Large Improvements in Health-Related Quality of Life and Physical Fitness during Multidisciplinary Inpatient Rehabilitation for Pediatric Cancer Survivors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Sample and Recruitment
2.2. Rehabilitation Treatment
2.3. Patient-Reported Outcomes (PROs)
2.4. Observer-Reported and Performance Outcomes
2.5. Statistical Analysis
3. Results
3.1. HRQOL Reports Prior to Rehabilitation
3.2. Improvement in HRQOL during Rehabilitation Treatment
3.2.1. Child Self-Report
3.2.2. Proxy Ratings
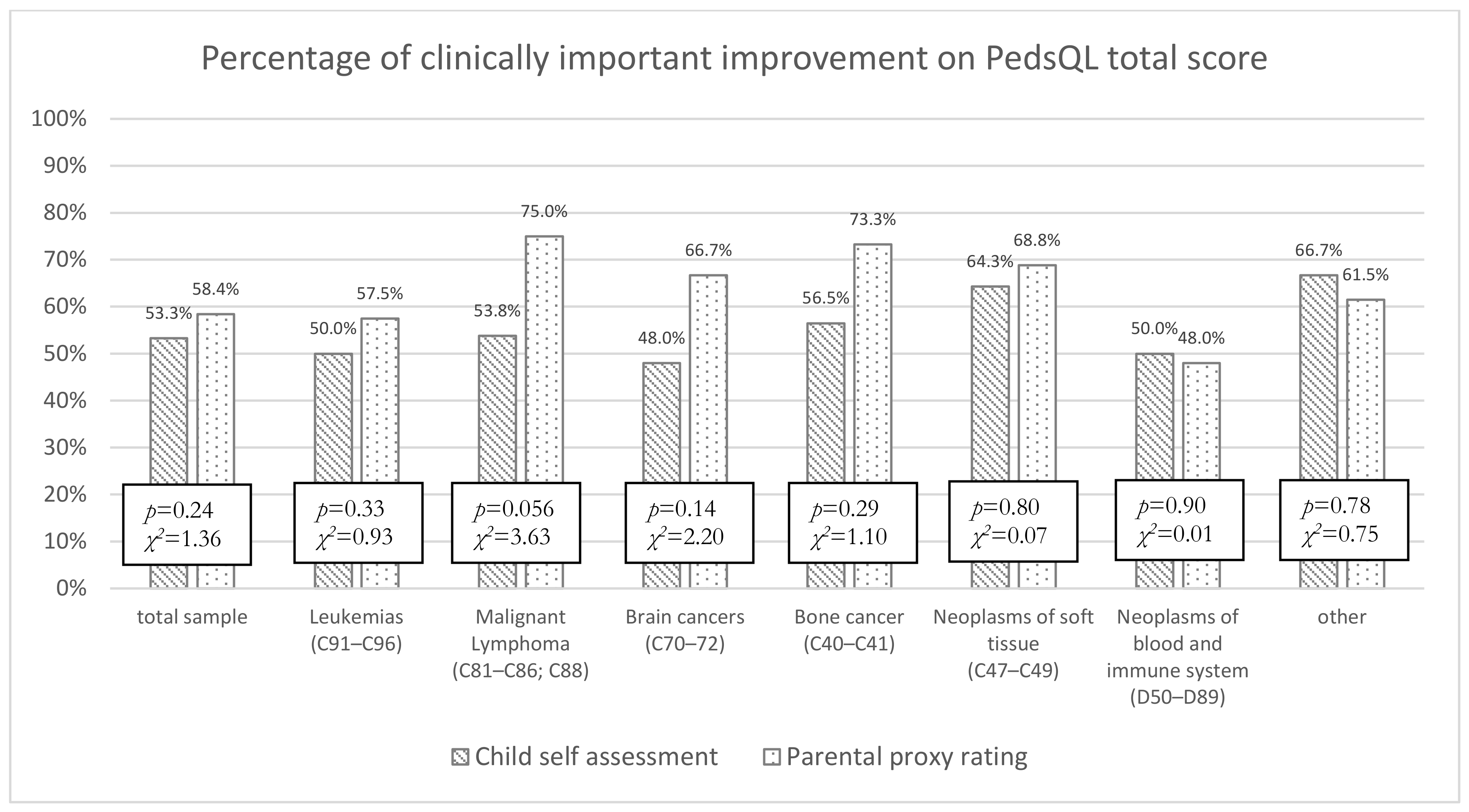
3.3. Improvement in Health Status and Goal Achievement during the Rehabilitation
3.4. Patient–Proxy Discrepancies
3.5. Accordance of Mean HRQOL Change Ratings by Children and Parents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A. IICC-3 contributors. International incidence of childhood cancer, 2001–2010: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- Gatta, G.; Botta, L.; Rossi, S.; Aareleid, T.; Bielska-Lasota, M.; Clavel, J.; Dimitrova, N.; Jakab, Z.; Kaatsch, P.; Lacour, B.; et al. Childhood cancer survival in Europe 1999–2007: Results of EUROCARE-5—a population-based study. Lancet Oncol. 2014, 15, 35–47. [Google Scholar] [CrossRef]
- Cancer Research UK. Children’s Cancer Statistics. 2018. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/childrens-cancers (accessed on 3 October 2022).
