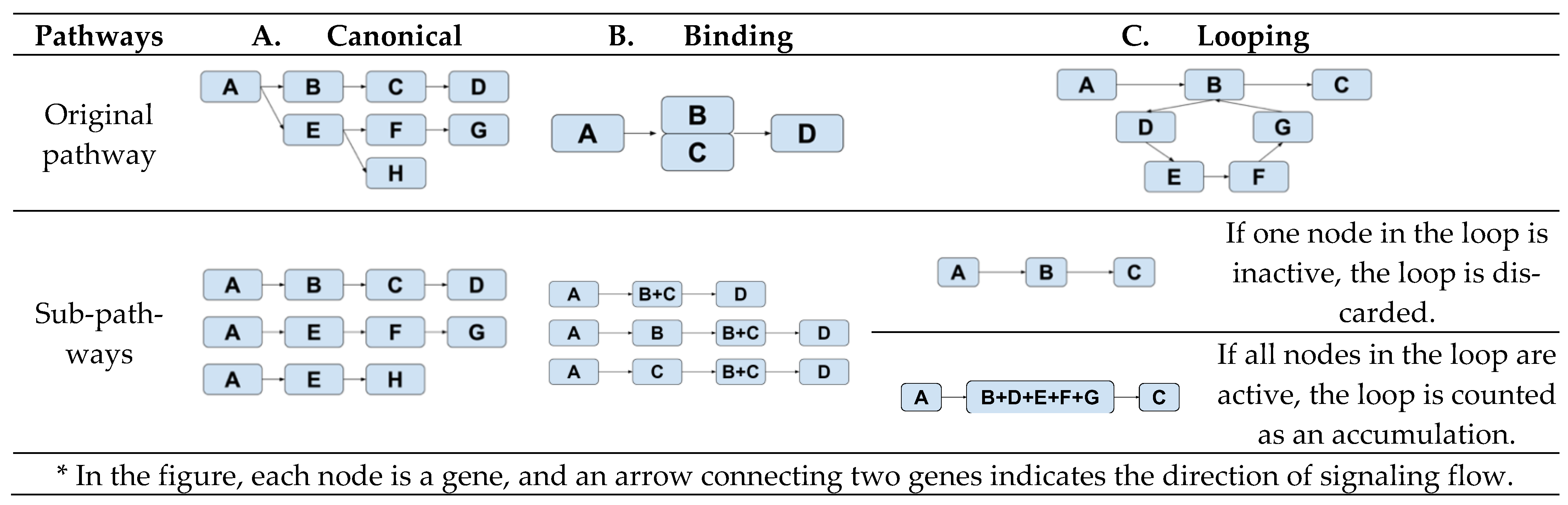
2.3. Analysis of Subpathway Data
3. Results
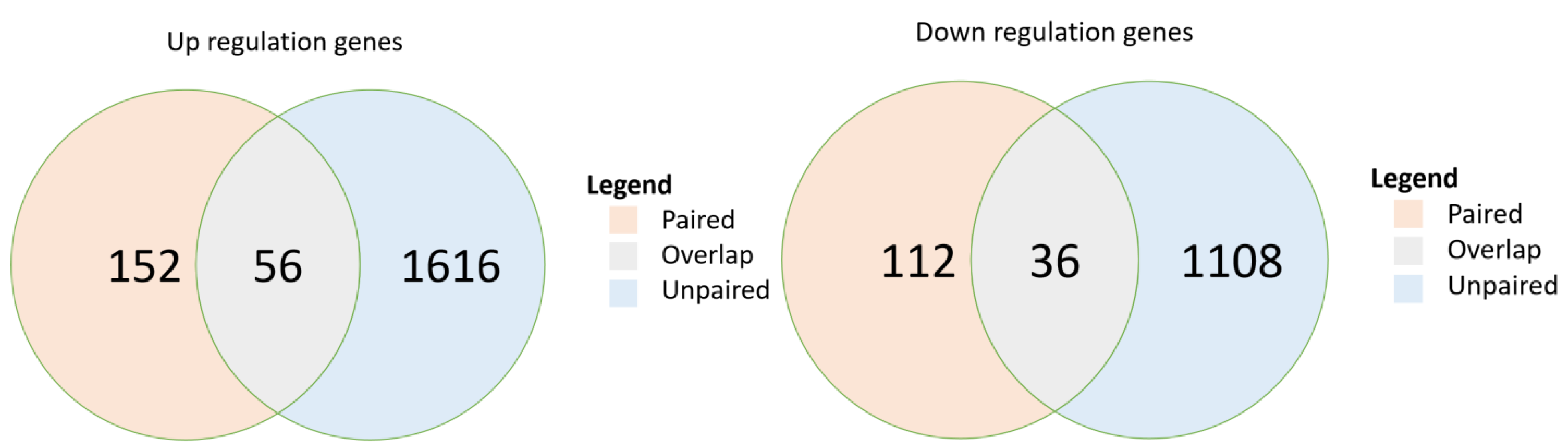
3.1. Genes Expressed Differentially between Pre- and Post-Chemotherapy among Patients with Breast Cancer
3.2. Pathways and Their Statistically Significantly Activated Subpathways from Pre- to Post-Chemotherapy in Both Paired and Unpaired Breast Cancer Sample Cohorts
3.3. Pathway and Sub-Pathway Analyses of Chemoresistance in Triple-Negative Breast Cancer
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Significant Contribution
References
- Breastcancer.org. Breast Cancer Facts and Statistics. Available online: https://www.breastcancer.org/symptoms/understand_bc/statistics (accessed on 14 August 2021).
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Sikov, W.M.; Berry, D.A.; Perou, C.M.; Singh, B.; Cirrincione, C.T.; Tolaney, S.M.; Kuzma, C.S.; Pluard, T.J.; Somlo, G.; Port, E.R.; et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J. Clin. Oncol. 2015, 33, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; O’Shaughnessy, J.; Untch, M.; Sikov, W.M.; Rugo, H.S.; McKee, M.D.; Huober, J.; Golshan, M.; von Minckwitz, G.; Maag, D.; et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomised, phase 3 trial. Lancet Oncol. 2018, 19, 497–509. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef]
- Robson, M.E.; Tung, N.; Conte, P.; Im, S.A.; Senkus, E.; Xu, B.; Masuda, N.; Delaloge, S.; Li, W.; Armstrong, A.; et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann. Oncol. 2019, 30, 558–566. [Google Scholar] [CrossRef]
- Litton, J.K.; Scoggins, M.E.; Hess, K.R.; Adrada, B.E.; Murthy, R.K.; Damodaran, S.; DeSnyder, S.M.; Brewster, A.M.; Barcenas, C.H.; Valero, V.; et al. Neoadjuvant talazoparib for patients with operable breast cancer with a germline BRCA pathogenic variant. J. Clin. Oncol. 2020, 38, 388–394. [Google Scholar] [CrossRef]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef]
