Pharmacotherapy for Advanced Non-Small Cell Lung Cancer with Performance Status 2 without Druggable Gene Alterations: Could Immune Checkpoint Inhibitors Be a Game Changer?
Abstract
Simple Summary
Abstract
1. Introduction
2. Current Evidence on Pharmacotherapy for NSCLC with PS 2
2.1. Cytotoxic Chemotherapy
2.2. Anti-PD-1/PD-L1 Antibody Monotherapy
2.3. Combination Therapy with ICI and Cytotoxic Chemotherapy
2.4. Combination Therapy with Nivolumab and Ipilimumab
| Phase | Histology | Line | Regimen | N | Median OS (Month) | 1-Year OS (%) | Median PFS (Month) | ORR (%) | TRAE with Grade 3–4 (%) | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CheckMate-171 | II | Sq NSCLC | ≥2 | Nivolumab | 98 | 5.4 | 27 | - | 11 | 6 | [22] |
| CheckMate-153 | III/IV | NSCLC | ≥2 | Nivolumab | 123 | 4.0 | 24 | - | - | 12 | [23] |
| TAIL | III/IV | NSCLC | ≥2 | Atezolizumab | 61 | 3.5 | 22 | 1.7 | 3 | 15 | [24] |
| PePS2 | II | NSCLC | 1, 2 | Pembrolizumab | 27 (TPS < 1%) | 8.1 | - | 3.7 | 11 | 28 | [25] |
| 15 (TPS 1–49%) | 12.6 | - | 8.3 | 33 | |||||||
| 15 (TPS 50%-) | 14.6 | - | 12.6 | 47 | |||||||
| IPSOS | III | NSCLC | 1 | Atezolizumab | 228 | (HR 0.86, 95%CI 0.67–1.10) | - | - | - | - | [26] |
| Gemcitabine or Vinorelbine | 116 | - | - | - | - | ||||||
| CheckMate-817 | IIIb | NSCLC | 1 | Nivolumab + Ipilimumab | 139 | 9.0 | 44 | 3.6 | 19 | 24 | [33] |
| Energy-GFPC 06-2015 | III | NSCLC | 1 | Nivolumab + Ipilimumab | 40 | 2.9 | - | - | - | - | [34] |
| Chemotherapy | 39 | 6.1 | - | - | - | - |
3. Challenges of ICI for NSCLC with PS 2
3.1. Why Is ICI Less Effective for Patients with PS 2?
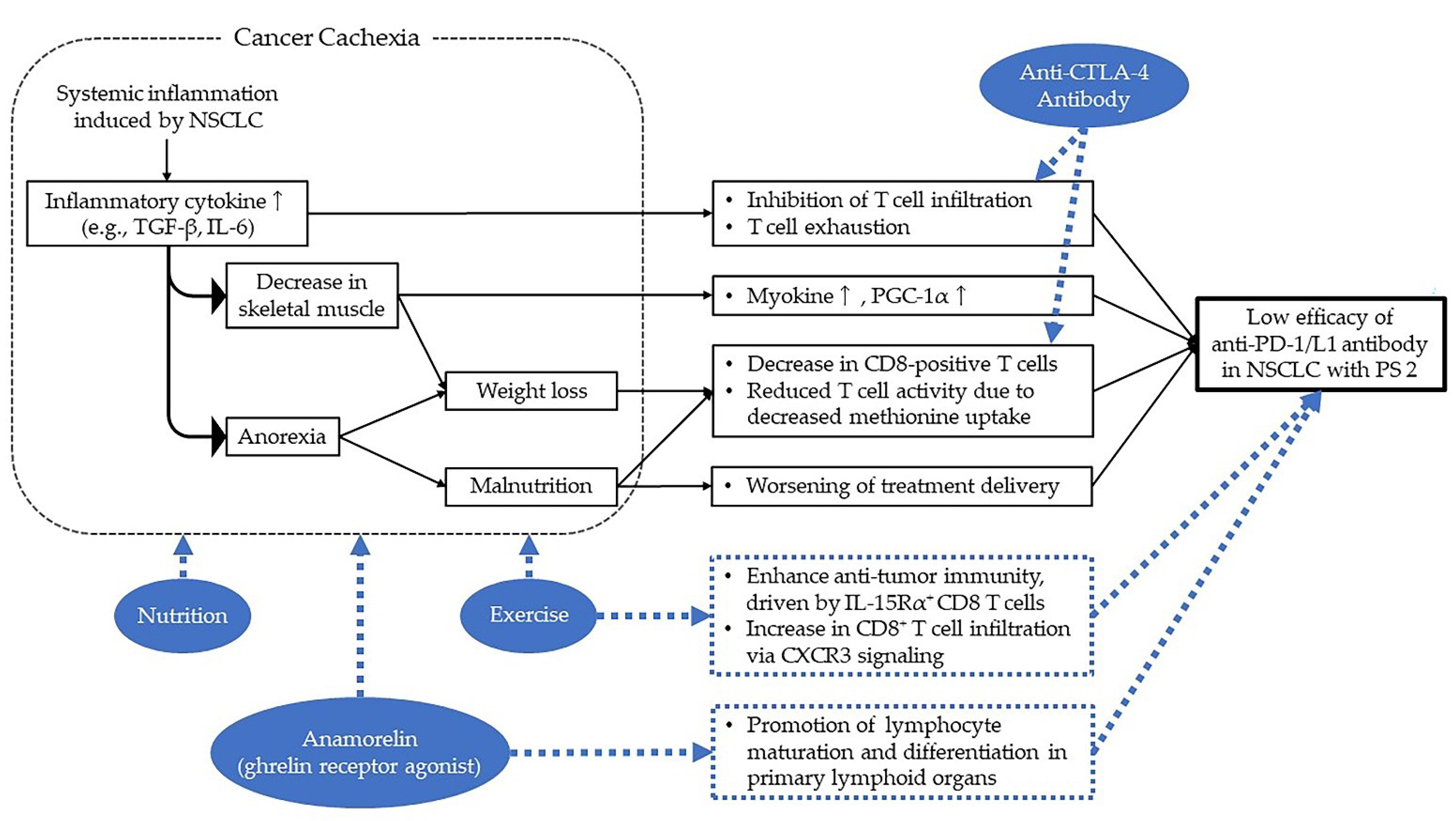
3.2. Impact of Cancer Cachexia on NSCLC with PS 2
3.3. How to Make ICI More Effective for NSCLC with PS 2?
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kawaguchi, T.; Takada, M.; Kubo, A.; Matsumura, A.; Fukai, S.; Tamura, A.; Saito, R.; Maruyama, Y.; Kawahara, M.; Ignatius Ou, S.H. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: A comprehensive analysis of 26,957 patients with NSCLC. J. Thorac. Oncol. 2010, 5, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Sculier, J.P.; Chansky, K.; Crowley, J.J.; Van Meerbeeck, J.; Goldstraw, P. International Staging Committee and Participating Institutions. The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the proposals for the 7th Edition. J. Thorac. Oncol. 2008, 3, 457–466. [Google Scholar] [PubMed]
- Sweeney, C.J.; Zhu, J.; Sandler, A.B.; Schiller, J.; Belani, C.P.; Langer, C.; Krook, J.; Harrington, D.; Johnson, D.H. Outcome of patients with a performance status of 2 in Eastern Cooperative Oncology Group Study E1594: A Phase II trial in patients with metastatic non-small cell lung carcinoma. Cancer 2001, 92, 2639–2647. [Google Scholar] [CrossRef]