- Kaatsch, P.; Grabow, D.; Spix, C. German Childhood Cancer Registry—Annual Report 2016 (1980–2015); Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI) at the University Center of the Johannes Gutenberg University Mainz: Mainz, Germany, 2016. [Google Scholar]
- Smith, M.A.; Altekruse, S.F.; Adamson, P.C.; Reaman, G.H.; Seibel, N.L. Declining childhood and adolescent cancer mortality. Cancer 2014, 120, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.A.; King, J.; Tai, E.; Buchanan, N.; Ajani, U.A.; Li, J. Cancer incidence rates and trends among children and adolescents in the United States, 2001–2009. Pediatrics 2014, 134, e945–e955. [Google Scholar] [CrossRef]
- National Cancer Institute. Children with Cancer—A Guide for Parents; National Institutes of Health: Bethesda, Maryland, 2015.
- Hudson, M.M.; Ness, K.K.; Gurney, J.G.; Mulrooney, D.A.; Chemaitilly, W.; Krull, K.R.; Green, D.M.; Armstrong, G.T.; Nottage, K.A.; Jones, K.E. Clinical ascertainment of health outcomes among adults treated for childhood cancer. Jama 2013, 309, 2371–2381. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef]
- World Health Organization, W. Health Topics: Rehabilitation; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- UNICEF. Convention on the Rights of the Child. 1990. Available online: https://www.ohchr.org/en/instruments-mechanisms/instruments/convention-rights-child (accessed on 10 March 2022).
- Gudbergsson, S.B.; Dahl, A.A.; Loge, J.H.; Thorsen, L.; Oldervoll, L.M.; Grov, E.K. What is covered by “cancer rehabilitation” in PubMed? A review of randomized controlled trials 1990–2011. J. Rehabil. Med. 2015, 47, 97–106. [Google Scholar] [CrossRef]
- Tanner, L.; Keppner, K.; Lesmeister, D.; Lyons, K.; Rock, K.; Sparrow, J. Cancer Rehabilitation in the Pediatric and Adolescent/Young Adult Population. Semin. Oncol. Nurs. 2020, 36, 150984. [Google Scholar] [CrossRef]
- Maehr, B.; Keilani, M.; Wiltschke, C.; Hassler, M.; Licht, T.; Marosi, C.; Huetterer, E.; Cenik, F.; Crevenna, R. Cancer rehabilitation in Austria-aspects of Physical Medicine and Rehabilitation. Wien. Med. Wochenschr. 2016, 166, 39–43. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Appendix 2 to The Guideline on The Evaluation of Anticancer Medicinal Products in Man: The Use of Patient-Reported Outcome (PRO) Measures in Oncology Studies. 2016. Available online: https://www.ema.europa.eu/en/documents/other/appendix-2-guideline-evaluation-anticancer-medicinal-products-man_en.pdf (accessed on 10 March 2022).
- U.S. Food and Drug Administration (FDA). Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims 2009; U.S. Food and Drug Administration: Rockville, MD, USA.
- Eiser, C.; Varni, J.W. Health-related quality of life and symptom reporting: Similarities and differences between children and their parents. Eur. J. Pediatr. 2013, 172, 1299–1304. [Google Scholar] [CrossRef]
- Ravens-Sieberer, U.; Karow, A.; Barthel, D.; Klasen, F. How to assess quality of life in child and adolescent psychiatry. Dialogues Clin. Neurosci. 2014, 16, 147–158. [Google Scholar] [CrossRef]
- Varni, J.W.; Limbers, C.A.; Burwinkle, T.M. Parent proxy-report of their children’s health-related quality of life: An analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual. Life Outcomes 2007, 5, 2. [Google Scholar] [CrossRef]
- Ronen, G.M.; Streiner, D.L.; Rosenbaum, P. Health-related quality of life in children with epilepsy: Development and validation of self-report and parent proxy measures. Epilepsia 2003, 44, 598–612. [Google Scholar] [CrossRef]
- Mack, J.W.; McFatrich, M.; Withycombe, J.S.; Maurer, S.H.; Jacobs, S.S.; Lin, L.; Lucas, N.R.; Baker, J.N.; Mann, C.M.; Sung, L.; et al. Agreement Between Child Self-report and Caregiver-Proxy Report for Symptoms and Functioning of Children Undergoing Cancer Treatment. JAMA Pediatr. 2020, 174, e202861. [Google Scholar] [CrossRef]