- Gucalp, A.; Tolaney, S.; Isakoff, S.J.; Ingle, J.N.; Liu, M.C.; Carey, L.A.; Blackwell, K.; Rugo, H.; Nabell, L.; Forero, A.; et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin. Cancer Res. 2013, 19, 5505–5512. [Google Scholar] [CrossRef]
- Kim, S.B.; Dent, R.; Im, S.A.; Espié, M.; Blau, S.; Tan, A.R.; Isakoff, S.J.; Oliveira, M.; Saura, C.; Wongchenko, M.J.; et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017, 18, 1360–1372. [Google Scholar] [CrossRef]
- Sanders, S.; Butcher, H.K.; Swails, P.; Power, J. Portraits of caregivers of end-stage dementia patients receiving hospice care. Death Stud. 2009, 33, 521–556. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.J.; Ohtani, S.; Im, Y.H.; Lee, E.S.; Yokota, I.; Kuroi, K.; Im, S.A.; Park, B.W.; Kim, S.B.; et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Balko, J.M.; Cook, R.S.; Vaught, D.B.; Kuba, M.G.; Miller, T.W.; Bhola, N.E.; Sanders, M.E.; Granja-Ingram, N.M.; Smith, J.J.; Meszoely, I.M.; et al. Profiling of residual breast cancers after neoadjuvant chemotherapy identifies DUSP4 deficiency as a mechanism of drug resistance. Nature Med. 2012, 18, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Shapiro, G.I.; Lorusso, P.M.; Cervantes, A.; Schwartz, G.K.; Weiss, G.J.; Paz-Ares, L.; Cho, D.C.; Infante, J.R.; Alsina, M.; et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013, 3, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Balko, J.M.; Schwarz, L.J.; Luo, N.; Estrada, M.V.; Giltnane, J.M.; Dávila-González, D.; Wang, K.; Sánchez, V.; Dean, P.T.; Combs, S.E.; et al. Triple-negative breast cancers with amplification of JAK2 at the 9p24 locus demonstrate JAK2-specific dependence. Sci. Transl. Med. 2016, 8, 334ra53. [Google Scholar] [CrossRef]
- Balko, J.M.; Giltnane, J.M.; Wang, K.; Schwarz, L.J.; Young, C.D.; Cook, R.S.; Owens, P.; Sanders, M.E.; Kuba, M.G.; Sánchez, V.; et al. Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discov. 2014, 4, 232–245. [Google Scholar] [CrossRef]