- Sørensen, J.B.; Klee, M.; Palshof, T.; Hansen, H.H. Performance status assessment in cancer patients. An inter-observer variability study. Br. J. Cancer 1993, 67, 773–775. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.L.; Chen, G.; Feng, J.; Liu, X.Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomized, phase 3 study. Lancet Oncol. 2011, 12, 735–742. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small cell lung cancer (EURTAC): A multicentre, open-label, randomized phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar]
- Solomon, B.J.; Mok, T.; Kim, D.W.; Wu, Y.L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. PROFILE 1014 Investigators. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef]
- Wu, Y.L.; Lu, S.; Lu, Y.; Zhou, J.; Shi, Y.K.; Sriuranpong, V.; Ho, J.C.M.; Ong, C.K.; Tsai, C.M.; Chung, C.H.; et al. Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib versus Chemotherapy in East Asian Patients with ALK-Positive Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 1539–1548. [Google Scholar] [CrossRef]
- Iwama, E.; Goto, Y.; Murakami, H.; Harada, T.; Tsumura, S.; Sakashita, H.; Mori, Y.; Nakagaki, N.; Fujita, Y.; Seike, M.; et al. Alectinib for Patients with ALK Rearrangement-Positive Non-Small Cell Lung Cancer and a Poor Performance Status (Lung Oncology Group in Kyushu 1401). J. Thorac. Oncol. 2017, 12, 1161–1166. [Google Scholar] [CrossRef]
- Maemondo, M.; Minegishi, Y.; Inoue, A.; Kobayashi, K.; Harada, M.; Okinaga, S.; Morikawa, N.; Oizumi, S.; Tanaka, T.; Isobe, H.; et al. First-line gefitinib in patients aged 75 or older with advanced non-small cell lung cancer harboring epidermal growth factor receptor mutations: NEJ 003 study. J. Thorac. Oncol. 2012, 7, 1417–1422. [Google Scholar] [CrossRef]
- Baggstrom, M.Q.; Stinchcombe, T.E.; Fried, D.B.; Poole, C.; Hensing, T.A.; Socinski, M.A. Third-generation chemotherapy agents in the treatment of advanced non-small cell lung cancer: A meta-analysis. J. Thorac. Oncol. 2007, 2, 845–853. [Google Scholar] [CrossRef]
- Lilenbaum, R.C.; Herndon, J.E., II; List, M.A.; Desch, C.; Watson, D.M.; Miller, A.A.; Graziano, S.L.; Perry, M.C.; Saville, W.; Chahinian, P.; et al. Single-agent versus combination chemotherapy in advanced non-small cell lung cancer: The cancer and leukemia group B (study 9730). J. Clin. Oncol. 2005, 23, 190–196. [Google Scholar] [CrossRef]
