Should the Splenic Vein Be Preserved—Fate of Sinistral Portal Hypertension after Pancreatoduodenectomy with Vascular Re-Section for Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Enrolled Patients
2.2. Clinicopathological Data Collection
2.3. Imaging Assessment
2.4. Prediction Model for Postoperative Variceal Formation
2.5. Comparison of Varix-Related Parameters and Oncologic Outcomes According to SV Status
2.6. Statistics
3. Results
3.1. Clinicopathological Characteristics
3.2. Prediction Model for Postoperative Variceal Formation
3.3. Characteristics between SV Saving and SV Ligation
3.4. Comparison of Varix-Related Parameters According to SV Status
3.5. Oncologic Outcomes According to SV Status and SPH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Millikan, K.W.; Deziel, D.J.; Silverstein, J.C.; Kanjo, T.M.; Christein, J.D.; Doolas, A.; Prinz, R.A. Prognostic factors associated with resectable adenocarcinoma of the head of the pancreas. Am. Surg. 1999, 65, 618–623, discussion 623–614. [Google Scholar] [PubMed]
- Sadot, E.; Doussot, A.; O’Reilly, E.M.; Lowery, M.A.; Goodman, K.A.; Do, R.K.; Tang, L.H.; Gönen, M.; D’Angelica, M.I.; DeMatteo, R.P.; et al. FOLFIRINOX Induction Therapy for Stage 3 Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2015, 22, 3512–3521. [Google Scholar] [CrossRef] [PubMed]
- Janssen, Q.P.; Buettner, S.; Suker, M.; Beumer, B.R.; Addeo, P.; Bachellier, P.; Bahary, N.; Bekaii-Saab, T.; Bali, M.A.; Besselink, M.G.; et al. Neoadjuvant FOLFIRINOX in Patients With Borderline Resectable Pancreatic Cancer: A Systematic Review and Patient-Level Meta-Analysis. J. Natl. Cancer Inst. 2019, 111, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef]
- Kim, M.; Kang, T.W.; Cha, D.I.; Kim, Y.K.; Kim, S.H.; Jang, K.T.; Han, I.W.; Sohn, I. Prediction and clinical implications of portal vein/superior mesenteric vein invasion in patients with resected pancreatic head cancer: The significance of preoperative CT parameters. Clin. Radiol. 2018, 73, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Matsueda, K.; Koga, R.; Takahashi, Y.; Arita, J.; Takahashi, M.; Inoue, Y.; Unno, T.; Saiura, A. Sinistral portal hypertension after pancreaticoduodenectomy with splenic vein ligation. Br. J. Surg. 2015, 102, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Ito, H.; Ono, Y.; Matsueda, K.; Mise, Y.; Ishizawa, T.; Inoue, Y.; Takahashi, Y.; Hiratsuka, M.; Unno, T.; et al. Impact of portal vein resection with splenic vein reconstruction after pancreatoduodenectomy on sinistral portal hypertension: Who needs reconstruction? Surgery 2019, 165, 291–297. [Google Scholar] [CrossRef]