- Kim, C.; Gao, R.; Sei, E.; Brandt, R.; Hartman, J.; Hatschek, T.; Crosetto, N.; Foukakis, T.; Navin, N.E. Chemoresistance evolution in triple-negative breast cancer delineated by single-cell sequencing. Cell 2018, 173, 879–893.e13. [Google Scholar] [CrossRef]
- Vera-Ramirez, L.; Sánchez-Rovira, P.; Ramirez-Tortosa, C.L.; Quiles, J.L.; Ramirez-Tortosa, M.; Lorente, J.A. Transcriptional shift identifies a set of genes driving breast cancer chemoresistance. PLoS ONE 2013, 8, e53983. [Google Scholar] [CrossRef]
- Korde, L.A.; Lusa, L.; McShane, L.; Lebowitz, P.F.; Lukes, L.; Camphausen, K.; Parker, J.S.; Swain, S.; Hunter, K.; Zujewski, J.A. Gene expression pathway analysis to predict response to neoadjuvant docetaxel and capecitabine for breast cancer. Breast Cancer Res. Treat. 2010, 119, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.P.; Richardson, A.L.; Eklund, A.C.; Wang, Z.C.; Szallasi, Z.; Li, Q.; Juul, N.; Leong, C.-O.; Calogrias, D.; Buraimoh, A.; et al. Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J. Clin. Oncol. 2010, 28, 1145–1153. [Google Scholar] [CrossRef]
- Stover, D.G.; Coloff, J.L.; Barry, W.T.; Brugge, J.S.; Winer, E.P.; Selfors, L.M. The role of proliferation in determining response to neoadjuvant chemotherapy in breast cancer: A gene expression-based meta-analysis. Clin. Cancer Res. 2016, 22, 6039–6050. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shojaie, A.; Michailidis, G. A comparative study of topology-based pathway enrichment analysis methods. BMC Bioinform. 2019, 20, 546. [Google Scholar] [CrossRef]
- Amadoz, A.; Hidalgo, M.R.; Cubuk, C.; Carbonell-Caballero, J.; Dopazo, J. A comparison of mechanistic signaling pathway activity analysis methods. Brief. Bioinform. 2019, 20, 1655–1668. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine. Gene Expression Omnibus. Available online: https://www.ncbi.nlm.nih.gov/geo/ (accessed on 14 August 2021).