- Kosmidis, P.A.; Dimopoulos, M.A.; Syrigos, K.; Nicolaides, C.; Aravantinos, G.; Boukovinas, I.; Pectasides, D.; Fountzilas, G.; Bafaloukos, D.; Bacoyiannis, C.; et al. Gemcitabine versus gemcitabine-carboplatin for patients with advanced non-small cell lung cancer and a performance status of 2: A prospective randomized phase II study of the Hellenic Cooperative Oncology Group. J. Thorac. Oncol. 2007, 2, 135–140. [Google Scholar] [CrossRef]
- Langer, C.; Li, S.; Schiller, J.; Tester, W.; Rapoport, B.L.; Johnson, D.H.; Eastern Cooperative Oncology Group. Randomized phase II trial of paclitaxel plus carboplatin or gemcitabine plus cisplatin in Eastern Cooperative Oncology Group performance status 2 non-small cell lung cancer patients: ECOG 1599. J. Clin. Oncol. 2007, 25, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.; Obasaju, C.; Schell, M.J.; Li, X.; Zheng, Z.; Boulware, D.; Caton, J.R.; Demarco, L.C.; O’Rourke, M.A.; Shaw Wright, G.; et al. Randomized phase III trial of gemcitabine-based chemotherapy with in situ RRM1 and ERCC1 protein levels for response prediction in non-small cell lung cancer. J. Clin. Oncol. 2009, 27, 5808–5815. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Nakagawa, K.; Takeda, K.; Iwamoto, Y.; Ando, M.; Maeda, M.; Katakami, N.; Nakano, T.; Kurata, T.; Fukuoka, M. Randomized phase II study of carboplatin-paclitaxel or gemcitabine-vinorelbine in patients with advanced nonsmall cell lung cancer and a performance status of 2: West Japan Thoracic Oncology Group 0004. Am. J. Clin. Oncol. 2012, 35, 58–63. [Google Scholar] [CrossRef]
- Morabito, A.; Gebbia, V.; Di Maio, M.; Cinieri, S.; Viganò, M.G.; Bianco, R.; Barbera, S.; Cavanna, L.; De Marinis, F.; Montesarchio, V.; et al. Randomized phase III trial of gemcitabine and cisplatin vs. gemcitabine alone in patients with advanced non-small cell lung cancer and a performance status of 2: The CAPPA-2 study. Lung Cancer 2013, 81, 77–83. [Google Scholar] [CrossRef]
- Zukin, M.; Barrios, C.H.; Pereira, J.R.; Ribeiro, R.D.A.; Beato, C.A.; do Nascimento, Y.N.; Murad, A.; Franke, F.A.; Precivale, M.; Araujo, L.H.; et al. Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J. Clin. Oncol. 2013, 31, 2849–2853. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Ardizzoni, A.; Ciuleanu, T.; Cobo, M.; Laktionov, K.; Szilasi, M.; Califano, R.; Carcereny, E.; Griffiths, R.; Paz-Ares, L.; et al. CheckMate 171: A phase 2 trial of nivolumab in patients with previously treated advanced squamous non-small cell lung cancer, including ECOG PS 2 and elderly populations. Eur. J. Cancer. 2020, 127, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; McCleod, M.; Jotte, R.M.; Einhorn, L.; Horn, L.; Waterhouse, D.M.; Creelan, B.; Babu, S.; Leighl, N.B.; Chandler, J.C.; et al. Safety, Efficacy, and Patient-Reported Health-Related Quality of Life and Symptom Burden with Nivolumab in Patients with Advanced Non-Small Cell Lung Cancer, Including Patients Aged 70 Years or Older or with Poor Performance Status (CheckMate 153). J. Thorac. Oncol. 2019, 14, 1628–1639. [Google Scholar] [CrossRef] [PubMed]