- Ono, Y.; Inoue, Y.; Kato, T.; Matsueda, K.; Oba, A.; Sato, T.; Ito, H.; Saiura, A.; Takahashi, Y. Sinistral Portal Hypertension after Pancreaticoduodenectomy with Splenic Vein Resection: Pathogenesis and Its Prevention. Cancers 2021, 13, 5334. [Google Scholar] [CrossRef]
- Gyoten, K.; Mizuno, S.; Nagata, M.; Ogura, T.; Usui, M.; Isaji, S. Significance of Simultaneous Splenic Artery Resection in Left-Sided Portal Hypertension After Pancreaticoduodenectomy with Combined Portal Vein Resection. World J. Surg. 2017, 41, 2111–2120. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C. AJCC Cancer Staging Manual; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- Al-Asari, S.F.; Lim, D.; Min, B.S.; Kim, N.K. The relation between inferior mesenteric vein ligation and collateral vessels to splenic flexure: Anatomical landmarks, technical precautions and clinical significance. Yonsei Med. J. 2013, 54, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, J.M.; Choi, J.Y.; Suh, K.S.; Yi, N.J.; Han, J.K.; Choi, B.I. Changes of portosystemic collaterals and splenic volume on CT after liver transplantation and factors influencing those changes. AJR Am. J. Roentgenol. 2008, 191, W8–W16. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.J.; Taylor, M.A.; McKie, L.D.; Diamond, T. Sinistral portal hypertension. Ulster Med. J. 2006, 75, 175–177. [Google Scholar] [PubMed]
- Addeo, P.; De Mathelin, P.; Averous, G.; Tambou-Nguipi, M.; Terrone, A.; Schaaf, C.; Dufour, P.; Bachellier, P. The left splenorenal venous shunt decreases clinical signs of sinistral portal hypertension associated with splenic vein ligation during pancreaticoduodenectomy with venous resection. Surgery 2020, 168, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Fujii, T.; Yamada, S.; Inokawa, Y.; Suenaga, M.; Takami, H.; Kanda, M.; Sugimoto, H.; Nomoto, S.; Murotani, K.; et al. Significance of the Splenic Vein and Its Branches in Pancreatoduodenectomy with Resection of the Portal Vein System. Dig. Surg. 2015, 32, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Kato, H.; Yamaue, H.; Fujii, T.; Satoi, S.; Saiura, A.; Murakami, Y.; Sho, M.; Yamamoto, M.; Isaji, S. Left-sided Portal Hypertension After Pancreaticoduodenectomy With Resection of the Portal Vein/Superior Mesenteric Vein Confluence in Patients With Pancreatic Cancer: A Project Study by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. Ann. Surg. 2021, 274, e36–e44. [Google Scholar] [CrossRef] [PubMed]