- Liu, W.-M.; Mei, R.; Di, X.; Ryder, T.B.; Hubbell, E.; Dee, S.; Webster, T.A.; Harrington, C.A.; Ho, M.-H.; Baid, J.; et al. Analysis of high density expression microarrays with signed-rank call algorithms. Bioinformatics 2002, 18, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- McNemar, Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika 1947, 12, 153–157. [Google Scholar] [CrossRef]
- Pearson, K. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1900, 50, 157–175. [Google Scholar] [CrossRef]
- Sebastián-León, P.; Carbonell, J.; Salavert, F.; Sanchez, R.; Medina, I.; Dopazo, J. Inferring the functional effect of gene expression changes in signaling pathways. Nucleic Acids Res. 2013, 41, W213–W217. [Google Scholar] [CrossRef]
- Hidalgo, M.R.; Cubuk, C.; Amadoz, A.; Salavert, F.; Carbonell-Caballero, J.; Dopazo, J. High throughput estimation of functional cell activities reveals disease mechanisms and predicts relevant clinical outcomes. Oncotarget 2017, 8, 5160–5178. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qian, J.; Li, J.; Zhu, C. Knockdown of lncRNA-HOTAIR downregulates the drug-resistance of breast cancer cells to doxorubicin via the PI3K/AKT/mTOR signaling pathway. Exp. Ther. Med. 2019, 18, 435–442. [Google Scholar] [CrossRef]
- Knuefermann, C.; Lu, Y.; Liu, B.; Jin, W.; Liang, K.; Wu, L.; Schmidt, M.; Mills, G.B.; Mendelsohn, J.; Fan, Z. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene 2003, 22, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, Y.; Liang, K.; Liu, B.; Fan, Z. Differential responses to doxorubicin-induced phosphorylation and activation of Akt in human breast cancer cells. Breast Cancer Res. 2005, 7, R589–R597. [Google Scholar] [CrossRef]
- Chang, L.; Hu, Z.; Zhou, Z.; Zhang, H. Linc00518 contributes to multidrug resistance through regulating the MiR-199a/MRP1 axis in breast cancer. Cell. Physiol. Biochem. 2018, 48, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Antoon, J.W.; White, M.D.; Slaughter, E.M.; Driver, J.L.; Khalili, H.S.; Elliott, S.; Smith, C.D.; Burow, M.E.; Beckman, B.S. Targeting NFkB mediated breast cancer chemoresistance through selective inhibition of sphingosine kinase-2. Cancer Biol. Ther. 2011, 11, 678–689. [Google Scholar] [CrossRef]
- Christowitz, C.; Davis, T.; Isaacs, A.; Van Niekerk, G.; Hattingh, S.; Engelbrecht, A.-M. Mechanisms of doxorubicin-induced drug resistance and drug resistant tumour growth in a murine breast tumour model. BMC Cancer 2019, 19, 757. [Google Scholar] [CrossRef]
- Hasna, J.; Hague, F.; Despoix, L.; Geerts, D.; Leroy, C.; Tulasne, D.; Ouadid-Ahidouch, H.; Kischel, P. Orai3 calcium channel and resistance to chemotherapy in breast cancer cells: The p53 connection. Cell Death Differ. 2018, 25, 693–707. [Google Scholar] [CrossRef]
- Hien, T.T.; Kim, H.G.; Han, E.H.; Kang, K.W.; Jeong, H.G. Molecular mechanism of suppression of MDR1 by puerarin from Pueraria lobata via NF-kappaB pathway and cAMP-responsive element transcriptional activity-dependent up-regulation of AMP-activated protein kinase in breast cancer MCF-7/adr cells. Mol. Nutr. Food Res. 2010, 54, 918–928. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Wu, J.F.; Luo, Q.C.; Liu, Q.F.; Wu, Q.W.; Ye, G.D.; She, H.Q.; Li, B.A. Pygo2 activates MDR1 expression and mediates chemoresistance in breast cancer via the Wnt/beta-catenin pathway. Oncogene 2016, 35, 4787–4797. [Google Scholar] [CrossRef]