- Parsons, S.K.; Fairclough, D.L.; Wang, J.; Hinds, P.S. Comparing longitudinal assessments of quality of life by patient and parent in newly diagnosed children with cancer: The value of both raters’ perspectives. Qual. Life Res. 2012, 21, 915–923. [Google Scholar] [CrossRef]
- Weaver, M.S.; Jacobs, S.S.; Withycombe, J.S.; Wang, J.; Greenzang, K.A.; Baker, J.N.; Hinds, P.S. Profile Comparison of Patient-Reported and Proxy-Reported Symptoms in Pediatric Patients With Cancer Receiving Chemotherapy. JAMA Netw. Open 2022, 5, e221855. [Google Scholar] [CrossRef]
- Upton, P.; Lawford, J.; Eiser, C. Parent-child agreement across child health-related quality of life instruments: A review of the literature. Qual. Life Res. 2008, 17, 895–913. [Google Scholar] [CrossRef]
- Davis, E.; Davies, B.; Waters, E.; Priest, N. The relationship between proxy reported health-related quality of life and parental distress: Gender differences. Child Care Health Dev. 2008, 34, 830–837. [Google Scholar] [CrossRef]
- Waters, E.; Doyle, J.; Wolfe, R.; Wright, M.; Wake, M.; Salmon, L. Influence of parental gender and self-reported health and illness on parent-reported child health. Pediatrics 2000, 106, 1422–1428. [Google Scholar] [CrossRef]
- Yeh, C.H.; Chang, C.W.; Chang, P.C. Evaluating quality of life in children with cancer using children’s self-reports and parent-proxy reports. Nurs. Res. 2005, 54, 354–362. [Google Scholar] [CrossRef]
- Meryk, A.; Kropshofer, G.; Hetzer, B.; Riedl, D.; Lehmann, J.; Rumpold, G.; Haid, A.; Schneeberger-Carta, V.; Salvador, C.; Rabensteiner, E.; et al. Disagreement between mothers’ and fathers’ rating of health-related quality of life in children with cancer. Qual. Life Res. 2022. submitted. [Google Scholar]
- Riedl, D.; Giesinger, J.M.; Wintner, L.M.; Loth, F.L.; Rumpold, G.; Greil, R.; Nickels, A.; Licht, T.; Holzner, B. Improvement of quality of life and psychological distress after inpatient cancer rehabilitation: Results of a longitudinal observational study. Wien. Klin. Wochenschr. 2017, 129, 692–701. [Google Scholar] [CrossRef]
- Licht, T.; Nickels, A.; Rumpold, G.; Holzner, B.; Riedl, D. Evaluation by electronic patient-reported outcomes of cancer survivors’ needs and the efficacy of inpatient cancer rehabilitation in different tumor entities. Support. Care Cancer 2021, 29, 5853–5864. [Google Scholar] [CrossRef]
- Klocker, J.; Klocker-Kaiser, U.; Pipam, W.; Geissler, D. Long-term improvement of the bio-psycho-social state of cancer patients after 3 weeks of inpatient oncological rehabilitation: A long-term study at the Humanomed Zentrum Althofen. Wien. Med. Wochenschr. 2018, 168, 350–360. [Google Scholar] [CrossRef]
- Lehmann, J.; Rothmund, M.; Riedl, D.; Rumpold, G.; Grote, V.; Fischer, M.J.; Holzner, B. Clinical Outcome Assessment in Cancer Rehabilitation and the Central Role of Patient-Reported Outcomes. Cancers 2021, 14, 84. [Google Scholar] [CrossRef]
- Holzner, B.; Giesinger, J.M.; Pinggera, J.; Zugal, S.; Schöpf, F.; Oberguggenberger, A.S.; Gamper, E.M.; Zabernigg, A.; Weber, B.; Rumpold, G. The Computer-based Health Evaluation Software (CHES): A software for electronic patient-reported outcome monitoring. BMC Med. Inform. Decis. Mak. 2012, 12, 126. [Google Scholar] [CrossRef]
- Varni, J.W.; Burwinkle, T.M.; Seid, M.; Skarr, D. The PedsQL 4.0 as a pediatric population health measure: Feasibility, reliability, and validity. Ambul. Pediatr. 2003, 3, 329–341. [Google Scholar] [CrossRef]
- Varni, J.W.; Burwinkle, T.M.; Katz, E.R.; Meeske, K.; Dickinson, P. The PedsQL in pediatric cancer: Reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer 2002, 94, 2090–2106. [Google Scholar] [CrossRef]
- WHO. International Classification of Functioning, Disability and Health (ICF). 2001. Available online: https://www.who.int/classifications/international-classification-of-functioning-disability-and-health (accessed on 10 August 2022).
