FOXC2 Promotes Vasculogenic Mimicry in Ovarian Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
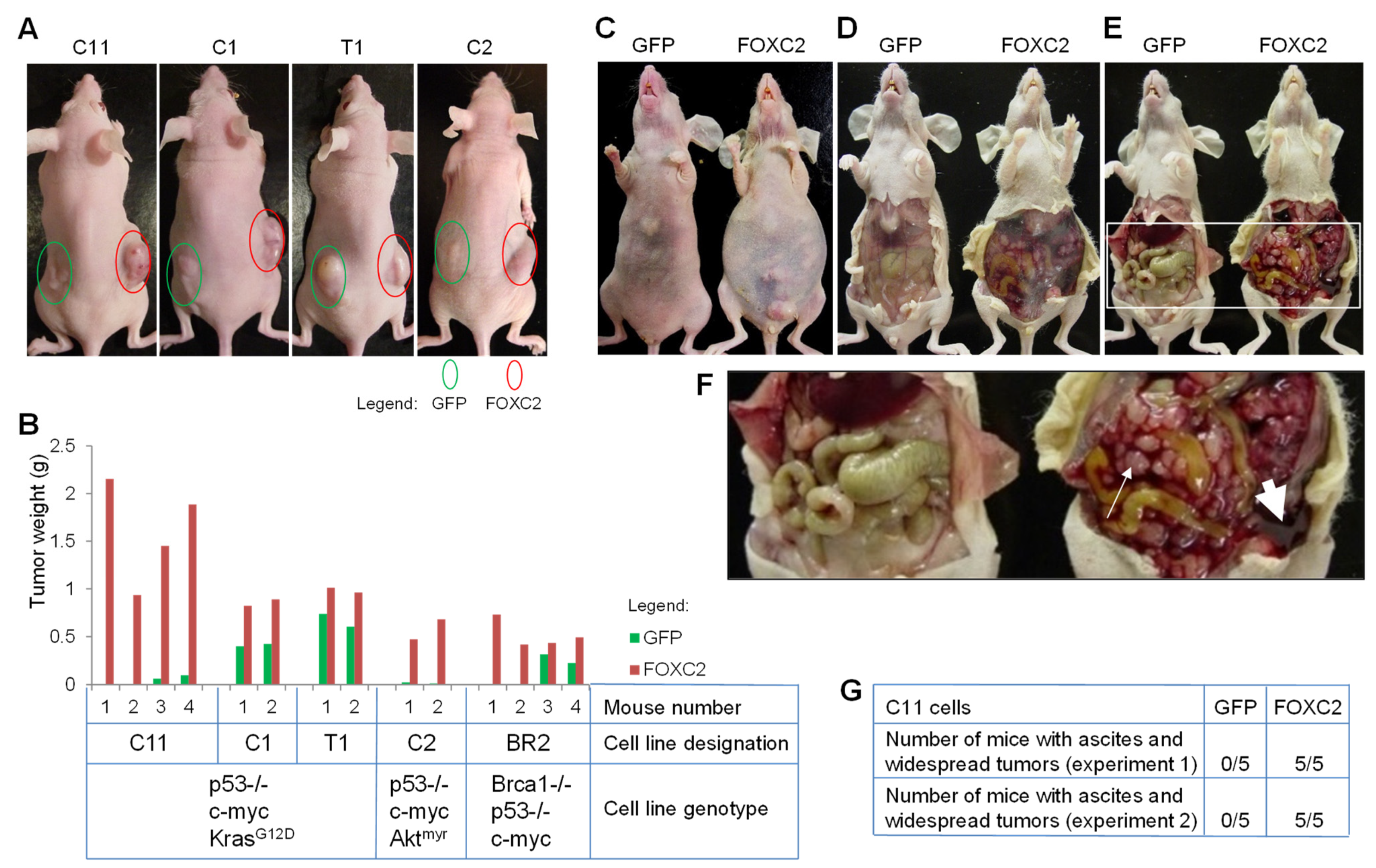
2.1. Xenograft Experiments in Mice
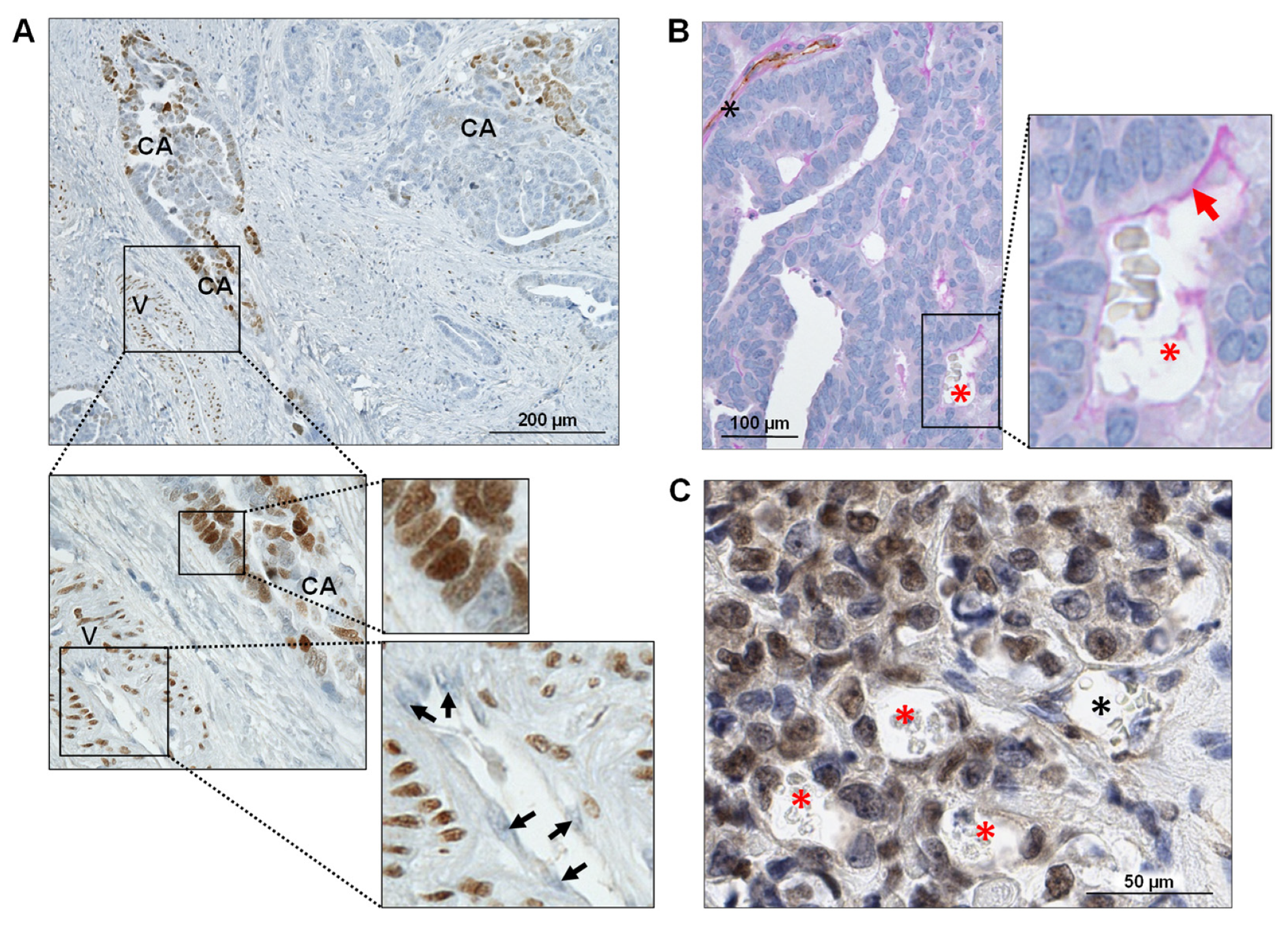
2.2. Immunohistochemical Staining of Human Specimens and Immunofluorescence Staining of Cells in Culture
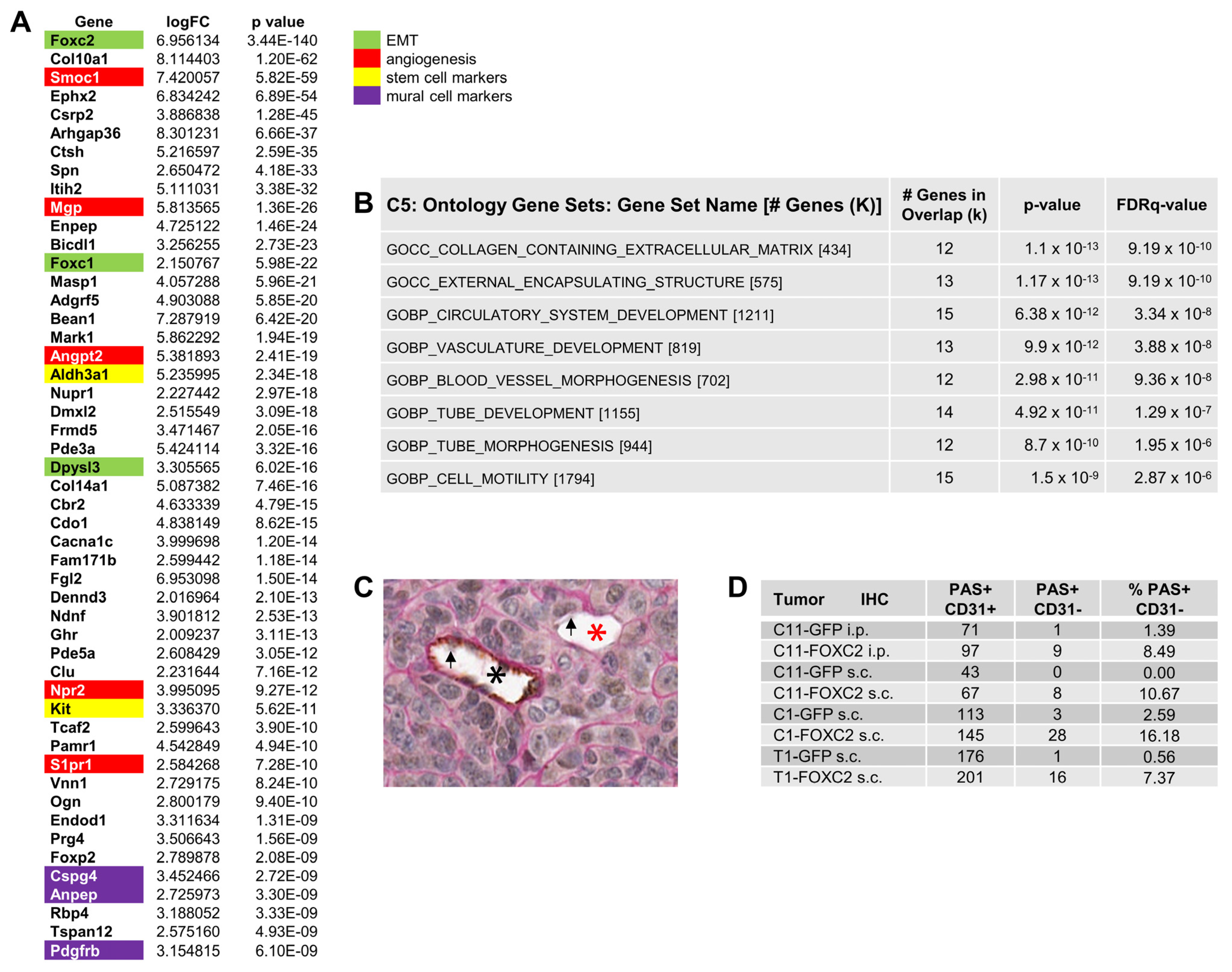
2.3. Periodic Acid Schiff (PAS) and CD31 Double Staining for VM Detection
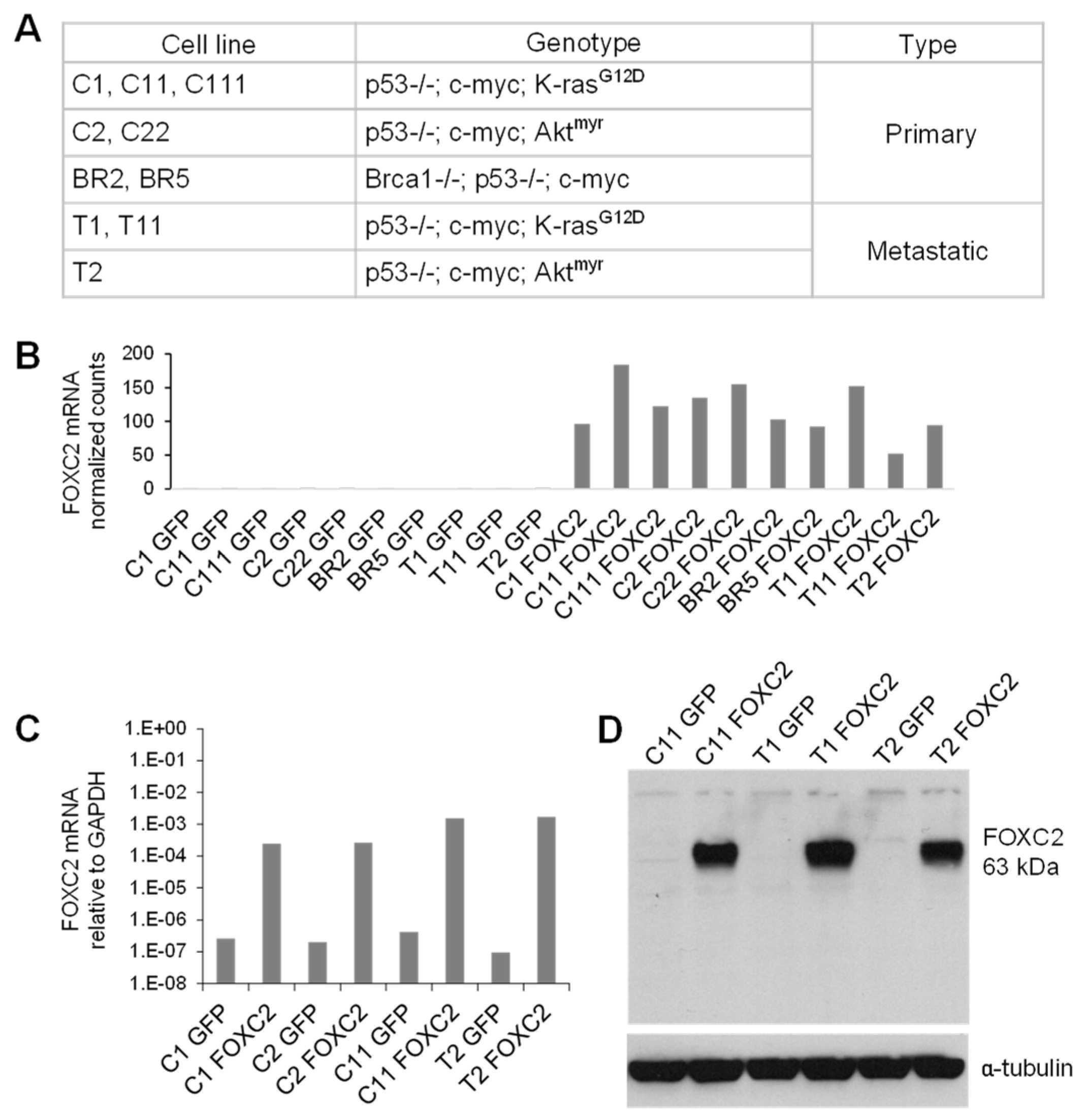
2.4. Cell Lines
2.5. Retroviral and Lentiviral Infection
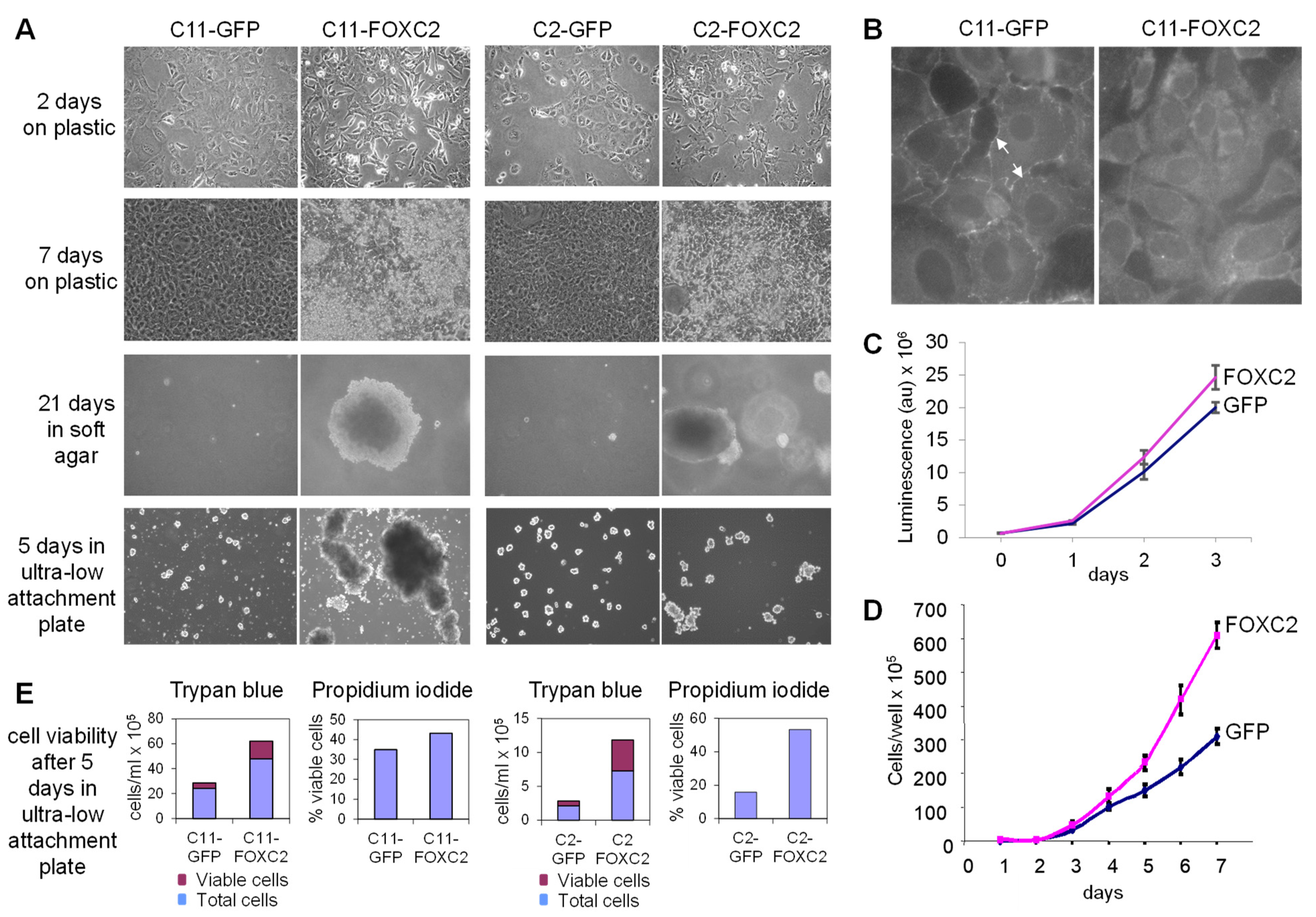
2.6. Cell Proliferation
2.7. Soft Agar Assay
2.8. Anchorage-Independent Growth Assay
2.9. Wound-Healing Scratch Assay
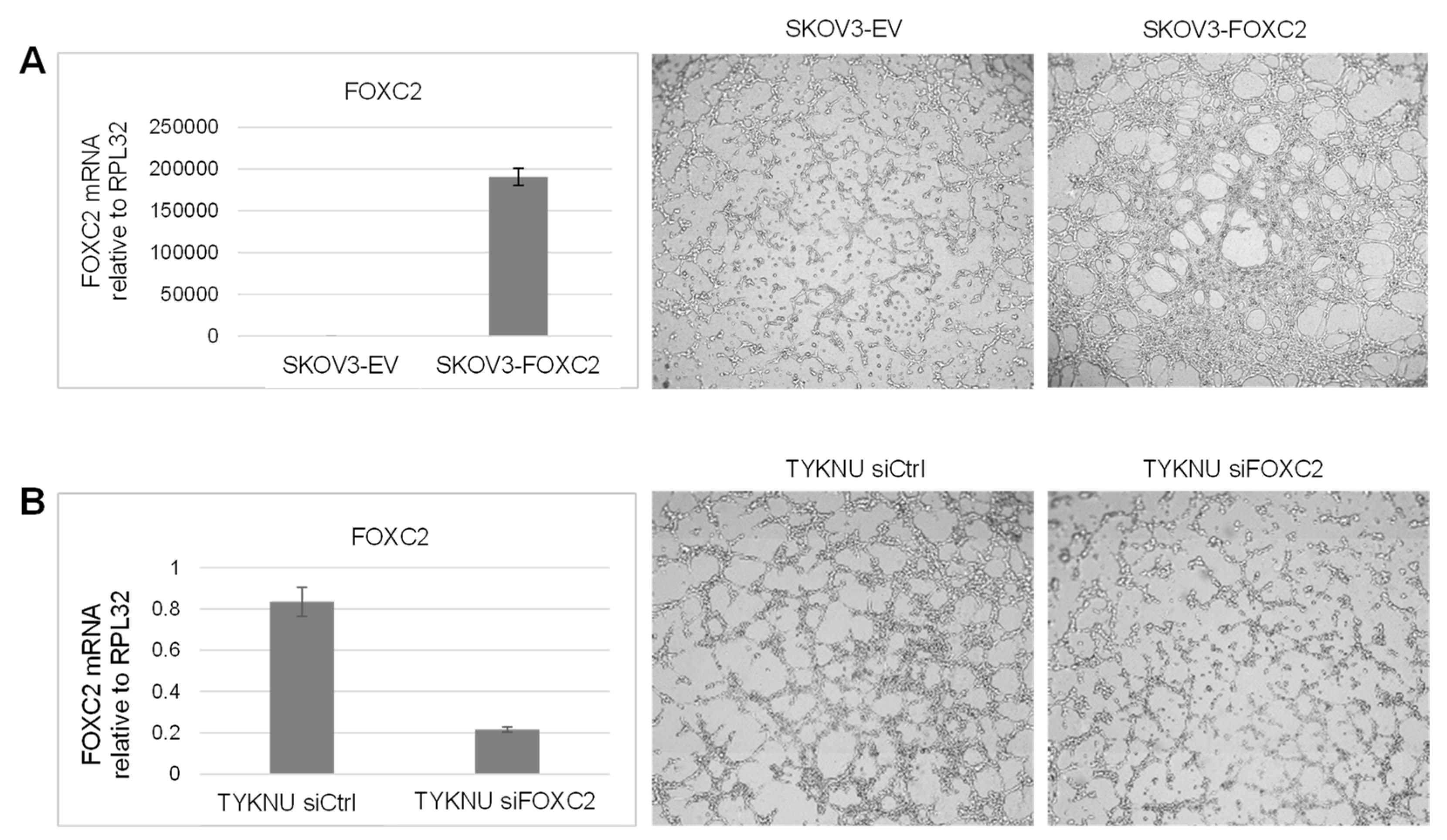
2.10. Vasculogenic Tube Formation Assay
2.11. Western Blot Analysis
2.12. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.13. RNA Sequencing (RNA-Seq) and Differential Gene Expression Analysis
2.14. Statistical Analysis
3. Results
3.1. Ectopic Expression of FOXC2 in Mouse Ovarian Cancer Cells Alters Cell Morphology and Increases Cell Proliferation, Anchorage-Independent Growth, and Resistance to Anoikis
3.2. Ectopic Expression of FOXC2 in Mouse Ovarian Cancer Cells Enhances Tumor Growth
3.3. Ectopic Expression of FOXC2 in Mouse Ovarian Cancer Cells Induces Expression of EMT, CSC, and Angiogenesis-Related Genes
3.4. FOXC2 Expression Is Associated with VM in Ovarian Cancer
3.5. Manipulation of FOXC2 Expression Levels in Human Ovarian Cancer Cell Lines Alters Their Vasculogenic Activity In Vitro
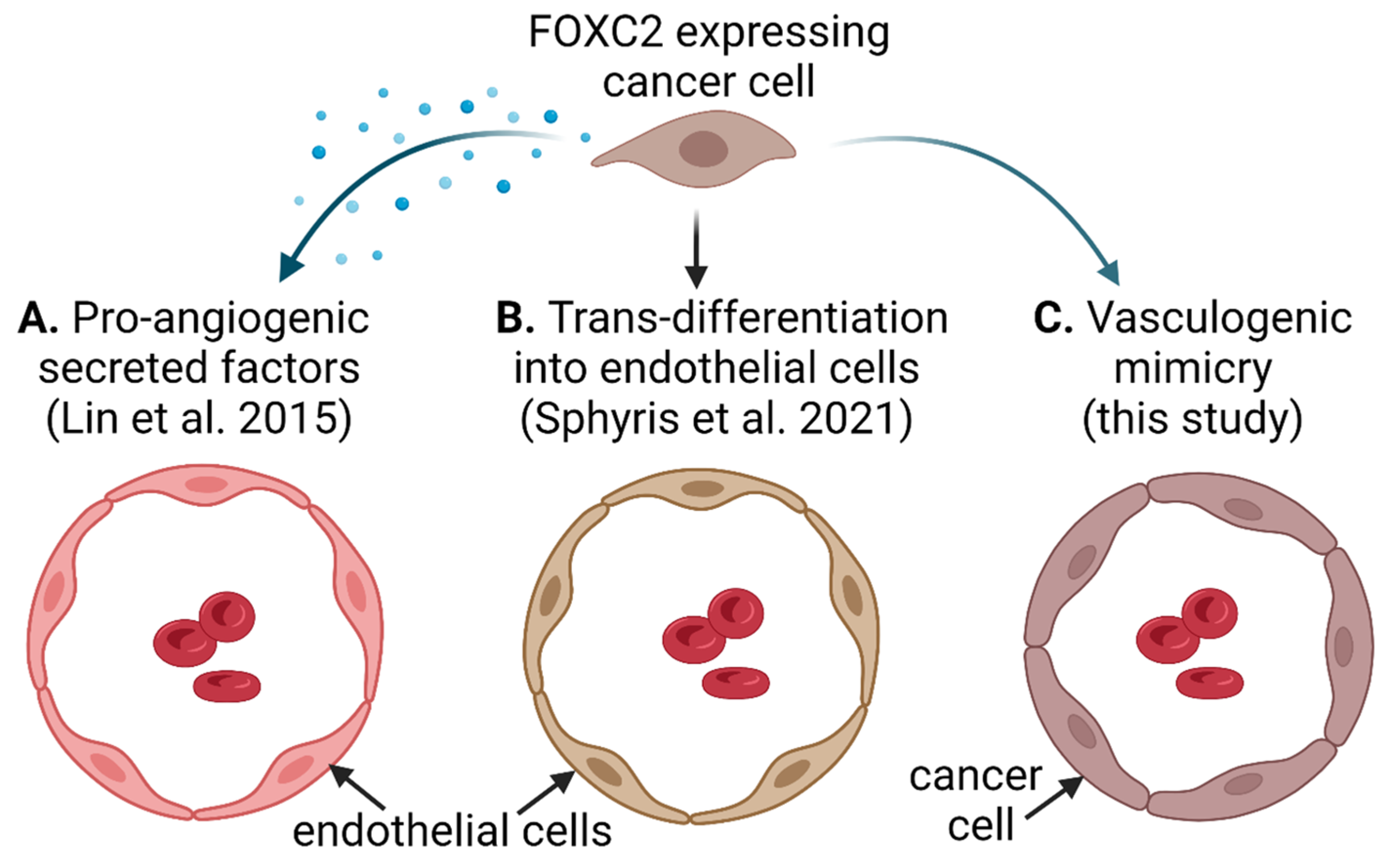
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miura, N.; Wanaka, A.; Tohyama, M.; Tanaka, K. MFH-1, a new member of the fork head domain family, is expressed in developing mesenchyme. FEBS Lett. 1993, 326, 171–176. [Google Scholar] [CrossRef]
- Iida, K.; Koseki, H.; Kakinuma, H.; Kato, N.; Mizutani-Koseki, Y.; Ohuchi, H.; Yoshioka, H.; Noji, S.; Kawamura, K.; Kataoka, Y.; et al. Essential roles of the winged helix transcription factor MFH-1 in aortic arch patterning and skeletogenesis. Development 1997, 124, 4627–4638. [Google Scholar] [CrossRef] [PubMed]
- Kume, T.; Jiang, H.; Topczewska, J.M.; Hogan, B.L. The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev. 2001, 15, 2470–2482. [Google Scholar] [CrossRef]
- Seo, S.; Fujita, H.; Nakano, A.; Kang, M.; Duarte, A.; Kume, T. The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev. Biol. 2006, 294, 458–470. [Google Scholar] [CrossRef]