- Ardizzoni, A.; Azevedo, S.; Rubio-Viqueira, B.; Rodríguez-Abreu, D.; Alatorre-Alexander, J.; Smit, H.J.M.; Yu, J.; Syrigos, K.; Trunzer, K.; Patel, H.; et al. Primary results from TAIL: A global single-arm safety study of atezolizumab monotherapy in a diverse population of patients with previously treated advanced non-small cell lung cancer. J. Immunother. Cancer 2021, 9, e001865. [Google Scholar] [CrossRef]
- Middleton, G.; Brock, K.; Savage, J.; Mant, R.; Summers, Y.; Connibear, J.; Shah, R.; Ottensmeier, C.; Shaw, P.; Lee, S.M.; et al. Pembrolizumab in patients with non-small cell lung cancer of performance status 2 (PePS2): A single arm, phase 2 trial. Lancet Respir. Med. 2020, 8, 895–904. [Google Scholar] [CrossRef]
- Lee, S.M.; Schulz, C.; Prabhash, K.; Han, B.; Szczesna, A.; Cortinovis, D.; Rittmeyer, A.; Vicente, D.; Califano, R.; Le, A.T.; et al. IPSOS: Results from a phase III study of first-line (1L) atezolizumab (atezo) vs. single-agent chemotherapy (chemo) in patients (pts) with NSCLC not eligible for a platinum-containing regimen. Ann. Oncol. 2022, 33, S808–S869. [Google Scholar] [CrossRef]
- Alessi, J.V.; Ricciuti, B.; Jiménez-Aguilar, E.; Hong, F.; Wei, Z.; Nishino, M.; Plodkowski, A.J.; Sawan, P.; Luo, J.; Rizvi, H. Outcomes to first-line pembrolizumab in patients with PD-L1-high (≥50%) non-small cell lung cancer and a poor performance status. J. Immunother. Cancer. 2020, 8, e001007. [Google Scholar] [CrossRef]
- Gadgeel, S.; Rodríguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Dómine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Impower150 Study Group. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Vicente, D.; Tafreshi, A.; Robinson, A.; Soto Parra, H.; Mazières, J.; Hermes, B.; Cicin, I.; Medgyasszay, B.; Rodríguez-Cid, J.; et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients with Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J. Thorac. Oncol. 2020, 15, 1657–1669. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Ciuleanu, T.E.; Lee, J.S.; Schenker, M.; Audigier-Valette, C.; Zurawski, B.; Linardou, H.; Otterson, G.A.; Salman, P.; Nishio, M.; et al. First-Line Nivolumab Plus Ipilimumab Versus Chemotherapy in Advanced NSCLC With 1% or Greater Tumor PD-L1 Expression: Patient-Reported Outcomes From CheckMate 227 Part 1. J. Thorac. Oncol. 2021, 16, 665–676. [Google Scholar] [CrossRef]