- Rosado, I.D.; Bhalla, S.; Sanchez, L.A.; Fields, R.C.; Hawkins, W.G.; Strasberg, S.M. Pattern of Venous Collateral Development after Splenic Vein Occlusion in an Extended Whipple Procedure (Whipple at the Splenic Artery) and Long-Term Results. J. Gastrointest. Surg. 2017, 21, 516–526. [Google Scholar] [CrossRef]
- Shiihara, M.; Higuchi, R.; Izumo, W.; Yazawa, T.; Uemura, S.; Furukawa, T.; Yamamoto, M. Retrospective evaluation of risk factors of postoperative varices after pancreaticoduodenectomy with combined portal vein resection. Pancreatology 2020, 20, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Nakao, A.; Oshima, K.; Iede, K.; Oshima, Y.; Kobayashi, H.; Kimura, Y. Splenic vein reconstruction is unnecessary in pancreatoduodenectomy combined with resection of the superior mesenteric vein-portal vein confluence according to short-term outcomes. HPB 2017, 19, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Yamada, D.; Takahashi, H.; Hama, N.; Toshiyama, R.; Asukai, K.; Hasegawa, S.; Wada, H.; Sakon, M.; Ishikawa, O. The clinical impact of splenic artery ligation on the occurrence of digestive varices after pancreaticoduodenectomy with combined portal vein resection: A retrospective study in two institutes. Langenbecks Arch. Surg. 2021, 406, 1469–1479. [Google Scholar] [CrossRef]
- Janssen, Q.P.; O’Reilly, E.M.; van Eijck, C.H.J.; Groot Koerkamp, B. Neoadjuvant Treatment in Patients With Resectable and Borderline Resectable Pancreatic Cancer. Front. Oncol. 2020, 10, 41. [Google Scholar] [CrossRef]
- Mauro, E.; Gadano, A. What’s new in portal hypertension? Liver Int. 2020, 40 (Suppl. 1), 122–127. [Google Scholar] [CrossRef] [PubMed]
- da Costa, W.L., Jr.; Tran Cao, H.S.; Gu, X.; Massarweh, N.N. Bayesian Approach to Understand the Association Between Treatment Down-staging and Survival for Patients With Pancreatic Adenocarcinoma. Ann. Surg. 2022, 275, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Karakayali, H.; Boyvat, F.; Coskun, M.; Isiklar, I.; Sözen, H.; Filik, L.; Yilmaz, U.; Gür, G.; Haberal, M. Venous complications after orthotopic liver transplantation. Transplant. Proc. 2006, 38, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Kuter, D.J. Managing thrombocytopenia associated with cancer chemotherapy. Oncology 2015, 29, 282–294. [Google Scholar]