- Norrmén, C.; Ivanov, K.I.; Cheng, J.; Zangger, N.; Delorenzi, M.; Jaquet, M.; Miura, N.; Puolakkainen, P.; Horsley, V.; Hu, J.; et al. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J. Cell Biol. 2009, 185, 439–457. [Google Scholar] [CrossRef]
- Davis, K.E.; Moldes, M.; Farmer, S.R. The Forkhead Transcription Factor FoxC2 Inhibits White Adipocyte Differentiation. J. Biol. Chem. 2004, 279, 42453–42461. [Google Scholar] [CrossRef] [PubMed]
- Gerin, I.; Bommer, G.; Lidell, M.E.; Cederberg, A.; Enerback, S.; MacDougald, O. On the Role of FOX Transcription Factors in Adipocyte Differentiation and Insulin-stimulated Glucose Uptake. J. Biol. Chem. 2009, 284, 10755–10763. [Google Scholar] [CrossRef]
- Cederberg, A.; Grønning, L.M.; Ahrén, B.; Taskén, K.; Carlsson, P.; Enerbäck, S. FOXC2 Is a Winged Helix Gene that Counteracts Obesity, Hypertriglyceridemia, and Diet-Induced Insulin Resistance. Cell 2001, 106, 563–573. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, H.-J.; Park, S.-Y.; Cederberg, A.; Westergren, R.; Nilsson, D.; Higashimori, T.; Cho, Y.-R.; Liu, Z.-X.; Dong, J.; et al. Adipocyte-Specific Overexpression of FOXC2 Prevents Diet-Induced Increases in Intramuscular Fatty Acyl CoA and Insulin Resistance. Diabetes 2005, 54, 1657–1663. [Google Scholar] [CrossRef]
- Gozo, M.; Aspuria, P.-J.; Cheon, D.-J.; Walts, A.E.; Berel, D.; Miura, N.; Karlan, B.Y.; Orsulic, S. Foxc2 induces Wnt4 and Bmp4 expression during muscle regeneration and osteogenesis. Cell Death Differ. 2013, 20, 1031–1042. [Google Scholar] [CrossRef]
- Yildirim-Toruner, C.; Subramanian, K.; El Manjra, L.; Chen, E.; Goldstein, S.; Vitale, E. A novel frameshift mutation ofFOXC2 gene in a family with hereditary lymphedema-distichiasis syndrome associated with renal disease and diabetes mellitus. Am. J. Med. Genet. 2004, 131A, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, E.; Almgren, P.; Hoffstedt, J.; Groop, L.; Ridderstrale, M. The FOXC2 C-512T polymorphism is associated with obesity and dyslipidemia. Obes. Res. 2004, 12, 1738. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, E.; Groop, L.; Ridderstråle, M. Role of the FOXC2 –512C>T polymorphism in type 2 diabetes: Possible association with the dysmetabolic syndrome. Int. J. Obes. 2005, 29, 268–274. [Google Scholar] [CrossRef][Green Version]
- Ridderstrale, M.; Carlsson, E.; Klannemark, M.; Cederberg, A.; Kosters, C.; Tornqvist, H.; Storgaard, H.; Vaag, A.; Enerback, S.; Groop, L. FOXC2 mRNA Expression and a 5′ Untranslated Region Polymorphism of the Gene Are Associated with Insulin Resistance. Diabetes 2002, 51, 3554–3560. [Google Scholar] [CrossRef]