- Ellis, P.D. The Essential Guide to Effect Sizes: Statistical Power, Meta-Analysis, and The Interpretation of Research Results; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2010. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Zdravkovic, A.; Grote, V.; Pirchl, M.; Stockinger, M.; Crevenna, R.; Fischer, M.J. Comparison of patient- and clinician-reported outcome measures in lower back rehabilitation: Introducing a new integrated performance measure (T2D). Qual. Life Res. 2022, 31, 303–315. [Google Scholar] [CrossRef]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences; Houghton Mifflin: Boston, MA, USA, 2003; Volume 5. [Google Scholar]
- Bily, W.; Jauker, J.; Nics, H.; Grote, V.; Pirchl, M.; Fischer, M.J. Associations between Patient-Reported and Clinician-Reported Outcome Measures in Patients after Traumatic Injuries of the Lower Limb. Int. J. Environ. Res. Public Health 2022, 19, 3140. [Google Scholar] [CrossRef] [PubMed]
- Grote, V.; Pirchl, M.; Fischer, M.J. A new perspective on stratified outcome evaluation. J. Int. Soc. Phys. Rehabil. Med. 2021, 4, 118. [Google Scholar]
- Eiser, C.; Morse, R. Can parents rate their child’s health-related quality of life? Results of a systematic review. Qual. Life Res. 2001, 10, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.; Hayashi, R.J. Participation and Self-Management Strategies of Young Adult Childhood Cancer Survivors. OTJR Occup. Particip. Health 2012, 33, 21–30. [Google Scholar] [CrossRef]
- Mitby, P.A.; Robison, L.L.; Whitton, J.A.; Zevon, M.A.; Gibbs, I.C.; Tersak, J.M.; Meadows, A.T.; Stovall, M.; Zeltzer, L.K.; Mertens, A.C. Childhood Cancer Survivor Study Steering Committee. Utilization of special education services and educational attainment among long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2003, 97, 1115–1126. [Google Scholar] [CrossRef]
- Ness, K.K.; Gurney, J.G.; Zeltzer, L.K.; Leisenring, W.; Mulrooney, D.A.; Nathan, P.C.; Robison, L.L.; Mertens, A.C. The impact of limitations in physical, executive, and emotional function on health-related quality of life among adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Arch. Phys. Med. Rehabil. 2008, 89, 128–136. [Google Scholar] [CrossRef]
- Taylor, O.D.; Ware, R.S.; Weir, K.A. Speech pathology services to children with cancer and nonmalignant hematological disorders. J. Pediatr. Oncol. Nurs. 2012, 29, 98–108. [Google Scholar] [CrossRef]
- Pang, J.W.; Friedman, D.L.; Whitton, J.A.; Stovall, M.; Mertens, A.C.; Robison, L.L.; Weiss, N.S. Employment status among adult survivors in the Childhood Cancer Survivor Study. Pediatr. Blood Cancer 2008, 50, 104–110. [Google Scholar] [CrossRef]
- Maurer, S.H.; Hinds, P.S.; Reeve, B.B.; Mack, J.W.; McFatrich, M.; Lin, L.; Withycombe, J.S.; Jacobs, S.S.; Baker, J.N.; Castellino, S.M. Patients, caregivers, and clinicians differ in performance status ratings: Implications for pediatric cancer clinical trials. Cancer 2021, 127, 3664–3670. [Google Scholar] [CrossRef]
- Rensen, N.; Steur, L.M.H.; Schepers, S.A.; Merks, J.H.M.; Moll, A.C.; Kaspers, G.J.L.; Van Litsenburg, R.R.L.; Grootenhuis, M.A. Determinants of health-related quality of life proxy rating disagreement between caregivers of children with cancer. Qual. Life Res. 2020, 29, 901–912. [Google Scholar] [CrossRef]