- Curry, M.C.; Luk, N.A.; Kenny, P.A.; Roberts-Thomson, S.J.; Monteith, G.R. Distinct regulation of cytoplasmic calcium signals and cell death pathways by different plasma membrane calcium ATPase isoforms in MDA-MB-231 breast cancer cells. J. Biol. Chem. 2012, 287, 28598–28608. [Google Scholar] [CrossRef] [PubMed]
- Paplomata, E.; O’Regan, R. The PI3K/AKT/mTOR pathway in breast cancer: Targets, trials and biomarkers. Ther. Adv. Med. Oncol. 2014, 6, 154–166. [Google Scholar] [CrossRef]
- Dowling, R.J.; Topisirovic, I.; Fonseca, B.D.; Sonenberg, N. Dissecting the role of mTOR: Lessons from mTOR inhibitors. Biochim. Biophys. Acta 2010, 1804, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Semiglazov, V.; Van Dam, P.; Manikhas, A.; Bellet, M.; Mayordomo, J.; Campone, M.; Kubista, E.; Greil, R.; Bianchi, G.; et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J. Clin. Oncol. 2009, 27, 2630–2637. [Google Scholar] [CrossRef] [PubMed]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer 2018, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Novak, C.M.; Horst, E.N.; Taylor, C.C.; Liu, C.Z.; Mehta, G. Fluid shear stress stimulates breast cancer cells to display invasive and chemoresistant phenotypes while upregulating PLAU in a 3D bioreactor. Biotechnol. Bioeng. 2019, 116, 3084–3097. [Google Scholar] [CrossRef]
- Parekh, A.; Das, S.; Parida, S.; Das, C.K.; Dutta, D.; Mallick, S.K.; Wu, P.-H.; Kumar, B.N.P.; Bharti, R.; Dey, G.; et al. Multi-nucleated cells use ROS to induce breast cancer chemo-resistance in vitro and in vivo. Oncogene 2018, 37, 4546–4561. [Google Scholar] [CrossRef]
- O’Reilly, E.A.; Gubbins, L.; Sharma, S.; Tully, R.; Guang, M.H.Z.; Weiner-Gorzel, K.; McCaffrey, J.; Harrison, M.; Furlong, F.; Kell, M.; et al. The fate of chemoresistance in triple negative breast cancer (TNBC). BBA Clin. 2015, 3, 257–275. [Google Scholar] [CrossRef]
- Taylor, J.R.; Lehmann, B.D.; Chappell, W.H.; Abrams, S.L.; Steelman, L.S.; McCubrey, J.A. Cooperative effects of Akt-1 and Raf-1 on the induction of cellular senescence in doxorubicin or tamoxifen treated breast cancer cells. Oncotarget 2011, 2, 610–626. [Google Scholar] [CrossRef]
- Han, C.-Y.; Cho, K.-B.; Choi, H.-S.; Han, H.-K.; Kang, K.-W. Role of FoxO1 activation in MDR1 expression in adriamycin-resistant breast cancer cells. Carcinogenesis 2008, 29, 1837–1844. [Google Scholar] [CrossRef]
- de Moraes, G.N.; Delbue, D.; Silva, K.L.; Robaina, M.C.; Khongkow, P.; Gomes, A.R.; Zona, S.; Crocamo, S.; Mencalha, A.L.; Magalhães, L.M.; et al. FOXM1 targets XIAP and survivin to modulate breast cancer survival and chemoresistance. Cell. Signal. 2015, 27, 2496–2505. [Google Scholar] [CrossRef] [PubMed]

| Publications 2010–2018 | Clinical Endpoint and Sample Size | Genomic Platform | Primary Genes and Pathways Discovered |
|---|---|---|---|
| Balko et al. 2012 [16] Balko et al. 2014 [17] Balko et al. 2016 [18] | Relapse-free survival (RFS) n = 74 | Targeted RNA and DNA sequencing | DUSP4 low expression, MYC high expression, and JAK2 amplification were associated with RFS. |
| Lips et al. 2015 [19] | Pathologic complete response (pCR) and RFS n = 56 | Targeted DNA sequencing | No statistically significant genes |