- Mani, S.A.; Yang, J.; Brooks, M.; Schwaninger, G.; Zhou, A.; Miura, N.; Kutok, J.L.; Hartwell, K.; Richardson, A.L.; Weinberg, R.A. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc. Natl. Acad. Sci. USA 2007, 104, 10069–10074. [Google Scholar] [CrossRef]
- Li, Y.; Yang, W.; Yang, Q.; Zhou, S. Nuclear localization of GLI1 and elevated expression of FOXC2 in breast cancer is associated with the basal-like phenotype. Histol. Histopathol. 2012, 27, 475. [Google Scholar] [PubMed]
- Børretzen, A.; Gravdal, K.; Haukaas, S.A.; Beisland, C.; Akslen, L.A.; Halvorsen, O.J. FOXC2 expression and epithelial-mesenchymal phenotypes are associated with castration resistance, metastasis and survival in prostate cancer. J. Pathol. Clin. Res. 2019, 5, 272. [Google Scholar] [CrossRef]
- Cui, L.; Dang, S.; Qu, J.; Mao, Z.; Wang, X.; Zhang, J.; Chen, J. FOXC2 is up-regulated in pancreatic ductal adenocarcinoma and promotes the growth and migration of cancer cells. Tumor Biol. 2016, 37, 8579–8585. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Wei, P.; Xu, Y.; Zhuo, C.; Wang, Y.; Li, D.; Cai, S. Overexpression of forkhead Box C2 promotes tumor metastasis and indicates poor prognosis in colon cancer via regulating epithelial-mesenchymal transition. Am. J. Cancer Res. 2015, 5, 2022–2034. [Google Scholar]
- Liu, H.; Zhang, Z.; Han, Y.; Fan, A.; Liu, H.; Zhang, X.; Liu, Y.; Zhang, R.; Liu, W.; Lu, Y.; et al. The FENDRR/FOXC2 Axis Contributes to Multidrug Resistance in Gastric Cancer and Correlates with Poor Prognosis. Front. Oncol. 2021, 11, 634579. [Google Scholar] [CrossRef]
- He, Y.; Xie, H.; Yu, P.; Jiang, S.; Wei, L. FOXC2 promotes epithelial–mesenchymal transition and cisplatin resistance of non-small cell lung cancer cells. Cancer Chemother. Pharmacol. 2018, 82, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, F.; An, J.; Dubinett, S.; Rettig, M. p120-Catenin Is Transcriptionally Downregulated by FOXC2 in Non–Small Cell Lung Cancer Cells. Mol. Cancer Res. 2010, 8, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Li, X.; Wang, X.; Sun, H.; Wang, Z.; Wang, X. Stanniocalcin 1 promotes metastasis, lipid metabolism and cisplatin chemoresistance via the FOXC2/ITGB6 signaling axis in ovarian cancer. J. Exp. Clin. Cancer Res. 2022, 41, 129. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ding, H.; Tian, J.; Wu, L.; Wang, Y.; Xing, Y.; Chen, M. Forkhead Box Protein C2 (FOXC2) Promotes the Resistance of Human Ovarian Cancer Cells to Cisplatin In Vitro and In Vivo. Cell. Physiol. Biochem. 2016, 39, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Mimori, K.; Yokobori, T.; Sudo, T.; Tanaka, F.; Shibata, K.; Ishii, H.; Doki, Y.; Mori, M. FOXC2 is a Novel Prognostic Factor in Human Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2010, 18, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Gozo, M.C.; Jia, D.; Aspuria, P.-J.; Cheon, D.-J.; Miura, N.; Walts, A.E.; Karlan, B.Y.; Orsulic, S. FOXC2 augments tumor propagation and metastasis in osteosarcoma. Oncotarget 2016, 7, 68792–68802. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Yin, C.-L.; Zhang, H.-Y.; Hao, J.-M.; Yang, Y.-Y.; Liao, H.; Jiao, B.-H. High Expression of Forkhead Box Protein C2 is Related to Poor Prognosis in Human Gliomas. Asian Pac. J. Cancer Prev. 2014, 15, 10621–10625. [Google Scholar] [CrossRef][Green Version]
- Li, W.; Fu, X.; Liu, R.; Wu, C.; Bai, J.; Xu, Y.; Zhao, Y.; Xu, Y. FOXC2 Often Overexpressed in Glioblastoma Enhances Proliferation and Invasion in Glioblastoma Cells. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2013, 21, 111–120. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Györffy, B.; Strong, E.W.; Tarnai, B.D.; Thompson, J.C.; Bushhouse, D.; Johnson, C.E.; Williams, C.J. The FOXC2 Transcription Factor Promotes Melanoma Outgrowth and Regulates Expression of Genes Associated with Drug Resistance and Interferon Responsiveness. Cancer Genom. Proteom. 2019, 16, 491–503. [Google Scholar] [CrossRef]
- Chen, J.; Rong, X.; Liu, X.; Zheng, D.; Rong, X.; Chen, F.; Zhao, P.; Liu, F.; Ruan, J. FOXC2 is a prognostic biomarker and contributes to the growth and invasion of human hepatocellular carcinoma. Cancer Cell Int. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Watanabe, A.; Suzuki, H.; Yokobori, T.; Altan, B.; Kubo, N.; Araki, K.; Wada, S.; Mochida, Y.; Sasaki, S.; Kashiwabara, K.; et al. Forkhead box protein C2 contributes to invasion and metastasis of extrahepatic cholangiocarcinoma, resulting in a poor prognosis. Cancer Sci. 2013, 104, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ding, H.; Tian, J.; Wu, L.; Wang, Y.; Xing, Y. Forkhead Box Protein C2 Promotes Epithelial-Mesenchymal Transition, Migration and Invasion in Cisplatin-Resistant Human Ovarian Cancer Cell Line (SKOV3/CDDP). Cell Physiol. Biochem. 2016, 39, 1098. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Han, S.-M.; Tang, X.-Y.; Han, L.; Li, C.-Z. Overexpressed FOXC2 in ovarian cancer enhances the epithelial-to-mesenchymal transition and invasion of ovarian cancer cells. Oncol. Rep. 2014, 31, 2545–2554. [Google Scholar] [CrossRef][Green Version]