- Davis, E.; Mackinnon, A.; Waters, E. Parent proxy-reported quality of life for children with cerebral palsy: Is it related to parental psychosocial distress? Child Care Health Dev. 2012, 38, 553–560. [Google Scholar] [CrossRef]
- Panepinto, J.A.; Hoffmann, R.G.; Pajewski, N.M. The effect of parental mental health on proxy reports of health-related quality of life in children with sickle cell disease. Pediatr. Blood Cancer 2010, 55, 714–721. [Google Scholar] [CrossRef]
- Krauth, K.A. Family-Oriented Rehabilitation (FOR) and Rehabilitation of Adolescents and Young Adults (AYA) in Pediatric Oncology. Oncol. Res. Treat. 2017, 40, 752–758. [Google Scholar] [CrossRef]
- Grote, V.; Unger, A.; Böttcher, E.; Muntean, M.; Puff, H.; Marktl, W.; Mur, E.; Kullich, W.; Holasek, S.; Hofmann, P. General and Disease-Specific Health Indicator Changes Associated with Inpatient Rehabilitation. J. Am. Med. Dir. Assoc. 2020, 21, 2017.e10–2017.e27. [Google Scholar] [CrossRef]
- Coughtrey, A.; Millington, A.; Bennett, S.; Christie, D.; Hough, R.; Su, M.T.; Constantinou, M.P.; Shafran, R. The Effectiveness of Psychosocial Interventions for Psychological Outcomes in Pediatric Oncology: A Systematic Review. J. Pain Symptom Manag. 2018, 55, 1004–1017. [Google Scholar] [CrossRef]
- Copay, A.G.; Subach, B.R.; Glassman, S.D.; Polly, D.W., Jr.; Schuler, T.C. Understanding the minimum clinically important difference: A review of concepts and methods. Spine J. 2007, 7, 541–546. [Google Scholar] [CrossRef]
- de Rojas, T.; Neven, A.; Towbin, A.J.; Carceller, F.; Bautista, F.; Riedl, D.; Sodergren, S.; Darlington, A.S.; Fernandez-Teijeiro, A.; Moreno, L. Clinical research tools in pediatric oncology: Challenges and opportunities. Cancer Metastasis Rev. 2020. ahead of print. [Google Scholar] [CrossRef]
- Riedl, D.; Rothmund, M.; Darlington, A.S.; Sodergren, S.; Crazzolara, R.; de Rojas, T.; EORTC Quality of Life Group. Rare use of patient-reported outcomes in childhood cancer clinical trials - a systematic review of clinical trial registries. Eur. J. Cancer 2021, 152, 90–99. [Google Scholar] [CrossRef]
- Rothmund, M.; Lehmann, J.; Moser, W.; de Rojas, T.; Sodergren, S.C.; Darlington, A.S.; Riedl, D. Patient-reported outcomes are under-utilised in evaluating supportive therapies in paediatric oncology—a systematic review of clinical trial registries. Crit. Rev. Oncol. Hematol. 2022, 176, 103755. [Google Scholar] [CrossRef]
| Treatment Modality | n | (%) | Treatment Frequency per Patient | |
|---|---|---|---|---|
| Median | IQR | |||
| Guidance and treatment by physician | 236 | (100.0%) | 6 | 5–7 |
| Nursing procedures | 236 | (100.0%) | 4 | 3–8 |
| Speech therapy | 206 | (87.3%) | 2 | 1–4 |
| Nutritional counseling | 236 | (100.0%) | 5 | 4–6 |
| Social counseling | 226 | (95.8%) | 2 | 1.75–2 |
| Physiotherapy | 236 | (100.0%) | 36 | 30–42 |
| Massage | 179 | (75.8%) | 3 | 2–4 |
| Functional occupational therapy | 235 | (99.6%) | 13 | 10–16 |
| Animal assisted therapy | 55 | (23.3%) | 1 | 1–2 |
| Psychological therapy | 236 | (100.0%) | 10.5 | 9–13 |
| Pedagogical interventions | 161 | (68.2%) | 3 | 2–5 |
| Mean age (SD) | 11.0 | (4.3) |
| (range) | (5–22) | |
| Sex | ||
| Male | 139 | 58.9% |
| Female | 97 | 41.1% |
| Mean body mass index, BMI (SD) | 20.0 | (5.0) |
| (range) | (12.7–33.1) | |
| Mean initial walking range in meter (SD) | 519.6 | (118.6) |
| (range) | (0–745) | |
| Karnofsky Performance Score | ||
| High level of functioning (80–100%) | 183 | 77.5% |