- Paz-Ares, L.G.; Ciuleanu, T.; Pluzanski, A.; Lee, J.S.; Gainor, J.F.; Otterson, G.A.; Audigier-Valette, C.; Ready, N.; Schenker, M.; Linardou, H.; et al. Safety of First-line Nivolumab Plus Ipilimumab in Patients with Metastatic Non-Small Cell Lung Cancer: A Pooled Analysis of CheckMate 227, CheckMate 568, and CheckMate 817. J. Thorac. Oncol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Lena, H.; Monnet, I.; Olivier, O.; Audigier-Valette, C.; Falchero, L.; Vergnenegre, A.; Demontrond, P.; Greillier, L.; Geier, M.; Guisier, F.; et al. Randomized phase III study of nivolumab and ipilimumab versus carboplatin-based doublet in first-line treatment of PS 2 or elderly (≥70 years) patients with advanced non–small cell lung cancer (Energy-GFPC 06-2015 study). J. Clin. Oncol. 2022, 40, 9011. [Google Scholar] [CrossRef]
- Facchinetti, F.; Mazzaschi, G.; Barbieri, F.; Passiglia, F.; Mazzoni, F.; Berardi, R.; Proto, C.; Cecere, F.L.; Pilotto, S.; Scotti, V.; et al. First-line pembrolizumab in advanced non-small cell lung cancer patients with poor performance status. Eur. J. Cancer 2020, 130, 155–167. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Fujieda, K.; Miyashita, A.; Fukushima, S.; Ikeda, T.; Kubo, Y.; Senju, S.; Ihn, H.; Nishimura, Y.; Oshiumi, H. Combined Blockade of IL6 and PD-1/PD-L1 Signaling Abrogates Mutual Regulation of Their Immunosuppressive Effects in the Tumor Microenvironment. Cancer Res. 2018, 78, 5011–5022. [Google Scholar] [CrossRef]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Lucia, A.; Ramírez, M. Muscling in on Cancer. N. Engl. J. Med. 2016, 375, 892–894. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Scharping, N.E.; Menk, A.V.; Moreci, R.S.; Whetstone, R.D.; Dadey, R.E.; Watkins, S.C.; Ferris, R.L.; Delgoffe, G.M. The Tumor Microenvironment Represses T Cell Mitochondrial Biogenesis to Drive Intratumoral T Cell Metabolic Insufficiency and Dysfunction. Immunity 2016, 45, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Chamoto, K.; Chowdhury, P.S.; Kumar, A.; Sonomura, K.; Matsuda, F.; Fagarasan, S.; Honjo, T. Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. Proc. Natl. Acad. Sci. USA 2017, 114, E761–E770. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef]
- Wang, Z.; Yip, L.Y.; Lee, J.H.J.; Wu, Z.; Chew, H.Y.; Chong, P.K.W.; Teo, C.C.; Ang, H.Y.; Peh, K.L.E.; Yuan, J.; et al. Methionine is a metabolic dependency of tumor-initiating cells. Nat. Med. 2019, 25, 825–837. [Google Scholar] [CrossRef]