- Hollier, B.G.; Tinnirello, A.A.; Werden, S.J.; Evans, K.W.; Taube, J.H.; Sarkar, T.R.; Sphyris, N.; Shariati, M.; Kumar, S.V.; Battula, V.L.; et al. FOXC2 Expression Links Epithelial–Mesenchymal Transition and Stem Cell Properties in Breast Cancer. Cancer Res. 2013, 73, 1981–1992. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, K.I.; Agalarov, Y.; Valmu, L.; Samuilova, O.; Liebl, J.; Houhou, N.; Hajjami, H.M.-E.; Norrmén, C.; Jaquet, M.; Miura, N.; et al. Phosphorylation Regulates FOXC2-Mediated Transcription in Lymphatic Endothelial Cells. Mol. Cell. Biol. 2013, 33, 3749–3761. [Google Scholar] [CrossRef]
- Sano, H.; LeBoeuf, J.P.; Novitskiy, S.V.; Seo, S.; Zaja-Milatovic, S.; Dikov, M.M.; Kume, T. The Foxc2 transcription factor regulates tumor angiogenesis. Biochem. Biophys. Res. Commun. 2010, 392, 201–206. [Google Scholar] [CrossRef][Green Version]
- Hayashi, H.; Sano, H.; Seo, S.; Kume, T. The Foxc2 transcription factor regulates angiogenesis via induction of integrin beta3 expression. J. Biol. Chem. 2008, 283, 23791. [Google Scholar] [CrossRef]
- Kume, T. The Role of FoxC2 Transcription Factor in Tumor Angiogenesis. J. Oncol. 2012, 2012, 204593. [Google Scholar] [CrossRef]
- González-Loyola, A.; Bovay, E.; Kim, J.; Lozano, T.W.; Sabine, A.; Renevey, F.; Arroz-Madeira, S.; Rapin, A.; Wypych, T.P.; Rota, G.; et al. FOXC2 controls adult lymphatic endothelial specialization, function, and gut lymphatic barrier preventing multiorgan failure. Sci. Adv. 2021, 7, eabf4335. [Google Scholar] [CrossRef]
- Liebl, J.; Zhang, S.; Moser, M.; Agalarov, Y.; Demir, C.S.; Hager, B.; Bibb, J.A.; Adams, R.H.; Kiefer, F.; Miura, N.; et al. Cdk5 controls lymphatic vessel development and function by phosphorylation of Foxc2. Nat. Commun. 2015, 6, 7274. [Google Scholar] [CrossRef]
- Fatima, A.; Wang, Y.; Uchida, Y.; Norden, P.; Liu, T.; Culver, A.; Dietz, W.H.; Culver, F.; Millay, M.; Mukouyama, Y.-S.; et al. Foxc1 and Foxc2 deletion causes abnormal lymphangiogenesis and correlates with ERK hyperactivation. J. Clin. Investig. 2016, 126, 2437–2451. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J. Clin. Oncol. 2019, 37, 2317–2328. [Google Scholar] [CrossRef] [PubMed]