| n = 94 | |
|---|---|
| Age (years) | 62.2 ± 9.1 |
| Sex (M:F) | 52:42 |
| BMI (kg/m2) | 22.8 ± 3.1 |
| Liver diseases | 6 (6.4%) |
| CA 19-9 (U/mL) | 98 (15–416) |
| Platelet count (103/uL) | 249 ± 87 |
| Splenic volume (cc) | 156 ± 101 |
| Neoadjuvant chemotherapy | 45 (47.9%) |
| IMV insertion type | |
| Type I | 8 (8.5%) |
| Type II | 38 (40.4%) |
| Type III | 48 (51.1%) |
| Postoperative PV or SMV stricture | 26 (27.7%) |
| Vascular resection method | |
| Tangential resection | 29 (30.9%) |
| Segmental resection | 65 (69.1%) |
| Splenic vein status | |
| Splenic vein ligation | 50 (53.2%) |
| Splenic vein saving | 44 (46.8%) |
| IMV ligation | 34 (36.2%) |
| Operation time (min) | 487 ± 116 |
| Blood loss (mL) | 888 ± 613 |
| Complication (CDC ≥ III) | 12 (12.8%) |
| POPF (≥Grade B) | 2 (2.1%) |
| Ascites | 12 (12.8%) |
| Thrombosis | 5 (5.3%) |
| R1 resection | 17 (18.1%) |
| T stage | |
| 0 | 6 (6.4%) |
| 1 | 30 (31.9%) |
| 2 | 54 (57.4%) |
| 3 | 4 (4.3%) |
| N stage | |
| 0 | 43 (45.7%) |
| 1 | 39 (41.5%) |
| 2 | 12 (12.8%) |
| TNM stage | |
| 0 | 6 (6.4%) |
| I | 36 (38.3%) |
| II | 40 (42.6%) |
| III | 12 (12.8%) |
| Adjuvant chemotherapy | 74 (78.7%) |
| Variceal formation | 22 (23.4%) |
| Variceal bleeding | 2 (2.1%) |
| Median follow-up (months) | 25 (16–37) |
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95%CI | p Value | OR | 95%CI | p Value | ||
| IMV insertion type | Type I | 0.93 | 0.35–2.51 | 0.931 | |||
| Type II | 0.43 | 0.05–3.85 | 0.429 | ||||
| Type III | (reference) | ||||||
| IMV ligation | 4.25 | 1.55–11.63 | 0.005 | ||||
| SV ligation | 4.29 | 1.54–11.91 | 0.005 | 4.56 | 1.53–13.00 | 0.006 | |
| Postoperative PV or SMV stricture | 3.80 | 1.38–10.44 | 0.010 | 3.98 | 1.36–11.69 | 0.012 | |
| Vascular resection method | SR | 6.00 | 1.30–27.71 | 0.022 | |||
| TR | (reference) | ||||||
| SV Saving (n = 36) | SV Ligation (n = 32) | p Value | |
|---|---|---|---|
| Age (years) | 62.5 ± 9.2 | 62.9 ± 9.0 | 0.866 |
| Sex (M:F) | 20:16 | 14:18 | 0.466 |
| BMI (kg/m2) | 23.4 ± 3.2 | 22.5 ± 2.3 | 0.191 |
| Liver diseases | 5 (13.9%) | 1 (3.1%) | 0.257 |
| CA 19-9 (U/mL) | 73 [10–391] | 86 [18–276] | 0.907 |
| Neoadjuvant chemotherapy | 15 (41.7%) | 15 (46.9%) | 0.852 |
| IMV insertion type | 0.600 | ||
| Type I | 2 (5.6%) | 3 (9.4%) | |
| Type II | 17 (47.2%) | 11 (34.4%) | |
| Type III | 17 (47.2%) | 18 (56.3%) | |
| IMV ligation | 0 (0.0%) | 23 (71.9%) | <0.001 |
| Vascular resection type | <0.001 | ||