| Medium level of functioning (50–80%) | 49 | 20.8% |
| Low level of functioning (0–50%) | 2 | 0.8% |
| Missing information | 2 | 0.8% |
| Cancer Entities | ||
| Leukemias (C91–C96) | 83 | 35.2% |
| Malignant lymphoma (C81–C86; C88) | 46 | 19.5% |
| Brain cancers (C70–72) | 32 | 13.6% |
| Bone cancer (C40–C41) | 27 | 11.4% |
| Neoplasms of soft tissue (C47–C49) | 18 | 7.6% |
| Neoplasms of blood and immune system (D50–D89) | 15 | 6.4% |
| Other | 15 | 6.4% |
| T1 (n = 236) | T2 (n = 182) | diff | |||||
|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | (T2-T1) | p-Value | η2 | |
| PedsQL generic core scale | |||||||
| Physical functioning | 73.9 | 19.6 | 81.4 | 15.6 | 7.5 | <0.001 | 0.183 |
| Emotional functioning | 76.2 | 20.7 | 81.5 | 18.6 | 5.3 | <0.001 | 0.088 |
| Social functioning | 79.9 | 16.5 | 84.2 | 17.4 | 4.3 | 0.005 | 0.043 |
| School functioning | 71.1 | 18.4 | 76.6 | 19.0 | 5.5 | <0.001 | 0.116 |
| Psychosocial functioning | 75.8 | 14.6 | 80.8 | 15.1 | 5.0 | <0.001 | 0.123 |
| Total score | 75.1 | 14.5 | 81.0 | 13.8 | 5.9 | <0.001 | 0.180 |
| PedsQL Cancer Module | |||||||
| Pain and hurt | 76.9 | 23.3 | 80.2 | 23.2 | 3.3 | 0.033 | 0.025 |
| Nausea | 79.1 | 18.1 | 81.8 | 16.7 | 2.7 | 0.025 | 0.028 |
| Procedural anxiety | 69.4 | 33.3 | 76.1 | 31.5 | 6.7 | 0.035 | 0.025 |
| Treatment anxiety | 86.3 | 20.5 | 87.5 | 20.9 | 1.2 | 0.27 | 0.007 |
| Worry | 77.7 | 24.9 | 79.6 | 21.6 | 1.9 | 0.056 | 0.020 |
| Cognitive problems | 76.4 | 20.8 | 79.0 | 21.3 | 2.6 | 0.035 | 0.024 |
| Perceived physical appearance | 79.6 | 22.4 | 80.2 | 22.0 | 0.6 | 0.88 | <0.01 |
| Communication | 78.6 | 23.7 | 83.2 | 22.3 | 4.6 | 0.010 | 0.037 |
| Total score | 78.0 | 14.2 | 80.9 | 14.8 | 2.9 | 0.004 | 0.045 |
| T1 (n = 478) | T2 (n = 478) | diff | |||||
|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | (T2-T1) | p-Value | η2 | |
| PedsQL generic core scale | |||||||
| Physical functioning | 70.7 | 20.5 | 78.0 | 19.4 | 7.3 | <0.001 | 0.215 |
| Emotional functioning | 64.7 | 18.6 | 74.3 | 17.9 | 9.6 | <0.001 | 0.276 |
| Social functioning | 73.3 | 18.7 | 78.6 | 18.9 | 5.3 | <0.001 | 0.124 |
| School functioning | 69.1 | 19.6 | 76.6 | 19.1 | 7.5 | <0.001 | 0.141 |
| Psychosocial functioning | 69.0 | 14.8 | 76.5 | 15.8 | 7.5 | <0.001 | 0.274 |
| Total score | 69.6 | 15.2 | 77.0 | 15.8 | 7.4 | <0.001 | 0.305 |
| PedsQL Cancer Module | |||||||
| Pain and hurt | 72.0 | 24.9 | 78.6 | 22.6 | 6.6 | <0.001 | 0.093 |
| Nausea | 74.3 | 19.5 | 79.0 | 18.4 | 4.7 | <0.001 | 0.084 |
| Procedural anxiety | 57.1 | 36.1 | 68.7 | 35.0 | 11.6 | <0.001 | 0.169 |
| Treatment anxiety | 79.4 | 23.5 | 84.5 | 22.1 | 5.1 | <0.001 | 0.070 |
| Worry | 76.3 | 24.9 | 82.2 | 23.0 | 5.9 | <0.001 | 0.090 |
| Cognitive problems | 65.5 | 22.2 | 68.5 | 22.8 | 3.0 | 0.009 | 0.020 |
| Perceived physical appearance | 77.0 | 24.7 | 81.3 | 22.0 | 4.3 | <0.001 | 0.048 |
| Communication | 57.7 | 28.8 | 57.4 | 31.1 | −0.2 | 0.84 | <0.01 |
| Total score | 69.7 | 14.7 | 74.8 | 15.5 | 5.1 | <0.001 | 0.176 |
| Patient | Mother | Father | Patient–Mother Dyad | Patient–Father Dyad | Mother–Father Dyad | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m | SD | m | SD | m | SD | rICC | 95% CI | p | rICC | 95% CI | p | rICC | 95% CI | p | |
| Functioning scales | |||||||||||||||
| PF | 78.2 | 19.1 | 72.0 | 18.4 | 68.1 | 19.8 | 0.69 | 0.51–0.80 | <0.001 | 0.65 | 0.37–0.79 | <0.001 | 0.76 | 0.64–0.84 | <0.001 |