- Maniotis, A.J.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.; Pe'Er, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J. Vascular Channel Formation by Human Melanoma Cells in Vivo and in Vitro: Vasculogenic Mimicry. Am. J. Pathol. 1999, 155, 739–752. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Q.; Li, X.Y.; Yang, Q.Y.; Xu, W.W.; Liu, G.L. Short-term anti-vascular endothelial growth factor treatment elicits vasculogenic mimicry formation of tumors to accelerate metastasis. J. Exp. Clin. Cancer Res. 2012, 31, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Zhou, H.; Fan, G.; Li, Q. Molecular Mechanisms and Anticancer Therapeutic Strategies in Vasculogenic Mimicry. J. Cancer 2019, 10, 6327–6340. [Google Scholar] [CrossRef]
- Yang, J.P.; Liao, Y.D.; Mai, D.M.; Xie, P.; Qiang, Y.Y.; Zheng, L.S.; Wang, M.Y.; Mei, Y.; Meng, D.F.; Xu, L.; et al. Tumor vasculogenic mimicry predicts poor prognosis in cancer patients: A meta-analysis. Angiogenesis 2016, 19, 191–200. [Google Scholar] [CrossRef]
- Du, J.; Sun, B.; Zhao, X.; Gu, Q.; Dong, X.; Mo, J.; Sun, T.; Wang, J.; Sun, R.; Liu, Y. Hypoxia promotes vasculogenic mimicry formation by inducing epithelial–mesenchymal transition in ovarian carcinoma. Gynecol. Oncol. 2014, 133, 575–583. [Google Scholar] [CrossRef]
- Sood, A.K.; Fletcher, M.S.; Coffin, J.E.; Yang, M.; Seftor, E.A.; Gruman, L.M.; Gershenson, D.M.; Hendrix, M.J. Functional role of matrix metalloproteinases in ovarian tumor cell plasticity. Am. J. Obstet. Gynecol. 2004, 190, 899–909. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, P.; Wang, X.; Mao, H. Role of X-linked inhibitor of apoptosis-associated factor-1 in vasculogenic mimicry in ovarian cancer. Mol. Med. Rep. 2017, 16, 325–330. [Google Scholar] [CrossRef][Green Version]
- Liang, J.; Yang, B.; Cao, Q.; Wu, X. Association of Vasculogenic Mimicry Formation and CD133 Expression with Poor Prognosis in Ovarian Cancer. Gynecol. Obstet. Investig. 2016, 81, 529–536. [Google Scholar] [CrossRef]
- Ayala-Domínguez, L.; Olmedo-Nieva, L.; Muñoz-Bello, J.O.; Contreras-Paredes, A.; Manzo-Merino, J.; Martínez-Ramírez, I.; Lizano, M. Mechanisms of Vasculogenic Mimicry in Ovarian Cancer. Front. Oncol. 2019, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Do, Y.; Kwon, B.S.; Chang, W.; Lee, M.-S.; Kim, J.; Cho, J.G. Angiogenesis and vasculogenic mimicry as therapeutic targets in ovarian cancer. BMB Rep. 2020, 53, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Mu, D.; Ergel, B.; Singavarapu, R.; Duan, Z.; Powers, S.; Oliva, E.; Orsulic, S. Hepsin colocalizes with desmosomes and induces progression of ovarian cancer in a mouse model. Int. J. Cancer 2008, 123, 2041–2047. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Lin, Y.-X.; Ma, W.; Zhang, H.-J.; Chen, K.-M.; He, G.-P.; Zhang, X.; Xu, M.; Feng, Q.-S.; Chen, M.-Y.; et al. Vasculogenic mimicry formation in EBV-associated epithelial malignancies. Nat. Commun. 2018, 9, 5009. [Google Scholar] [CrossRef]
- Valdivia, A.; Mingo, G.; Aldana, V.; Pinto, M.P.; Ramirez, M.; Retamal, C.; Gonzalez, A.; Nualart, F.; Corvalan, A.H.; Owen, G.I. Fact or Fiction, It Is Time for a Verdict on Vasculogenic Mimicry? Front. Oncol. 2019, 9, 680. [Google Scholar] [CrossRef]
- Orsulic, S.; Li, Y.; Soslow, R.A.; Vitale-Cross, L.A.; Gutkind, J.; Varmus, H.E. Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell 2002, 1, 53–62. [Google Scholar] [CrossRef]
- Xing, D.; Orsulic, S. A genetically defined mouse ovarian carcinoma model for the molecular characterization of pathway-targeted therapy and tumor resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 6936–6941. [Google Scholar] [CrossRef]
- Xing, D.; Orsulic, S. A Mouse Model for the Molecular Characterization of Brca1-Associated Ovarian Carcinoma. Cancer Res. 2006, 66, 8949–8953. [Google Scholar] [CrossRef]
- Xing, D.; Orsulic, S. Modeling resistance to pathway-targeted therapy in ovarian cancer. Cell Cycle 2005, 4, 1004–1006. [Google Scholar] [CrossRef]
- Alwosaibai, K.; Al-Hujaily, E.M.; Alamri, S.; Ghandorah, S.; Garson, K.; Vanderhyden, B.C. PAX2 induces vascular-like structures in normal ovarian cells and ovarian cancer. Exp. Ther. Med. 2022, 23, 1–12. [Google Scholar] [CrossRef]
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ—A comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 10, 11568. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zheng, L.; Wang, Q.; Hu, Y.-W. Emerging roles and mechanisms of FOXC2 in cancer. Clin. Chim. Acta 2018, 479, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wei, L.; Bai, Y.; Wu, S.; Han, S. FoxC1 promotes epithelial-mesenchymal transition through PBX1 dependent transactivation of ZEB2 in esophageal cancer. Am. J. Cancer Res. 2017, 7, 1642–1653. [Google Scholar] [PubMed]
- Cao, Q.; Wang, X.; Shi, Y.; Zhang, M.; Yang, J.; Dong, M.; Mi, Y.; Zhang, Z.; Liu, K.; Jiang, L.; et al. FOXC1 silencing inhibits the epithelial-to-mesenchymal transition of glioma cells: Involvement of β-catenin signaling. Mol. Med. Rep. 2019, 19, 251. [Google Scholar] [CrossRef]