| Tangential resection | 24 (66.7%) | 1 (3.1%) | |
| Segmental resection | 12 (33.3%) | 31 (96.9%) | |
| Operation time (min) | 453 ± 93 | 510 ± 117 | 0.028 |
| Blood loss (mL) | 843 ± 645 | 978 ± 622 | 0.385 |
| Complication (CDC≥III) | 5 (13.9%) | 5 (15.6%) | >0.999 |
| POPF (≥Grade B) | 1 (2.8%) | 1 (3.1%) | >0.999 |
| Ascites | 4 (11.1%) | 4 (12.5%) | >0.999 |
| Thrombosis | 2 (5.6%) | 1 (3.1%) | >0.999 |
| R1 resection | 7 (19.4%) | 6 (18.8%) | >0.999 |
| T stage | 0.967 | ||
| 0 | 2 (5.6%) | 1 (3.1%) | |
| 1 | 12 (33.3%) | 11 (34.4%) | |
| 2 | 21 (58.3%) | 18 (56.3%) | |
| 3 | 1 (2.8%) | 2 (6.3%) | |
| N stage | 0.514 | ||
| 0 | 14 (38.9%) | 13 (40.6%) | |
| 1 | 15 (41.7%) | 16 (50.0%) | |
| 2 | 7 (19.4%) | 3 (9.4%) | |
| TNM stage | 0.655 | ||
| 0 | 2 (5.6%) | 1 (3.1%) | |
| I | 12 (33.3%) | 12 (37.5%) | |
| II | 15 (41.7%) | 16 (50.0%) | |
| III | 7 (19.4%) | 3 (9.4%) | |
| Adjuvant chemotherapy | 31 (86.1%) | 25 (78.1%) | 0.587 |
| Variceal formation | 2 (5.6%) | 9 (28.1%) | 0.028 |
| Variceal bleeding | 0 (0.0%) | 1 (3.1%) | 0.953 |
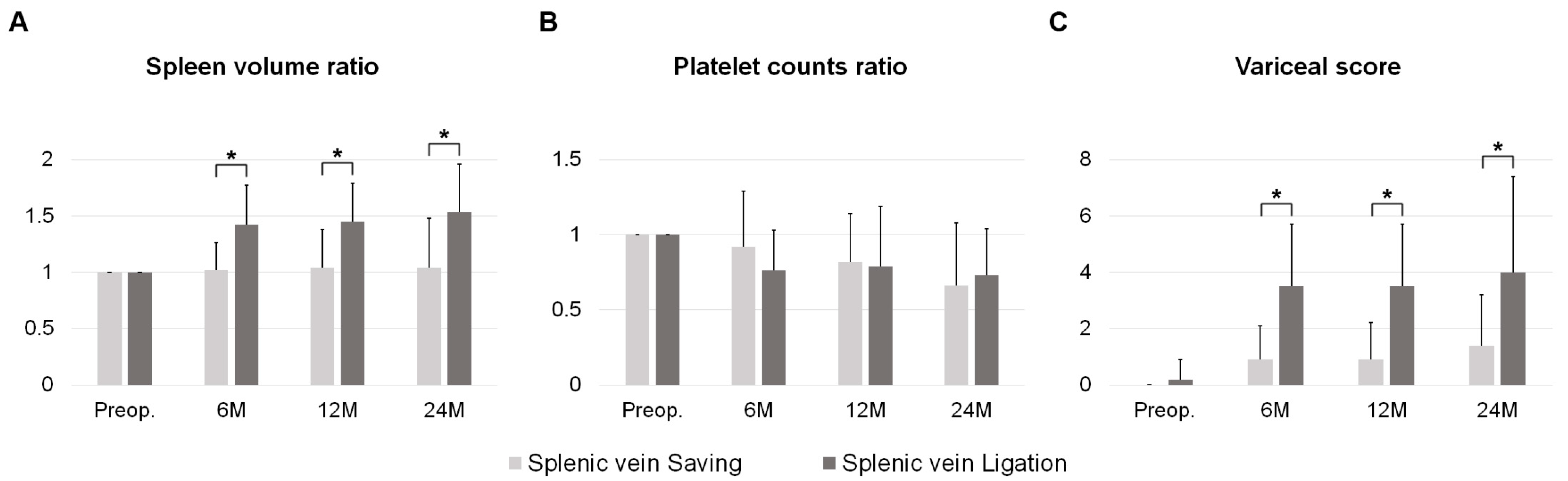
| Parameters | SV Saving (n = 36) | SV Ligation (n = 32) | p Value | |
|---|---|---|---|---|
| Preop. | Splenic volume (cc) | 149 ± 71 | 150 ± 68 | 0.938 |
| Platelet counts (103/uL) | 269 ± 96 | 227 ± 64 | 0.038 | |
| Platelet count < 100K | 0 (0.0%) | 0 (0.0%) | 1.000 | |
| Variceal score | 0.0 ± 0.0 | 0.2 ± 0.7 | 0.091 | |
| 6M | Splenic volume (cc) | 149 ± 78 | 213 ± 108 | 0.007 |
| Splenic volume (ratio) | 1.02 ± 0.24 | 1.42 ± 0.35 | <0.001 | |
| Platelet count (103/uL) | 227 ± 76 | 166 ± 54 | <0.001 | |
| Platelet count (ratio) | 0.92 ± 0.37 | 0.76 ± 0.27 | 0.059 | |
| Platelet count < 100K | 0 (0.0%) | 2 (6.3%) | 0.422 | |
| Variceal score | 0.9 ± 1.2 | 3.5 ± 2.2 | <0.001 | |
| 12M | Splenic volume (cc) | 137 ± 61 | 218 ± 114 | 0.002 |
| Splenic volume (ratio) | 1.04 ± 0.34 | 1.45 ± 0.34 | <0.001 | |
| Platelet count (103/uL) | 205 ± 56 | 161 ± 56 | 0.005 | |
| Platelet count < 100K | 1 (3.1%) | 5 (18.5%) | 0.129 | |
| Platelet count (ratio) | 0.82 ± 0.32 | 0.79 ± 0.40 | 0.677 | |
| Variceal score | 0.9 ± 1.3 | 3.5 ± 2.2 | <0.001 | |
| 24M | Splenic volume (cc) | 154 ± 160 | 250 ± 138 | 0.074 |
| Splenic volume (ratio) | 1.04 ± 0.44 | 1.53 ± 0.43 | 0.003 | |
| Platelet count (103/uL) | 184 ± 41 | 154 ± 54 | 0.086 | |
| Platelet count < 100K | 0 (0.0.%) | 1 (6.3%) | 0.975 | |
| Platelet count (ratio) | 0.66 ± 0.42 | 0.73 ± 0.31 | 0.583 | |