| EF | 76.7 | 19.7 | 60.8 | 19.9 | 64.6 | 19.7 | 0.58 | 0.08–0.78 | <0.001 | 0.57 | 0.25–74 | <0.001 | 0.86 | 0.79–0.91 | <0.001 |
| SF | 81.7 | 14.2 | 75.1 | 15.8 | 74.5 | 15.1 | 0.53 | 0.28–0.69 | <0.001 | 0.59 | 0.34–0.74 | <0.001 | 0.78 | 0.66–0.85 | <0.001 |
| SchF | 74.9 | 16.5 | 69.3 | 19.2 | 69.8 | 16.9 | 0.65 | 0.47–0.77 | <0.001 | 0.59 | 0.38–0.73 | <0.001 | 0.80 | 0.70–0.87 | <0.001 |
| PsF | 77.8 | 13.4 | 68.4 | 14.7 | 69.6 | 13.4 | 0.64 | 0.24–0.81 | <0.001 | 0.63 | 0.29–0.79 | <0.001 | 0.86 | 0.79–0.91 | <0.001 |
| TotF | 77.9 | 13.1 | 69.6 | 14.3 | 69.1 | 13.9 | 0.66 | 0.31–0.81 | <0.001 | 0.63 | 0.25–0.80 | <0.001 | 0.84 | 0.77–0.90 | <0.001 |
| Symptom scales | |||||||||||||||
| Pain | 80.4 | 21.8 | 69.3 | 24.0 | 71.6 | 25.7 | 0.73 | 0.48–0.85 | <0.001 | 0.66 | 0.46–0.78 | <0.001 | 0.81 | 0.71–0.87 | <0.001 |
| Nausea | 80.1 | 17.5 | 70.0 | 20.1 | 69.8 | 20.3 | 0.61 | 0.31–0.77 | <0.001 | 0.46 | 0.14–66 | <0.001 | 0.82 | 0.71–0.88 | <0.001 |
| Prod Anx | 67.7 | 33.2 | 59.5 | 35.0 | 63.2 | 33.0 | 0.84 | 0.74–0.90 | <0.001 | 0.78 | 0.66–0.85 | <0.001 | 0.82 | 0.74–0.88 | <0.001 |
| Treat Anx | 87.2 | 19.6 | 78.1 | 23.1 | 80.5 | 21.3 | 0.61 | 0.38–0.75 | <0.001 | 0.52 | 0.29–0.68 | <0.001 | 0.77 | 0.66–0.85 | <0.001 |
| Worry | 80.4 | 24.6 | 71.5 | 25.7 | 72.5 | 23.2 | 0.67 | 0.48–0.78 | <0.001 | 0.64 | 0.45–0.76 | <0.001 | 0.79 | 0.69–0.86 | <0.001 |
| Cogn | 79.3 | 19.9 | 65.2 | 22.4 | 63.3 | 20.1 | 0.49 | 0.15–0.68 | <0.001 | 0.40 | 0.03–0.63 | <0.001 | 0.61 | 0.41–0.74 | <0.001 |
| Perc Attr | 85.0 | 18.8 | 70.2 | 25.2 | 72.8 | 22.1 | 0.50 | 0.16–0.69 | <0.001 | 0.57 | 0.26–0.74 | <0.001 | 0.77 | 0.65–0.85 | <0.001 |
| Comm | 77.8 | 24.5 | 53.7 | 28.7 | 52.8 | 29.4 | −0.36 | −0.97–0.08 | 0.98 | –0.17 | −0.59–0.16 | 0.86 | 0.65 | 0.48–0.77 | <0.001 |
| TotSym | 79.5 | 13.4 | 69.6 | 14.3 | 69.1 | 13.9 | 0.52 | 0.15–0.71 | <0.001 | 0.47 | 0.09–0.68 | <0.001 | 0.84 | 0.77–0.90 | <0.001 |
| Patient | Mother | Father | Patient–Mother Dyad | Patient–Father Dyad | Mother–Father Dyad | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m | SD | m | SD | m | SD | rICC | 95% CI | p | rICC | 95% CI | p | rICC | 95% CI | p | |
| Functioning scales | |||||||||||||||
| PF | 83.5 | 14.5 | 80.4 | 16.3 | 78.3 | 18.4 | 0.86 | 0.76–0.91 | <0.001 | 0.78 | 0.58–0.88 | <0.001 | 0.86 | 0.78–0.91 | <0.001 |
| EF | 79.9 | 20.0 | 74.5 | 20.3 | 73.9 | 20.5 | 0.77 | 0.62–0.86 | <0.001 | 0.76 | 0.60–0.86 | <0.001 | 0.88 | 0.81–0.93 | <0.001 |
| SF | 85.3 | 16.4 | 82.9 | 16.9 | 80.5 | 17.6 | 0.84 | 0.73–0.90 | <0.001 | 0.80 | 0.62–0.89 | <0.001 | 0.82 | 0.71–0.89 | <0.001 |
| SchF | 78.3 | 19.5 | 78.4 | 20.5 | 77.1 | 19.8 | 0.85 | 0.75–0.91 | <0.001 | 0.81 | 0.68–0.88 | <0.001 | 0.79 | 0.67–0.87 | <0.001 |
| PsF | 81.0 | 15.1 | 78.6 | 17.1 | 77.3 | 17.1 | 0.87 | 0.78–0.92 | <0.001 | 0.83 | 0.70–0.90 | <0.001 | 0.86 | 0.77–0.91 | <0.001 |
| TotF | 81.9 | 13.9 | 79.2 | 15.8 | 77.7 | 16.2 | 0.88 | 0.80–0.93 | <0.001 | 0.85 | 0.69–0.92 | <0.001 | 0.88 | 0.81–0.93 | <0.001 |
| Symptom scales | |||||||||||||||
| Pain | 80.8 | 24.5 | 75.4 | 25.1 | 74.8 | 24.0 | 0.78 | 0.64–0.87 | <0.001 | 0.87 | 0.77–0.92 | <0.001 | 0.89 | 0.83–0.94 | <0.001 |
| Nausea | 82.5 | 17.9 | 78.1 | 22.3 | 77.1 | 22.1 | 0.65 | 0.40–0.80 | <0.001 | 0.74 | 0.55–0.86 | <0.001 | 0.84 | 0.72–0.90 | <0.001 |