| Kim et al. 2018 [20] | pCR was not defined after NACT n = 20 | Bulk DNA sequencing, single nucleus RNA- seq/DNA-seq | Chemoresistance gene signatures are enriched in EMT, CDH1, AKT1, hypoxia, angiogenesis, and extracellular matrix degradation signaling pathways. |
| Laura et al. 2013 [21] | pCR n = 106 | Affymetrix | Significant genes enriched in Wnt, HIF1, p53, and Rho GTPases signaling pathways were associated with poor response to chemotherapy drugs. |
| Korde et al. 2010 [22] | pCR n = 21 | Affymetrix | MAP-2, MACF1,VEGF-B, and EGFR showed high expression in patients without pCR after chemotherapy. |
| Silver et al. 2010 [23] | pCR n = 28 | Affymetrix | BRCA1 promoter methylation and E2F3 activation contribute to good cisplatin response. |
| Stover et al. 2015 [24] | pCR n = 446 | Affymetrix, Agilent | Low proliferation and immune-predicted resistance, with stem-like phenotype and Ras-Erk were associated with chemotherapy resistance. |
| Group Demographics Clinical Status | Pre-Chemo | Percentage (%) | Post-Chemo | Percentage (%) |
|---|---|---|---|---|
| Age < 55, Race = white ER+ PR+ HER2+ | 15 | 2.18 | 1 | 2.22 |
| Age < 55, Race = white ER+PR-/ER-PR+/ER-PR-HER2- | 89 | 12.90 | 5 | 11.11 |
| Age < 55, Race = white ER+PR-/ER-PR+/ER-PR-HER2+ | 21 | 3.04 | 2 | 4.44 |
| Age < 55, Race = white ER-PR-HER2- | 46 | 6.67 | 7 | 15.56 |
| Age < 55, Race = non-white ER+PR+HER2+ | 22 | 3.19 | 1 | 2.22 |
| Age < 55, Race = non-white ER+PR-/ER-PR+/ER-PR-HER2- | 52 | 7.53 | 6 | 13.33 |
| Age < 55, Race = non-white ER+PR-/ER-PR+/ER-PR-HER2+ | 35 | 5.07 | 4 | 8.89 |
| Age < 55, Race = non-white ER-PR-HER2- | 48 | 6.96 | 4 | 8.89 |
| Age > 55, Race = white ER+PR+HER2+ | 31 | 4.49 | 0 | 0 |
| Age > 55, Race = white ER+PR-/ER-PR+/ER-PR-HER2- | 94 | 13.62 | 6 | 13.33 |
| Age > 55, Race = white ER+PR-/ER-PR+/ER-PR-HER2+ | 30 | 4.35 | 3 | 6.66 |
| Age > 55, Race = white ER-PR-HER2- | 55 | 7.97 | 6 | 13.33 |
| Age > 55, Race = non-white ER+PR+HER2+ | 51 | 7.39 | 0 | 0 |
| Age > 55, Race = non-white ER+PR-/ER-PR+/ER-PR-HER2- | 30 | 4.34 | 0 | 0 |
| Age > 55, Race = non-white ER+PR-/ER-PR+/ER-PR-HER2+ | 25 | 3.62 | 0 | 0 |
| Age > 55, Race = non-white ER-PR-HER2- | 46 | 6.67 | 0 | 0 |
| Name | Relationship | Value |
|---|---|---|
| Activation | --> | 1 |
| Inhibition | --| | −1 |
| Expression | --> | 1 |
| Repression | --| | 1 |
| Indirect effect | ..> | 1 |
| State change | … | 1 |
| Binding/association | --- | 1 |
| Dissociation | -+- | −1 |
| Missing interaction | -/- | 1 |
| Phosphorylation | +p | 1 |
| Dephosphorylation | −p | −1 |
| Glycosylation | +g | 1 |
| Ubiquitination | +u | 1 |
| Methylation | +m | 1 |
| Pathway Name | Number of Genes in Pathway | DE Genes in Paired Samples | DE Genes in Unpaired Samples | p-Value (Paired Samples) | p-Value (Unpaired Samples) |
|---|---|---|---|---|---|
| Drug metabolism | 133 | 13 | 41 | 9.74 × 10−07 | 0.00041 |
| MAPK signaling pathway | 278 | 26 | 37 | 9.99 × 10−12 | 0.00228 |
| ErbB signaling pathway | 131 | 14 | 15 | 1.25 × 10−07 | 0.00615 |
| Calcium signaling pathway | 170 | 20 | 43 | 4.44 × 10−11 | 0.01012 |
| cGMP-PKG signaling pathway | 188 | 26 | 13 | 9.78 × 10−16 | 1.075 × 10−06 |
| Sphingolipid signaling pathway | 137 | 13 | 40 | 1.35 × 10−06 | 0.00134 |
| PI3K-Akt signaling pathway | 416 | 16 | 32 | 0.00287 | 1.52 × 10−11 |
| Cushing’s syndrome | 194 | 30 | 17 | 2.14 × 10−19 | 2.70 × 10−05 |