| Variceal score | 1.4 ± 1.8 | 4.0 ± 3.4 | 0.009 |
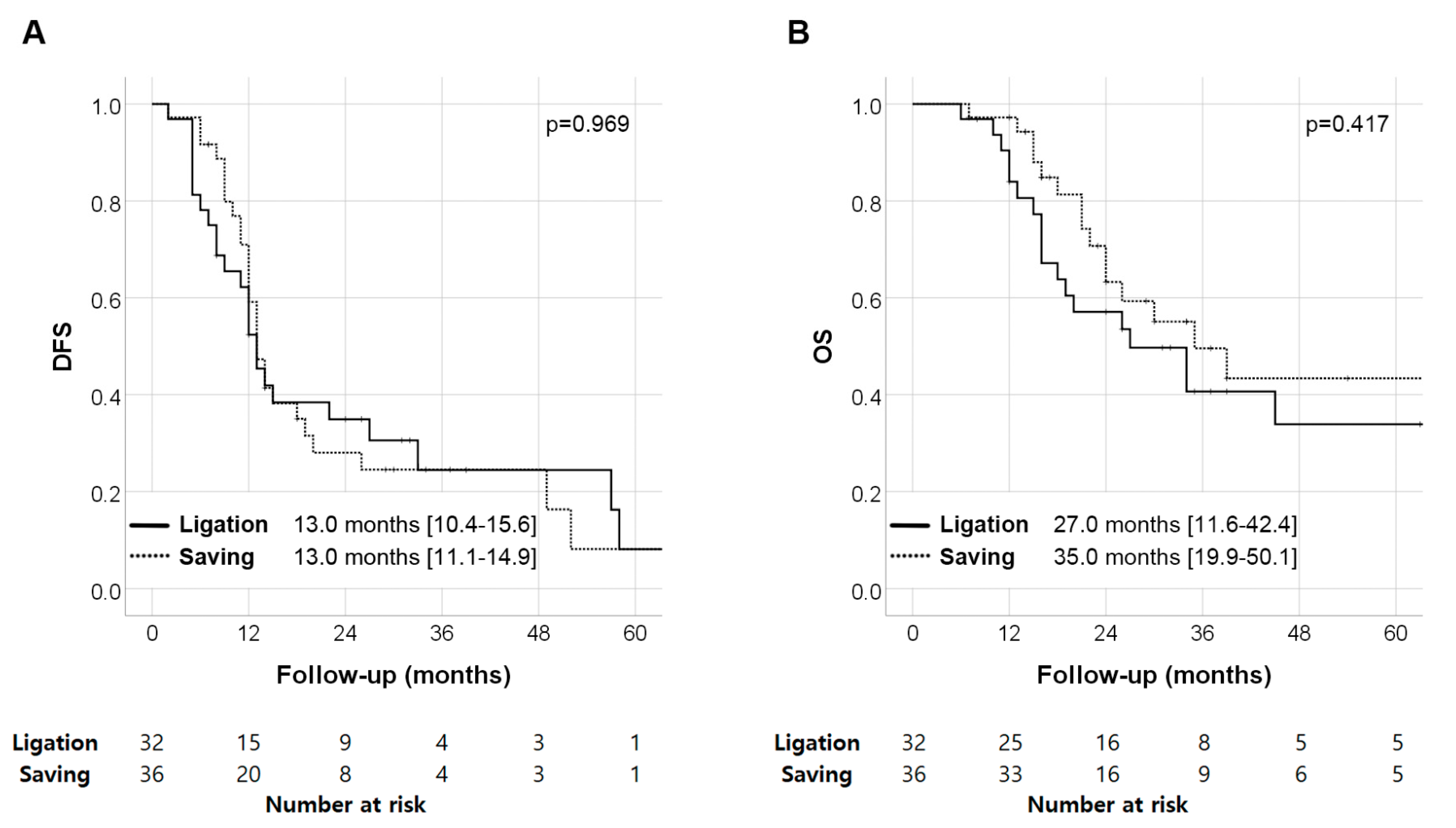
| Univariate Analysis | Multivariate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p Value | HR | 95%CI | p Value | |||
| DFS | Preop. CA 19-9 (log scale) | 1.00 | 0.78–1.26 | 0.989 | 1.02 | 0.81–1.30 | 0.845 | |
| TNM stage | III | 4.94 | 1.59–7.35 | <0.001 | 4.15 | 2.15–8.00 | <0.001 | |
| II | 3.42 | 2.74–8.2 | 0.002 | 3.09 | 1.32–7.23 | 0.009 | ||
| I | (reference) | (reference) | ||||||
| R1 resection | 1.24 | 0.74–2.10 | 0.420 | 1.35 | 0.67–2.68 | 0.396 | ||
| Adjuvant chemotherapy | 1.40 | 0.76–2.59 | 0.264 | 0.91 | 0.46–1.82 | 0.792 | ||
| Sinistral portal hypertension | 1.21 | 0.71–2.09 | 0.479 | 1.21 | 0.69–2.14 | 0.509 | ||
| OS | Preop. CA 19-9 (log scale) | 1.15 | 0.85–1.55 | 0.371 | 1.20 | 0.89–1.62 | 0.233 | |
| TNM stage | III | 4.45 | 2.42–8.19 | <0.001 | 3.32 | 1.53–7.21 | 0.002 | |
| II | 3.47 | 1.52–7.91 | 0.003 | 2.92 | 0.69–7.05 | 0.180 | ||
| I | (reference) | (reference) | ||||||
| R1 resection | 1.27 | 0.66–2.43 | 0.479 | 1.18 | 0.63–2.21 | 0.609 | ||
| Adjuvant chemotherapy | 1.31 | 0.61–2.82 | 0.495 | 0.88 | 0.38–2.03 | 0.756 | ||
| Sinistral portal hypertension | 1.32 | 0.76–2.29 | 0.328 | 1.03 | 0.62–1.73 | 0.902 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Kim, S.-S.; Hwang, H.K.; Lee, W.J.; Kang, C.M. Should the Splenic Vein Be Preserved—Fate of Sinistral Portal Hypertension after Pancreatoduodenectomy with Vascular Re-Section for Pancreatic Cancer. Cancers 2022, 14, 4853. https://doi.org/10.3390/cancers14194853
Kim SH, Kim S-S, Hwang HK, Lee WJ, Kang CM. Should the Splenic Vein Be Preserved—Fate of Sinistral Portal Hypertension after Pancreatoduodenectomy with Vascular Re-Section for Pancreatic Cancer. Cancers. 2022; 14(19):4853. https://doi.org/10.3390/cancers14194853
Chicago/Turabian StyleKim, Sung Hyun, Seung-Seob Kim, Ho Kyoung Hwang, Woo Jung Lee, and Chang Moo Kang. 2022. "Should the Splenic Vein Be Preserved—Fate of Sinistral Portal Hypertension after Pancreatoduodenectomy with Vascular Re-Section for Pancreatic Cancer" Cancers 14, no. 19: 4853. https://doi.org/10.3390/cancers14194853
APA StyleKim, S. H., Kim, S.-S., Hwang, H. K., Lee, W. J., & Kang, C. M. (2022). Should the Splenic Vein Be Preserved—Fate of Sinistral Portal Hypertension after Pancreatoduodenectomy with Vascular Re-Section for Pancreatic Cancer. Cancers, 14(19), 4853. https://doi.org/10.3390/cancers14194853