| Prod Anx | 75.4 | 33.3 | 71.0 | 35.5 | 74.2 | 32.2 | 0.88 | 0.81–0.93 | <0.001 | 0.91 | 0.85–0.95 | <0.001 | 0.86 | 0.77–0.91 | <0.001 |
| Treat Anx | 86.9 | 23.1 | 81.1 | 25.9 | 83.6 | 24.8 | 0.75 | 0.59–0.84 | <0.001 | 0.82 | 0.71–0.89 | <0.001 | 0.85 | 0.76–0.91 | <0.001 |
| Worry | 81.0 | 21.8 | 79.0 | 23.9 | 74.1 | 26.0 | 0.81 | 0.68–0.88 | <0.001 | 0.77 | 0.62–0.87 | <0.001 | 0.79 | 0.68–0.87 | <0.001 |
| Cogn | 80.7 | 20.5 | 68.4 | 23.9 | 65.1 | 22.3 | 0.64 | 0.32–0.80 | <0.001 | 0.40 | 0.01–0.64 | 0.004 | 0.50 | 0.21–0.69 | <0.001 |
| Perc Attr | 84.0 | 20.2 | 80.2 | 23.8 | 75.8 | 25.4 | 0.74 | 0.58–0.84 | <0.001 | 0.64 | 0.41–0.79 | <0.001 | 0.82 | 0.72–0.89 | <0.001 |
| Comm | 81.2 | 25.3 | 54.1 | 32.3 | 46.6 | 30.2 | −0.36 | −10.1–0.13 | 0.96 | 0.01 | −0.27–0.27 | 0.47 | 0.46 | 0.15–0.66 | 0.005 |
| TotSym | 81.4 | 16.0 | 79.2 | 15.8 | 78.8 | 15.1 | 0.79 | 0.65–0.87 | <0.001 | 0.82 | 0.69–0.89 | <0.001 | 0.88 | 0.81–0.93 | <0.001 |
| Delta (n = 149) | Performance Score (n = 149) | |||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
| PedsQL generic core scale | ||||
| Physical functioning | 0.51 | <0.001 | 0.59 | <0.001 |
| Emotional functioning | 0.36 | <0.001 | 0.52 | <0.001 |
| Social functioning | 0.37 | <0.001 | 0.51 | <0.001 |
| School functioning | 0.45 | <0.001 | 0.60 | <0.001 |
| Psychosocial functioning | 0.53 | <0.001 | 0.65 | <0.001 |
| Total score | 0.59 | <0.001 | 0.68 | <0.001 |
| PedsQL Cancer Module | ||||
| Pain and hurt | 0.28 | 0.004 | 0.42 | <0.001 |
| Nausea | 0.19 | 0.10 | 0.40 | <0.001 |
| Procedural anxiety | 0.20 | 0.043 | 0.60 | <0.001 |
| Treatment anxiety | 0.24 | 0.016 | 0.50 | <0.001 |
| Worry | 0.09 | 0.37 | 0.33 | 0.001 |
| Cognitive problems | 0.26 | 0.009 | 0.29 | 0.003 |
| Perceived physical appearance | 0.43 | <0.001 | 0.49 | <0.001 |
| Communication | <0.01 | 0.99 | −0.09 | 0.35 |
| Total score | 0.45 | <0.001 | 0.60 | <0.001 |
| Mean r-scores | 0.34 | 0.50 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riedl, D.; Licht, T.; Nickels, A.; Rothmund, M.; Rumpold, G.; Holzner, B.; Grote, V.; Fischer, M.J.; Fischmeister, G. Large Improvements in Health-Related Quality of Life and Physical Fitness during Multidisciplinary Inpatient Rehabilitation for Pediatric Cancer Survivors. Cancers 2022, 14, 4855. https://doi.org/10.3390/cancers14194855
Riedl D, Licht T, Nickels A, Rothmund M, Rumpold G, Holzner B, Grote V, Fischer MJ, Fischmeister G. Large Improvements in Health-Related Quality of Life and Physical Fitness during Multidisciplinary Inpatient Rehabilitation for Pediatric Cancer Survivors. Cancers. 2022; 14(19):4855. https://doi.org/10.3390/cancers14194855
Chicago/Turabian StyleRiedl, David, Thomas Licht, Alain Nickels, Maria Rothmund, Gerhard Rumpold, Bernhard Holzner, Vincent Grote, Michael J. Fischer, and Gustav Fischmeister. 2022. "Large Improvements in Health-Related Quality of Life and Physical Fitness during Multidisciplinary Inpatient Rehabilitation for Pediatric Cancer Survivors" Cancers 14, no. 19: 4855. https://doi.org/10.3390/cancers14194855
APA StyleRiedl, D., Licht, T., Nickels, A., Rothmund, M., Rumpold, G., Holzner, B., Grote, V., Fischer, M. J., & Fischmeister, G. (2022). Large Improvements in Health-Related Quality of Life and Physical Fitness during Multidisciplinary Inpatient Rehabilitation for Pediatric Cancer Survivors. Cancers, 14(19), 4855. https://doi.org/10.3390/cancers14194855