- Fujio, T.; Nakashima, K.; Naito, T.; Kobayashi, H.; Omori, S.; Wakuda, K.; Ono, A.; Kenmotsu, H.; Murakami, H.; Takahashi, T. Platinum Combination Chemotherapy Is Poorly Tolerated in Malnourished Advanced Lung Cancer Patients with Poor Performance Status. Nutr. Cancer 2019, 71, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Antoun, S.; Morel, H.; Souquet, P.J.; Surmont, V.; Planchard, D.; Bonnetain, F.; Foucher, P.; Egenod, T.; Krakowski, I.; Gaudin, H.; et al. Staging of nutrition disorders in non-small cell lung cancer patients: Utility of skeletal muscle mass assessment. J. Cachexia Sarcopenia Muscle 2019, 10, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, T.; Naito, T.; Kodama, A.; Nishioka, N.; Miyawaki, E.; Mamesaya, N.; Kawamura, T.; Kobayashi, H.; Omori, S.; Wakuda, K.; et al. Desensitizing Effect of Cancer Cachexia on Immune Checkpoint Inhibitors in Patients with Advanced NSCLC. JTO Clin. Res. Rep. 2020, 1, 100020. [Google Scholar] [CrossRef]
- Ross, P.J.; Ashley, S.; Norton, A.; Priest, K.; Waters, J.S.; Eisen, T.; Smith, I.E.; O’Brien, M.E. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br. J. Cancer 2004, 90, 1905–1911. [Google Scholar] [CrossRef]
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280. [Google Scholar] [CrossRef]
- Wei, S.C.; Levine, J.H.; Cogdill, A.P.; Zhao, Y.; Anang, N.A.S.; Andrews, M.C.; Sharma, P.; Wang, J.; Wargo, J.A.; Pe’er, D.; et al. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell 2017, 170, 1120–1133. [Google Scholar] [CrossRef]
- Fane, M.; Weeraratna, A.T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 2020, 20, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.M.; Lenardo, M.J. Development of immune checkpoint therapy for cancer. J. Exp. Med. 2019, 216, 1244–1254. [Google Scholar] [CrossRef]
- Katakami, N.; Uchino, J.; Yokoyama, T.; Naito, T.; Kondo, M.; Yamada, K.; Kitajima, H.; Yoshimori, K.; Sato, K.; Saito, H.; et al. Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: Results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer 2018, 124, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Li, W.G.; Gavrila, D.; Liu, X.; Wang, L.; Gunnlaugsson, S.; Stoll, L.L.; McCormick, M.L.; Sigmund, C.D.; Tang, C.; Weintraub, N.L. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation 2004, 109, 2221–2226. [Google Scholar] [CrossRef] [PubMed]
- Baatar, D.; Patel, K.; Taub, D.D. The effects of ghrelin on inflammation and the immune system. Mol. Cell. Endocrinol. 2011, 340, 44–58. [Google Scholar] [CrossRef]
- Kurz, E.; Hirsch, C.A.; Dalton, T.; Shadaloey, S.A.; Khodadadi-Jamayran, A.; Miller, G.; Pareek, S.; Rajaei, H.; Mohindroo, C.; Baydogan, S.; et al. Exercise-induced engagement of the IL-15/IL-15Rα axis promotes anti-tumor immunity in pancreatic cancer. Cancer Cell 2022, 40, 720–737. [Google Scholar] [CrossRef]