| Human papillomavirus infection | 387 | 22 | 18 | 2.43 × 10−06 | 1.81 × 10−17 |
| Proteoglycans in cancer | 332 | 16 | 44 | 0.000330 | 0.000977 |
| Pathway Name | Number of Subpathways (p < 0.05, Paired) | Number of Subpathways (p < 0.05, Un-Paired) | Overlapped Upregulated Subpathways (Same Direction) | False Discovery Rate (Overlap) | Significant Genes (Up-Regulated) * |
|---|---|---|---|---|---|
| Fluid shear stress and atherosclerosis | 1085 | 1694 | 277 | 0.10 | MAP3K5 |
| MAPK signaling pathway | 1947 | 1541 | 358 | 0.11 | MAP2K2, MAP3K1, MAP3K5, MAP4K1 |
| PI3K-Akt signaling pathway | 1470 | 1325 | 254 | 0.13 | NR4A1, NRAS, PIK3R3, OSMR |
| Wnt signaling pathway | 1775 | 2635 | 315 | 0.14 | MAP2K1, MAPK1 |
| FoxO signaling pathway | 1432 | 2175 | 246 | 0.15 | FOXO6, FBXO25 FOXO1 |
| ECM-receptor interaction | 1033 | 1876 | 167 | 0.15 | MYL9, IRS2 |
| Ras signaling pathway | 1019 | 1460 | 164 | 0.16 | RAC3 |
| Rap1 signaling pathway | 861 | 1505 | 132 | 0.16 | CTNNB1, MAGI3, RAPGEF6, RAP1B, ARAP3 |
| mTOR signaling pathway | 831 | 1253 | 126 | 0.16 | MAPK3, GSK3B |
| Calcium signaling pathway | 820 | 1238 | 124 | 0.16 | PLCG1, PLCG2, PRKCG |
| Cellular senescence | 684 | 1341 | 97 | 0.18 | TP53, CDKN1A |
| cAMP signaling pathway | 1937 | 2598 | 247 | 0.20 | E2F1, FOXM1 |
| Pathway Name | Number of Subpathways | Number of Subpathways (p < 0.01) | False Discovery Rate |
|---|---|---|---|
| Calcium signaling pathway | 12,056 | 1598 | 0.075 |
| Fluid shear stress and atherosclerosis | 12,337 | 1347 | 0.091 |
| cAMP signaling pathway | 19,409 | 1602 | 0.121 |
| Cellular senescence | 18,190 | 1040 | 0.174 |
| mTOR signaling pathway | 19,518 | 1097 | 0.177 |
| MAPK signaling pathway | 30,652 | 1553 | 0.197 |
| ECM-receptor interaction | 15,162 | 658 | 0.230 |
| PI3K-Akt signaling pathway | 34,998 | 1436 | 0.243 |
| Focal adhesion | 22,580 | 853 | 0.264 |
| Wnt signaling pathway | 28,687 | 835 | 0.346 |
| Ras signaling pathway | 40,147 | 1092 | 0.367 |
| Rap1 signaling pathway | 20,606 | 439 | 0.469 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, Y.; Shao, S.; Liu, E.; Li, J.; Tian, Z.; Wu, X.; Zhang, S.; Stover, D.; Wu, H.; Cheng, L.; et al. Subpathway Analysis of Transcriptome Profiles Reveals New Molecular Mechanisms of Acquired Chemotherapy Resistance in Breast Cancer. Cancers 2022, 14, 4878. https://doi.org/10.3390/cancers14194878
Huo Y, Shao S, Liu E, Li J, Tian Z, Wu X, Zhang S, Stover D, Wu H, Cheng L, et al. Subpathway Analysis of Transcriptome Profiles Reveals New Molecular Mechanisms of Acquired Chemotherapy Resistance in Breast Cancer. Cancers. 2022; 14(19):4878. https://doi.org/10.3390/cancers14194878
Chicago/Turabian StyleHuo, Yang, Shuai Shao, Enze Liu, Jin Li, Zhen Tian, Xue Wu, Shijun Zhang, Daniel Stover, Huanmei Wu, Lijun Cheng, and et al. 2022. "Subpathway Analysis of Transcriptome Profiles Reveals New Molecular Mechanisms of Acquired Chemotherapy Resistance in Breast Cancer" Cancers 14, no. 19: 4878. https://doi.org/10.3390/cancers14194878
APA StyleHuo, Y., Shao, S., Liu, E., Li, J., Tian, Z., Wu, X., Zhang, S., Stover, D., Wu, H., Cheng, L., & Li, L. (2022). Subpathway Analysis of Transcriptome Profiles Reveals New Molecular Mechanisms of Acquired Chemotherapy Resistance in Breast Cancer. Cancers, 14(19), 4878. https://doi.org/10.3390/cancers14194878