- Gomes-Santos, I.L.; Amoozgar, Z.; Kumar, A.S.; Ho, W.W.; Roh, K.; Talele, N.P.; Curtis, H.; Kawaguchi, K.; Jain, R.K.; Fukumura, D. Exercise Training Improves Tumor Control by Increasing CD8+ T-cell Infiltration via CXCR3 Signaling and Sensitizes Breast Cancer to Immune Checkpoint Blockade. Cancer Immunol. Res. 2021, 9, 765–778. [Google Scholar] [CrossRef]
| Heading | Phase | Regimen | N | Median OS (Month) | 1-Year OS (%) | Median PFS (Month) | ORR (%) | Ref |
|---|---|---|---|---|---|---|---|---|
| Lienbaum et al. (CALGB 9730) | III | PTX | 50 | 2.4 | 10 | - | 10 | [12] |
| CBDCA + PTX | 49 | 4.7 | 18 | - | 24 | |||
| Kosmidis et al. | II | GEM | 47 | 4.8 | 18 | 3 | 4 | [13] |
| CBDCA + GEM | 43 | 6.7 | 20 | 4.1 | 14 | |||
| Langer et al. (ECOG 1599) | II | CBDCA + PTX | 49 | 6.2 | 19 | 3.5 | 14 | [14] |
| CDDP + GEM | 54 | 6.9 | 25 | 3 | 23 | |||
| Reynolds et al. | III | GEM | 85 | 5.1 | 21 | 2.7 | 16 | [15] |
| CBDCA + GEM | 85 | 6.7 | 31 | 3.8 | 43 | |||
| Saito et al. (WJTOG0004) | II | GEM + VNR | 43 | 6.0 | 28 | 2.7 | 21 | [16] |
| CBDCA + PTX | 41 | 5.9 | 22 | 2.9 | 29 | |||
| Morabito et al. (CAPPA-2) | III | GEM | 28 | 3.0 | NR | 1.7 | 4 | [17] |
| CDDP + GEM | 28 | 5.9 | NR | 3.3 | 18 | |||
| Zukin et al. | III | PEM | 102 | 5.3 | 22 | 2.8 | 11 | [18] |
| CBDCA + PEM | 103 | 9.3 | 40 | 5.8 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, S.; Naito, T.; Miura, S.; Ito, K.; Furuya, N.; Misumi, T.; Ogura, T.; Kato, T. Pharmacotherapy for Advanced Non-Small Cell Lung Cancer with Performance Status 2 without Druggable Gene Alterations: Could Immune Checkpoint Inhibitors Be a Game Changer? Cancers 2022, 14, 4861. https://doi.org/10.3390/cancers14194861
Ikeda S, Naito T, Miura S, Ito K, Furuya N, Misumi T, Ogura T, Kato T. Pharmacotherapy for Advanced Non-Small Cell Lung Cancer with Performance Status 2 without Druggable Gene Alterations: Could Immune Checkpoint Inhibitors Be a Game Changer? Cancers. 2022; 14(19):4861. https://doi.org/10.3390/cancers14194861
Chicago/Turabian StyleIkeda, Satoshi, Tateaki Naito, Satoru Miura, Kentaro Ito, Naoki Furuya, Toshihiro Misumi, Takashi Ogura, and Terufumi Kato. 2022. "Pharmacotherapy for Advanced Non-Small Cell Lung Cancer with Performance Status 2 without Druggable Gene Alterations: Could Immune Checkpoint Inhibitors Be a Game Changer?" Cancers 14, no. 19: 4861. https://doi.org/10.3390/cancers14194861
APA StyleIkeda, S., Naito, T., Miura, S., Ito, K., Furuya, N., Misumi, T., Ogura, T., & Kato, T. (2022). Pharmacotherapy for Advanced Non-Small Cell Lung Cancer with Performance Status 2 without Druggable Gene Alterations: Could Immune Checkpoint Inhibitors Be a Game Changer? Cancers, 14(19), 4861. https://doi.org/10.3390/cancers14194861