- Matsunuma, R.; Chan, D.W.; Kim, B.-J.; Singh, P.; Han, A.; Saltzman, A.B.; Cheng, C.; Lei, J.T.; Wang, J.; da Silva, L.R.; et al. DPYSL3 modulates mitosis, migration, and epithelial-to-mesenchymal transition in claudin-low breast cancer. Proc. Natl. Acad. Sci. USA 2018, 115, E11978–E11987. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, Y.; Xie, D.; Liu, M.; Song, N.; Zhu, J.; Fan, J.; Zhu, C. Inhibition of cell-adhesion protein DPYSL3 promotes metastasis of lung cancer. Respir. Res. 2018, 19, 1–7. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.-J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Awwad, K.; Hu, J.; Shi, L.; Mangels, N.; Malik, R.A.; Zippel, N.; Fisslthaler, B.; Eble, J.A.; Pfeilschifter, J.; Popp, R.; et al. Role of secreted modular calcium-binding protein 1 (SMOC1) in transforming growth factor β signalling and angiogenesis. Cardiovasc. Res. 2015, 106, 284–294. [Google Scholar] [CrossRef]
- Rocnik, E.F.; Liu, P.; Sato, K.; Walsh, K.; Vaziri, C. The Novel SPARC Family Member SMOC-2 Potentiates Angiogenic Growth Factor Activity. J. Biol. Chem. 2006, 281, 22855–22864. [Google Scholar] [CrossRef]
- Boström, K.; Zebboudj, A.F.; Yao, Y.; Lin, T.S.; Torres, A. Matrix GLA protein stimulates VEGF expression through increased transforming growth factor-beta1 activity in endothelial cells. J. Biol. Chem. 2004, 279, 52904. [Google Scholar] [CrossRef]
- Yao, Y.; Zebboudj, A.F.; Shao, E.; Perez, M.; Boström, K. Regulation of Bone Morphogenetic Protein-4 by Matrix GLA Protein in Vascular Endothelial Cells Involves Activin-like Kinase Receptor 1. J. Biol. Chem. 2006, 281, 33921–33930. [Google Scholar] [CrossRef] [PubMed]
- Akwii, R.G.; Sajib, M.S.; Zahra, F.T.; Mikelis, C.M. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 2019, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-Y.; Wei, J.-X.; Lv, L.-H.; Han, Q.-F.; Yang, W.-B.; Li, G.-L.; Wang, P.-X.; Wu, S.-B.; Duan, J.-X.; Zhuo, W.-F.; et al. Angiopoietin-2 induces angiogenesis via exosomes in human hepatocellular carcinoma. Cell Commun. Signal. 2020, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Coma, S.; Allard-Ratick, M.; Akino, T.; van Meeteren, L.A.; Mammoto, A.; Klagsbrun, M. GATA2 and Lmo2 control angiogenesis and lymphangiogenesis via direct transcriptional regulation of neuropilin-2. Angiogenesis 2013, 16, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Ragunathrao, V.A.B.; Anwar, M.; Akhter, M.Z.; Chavez, A.; Natarajan, V.; Lakshmikanthan, S.; Chrzanowska-Wodnicka, M.; Dudek, A.Z.; Claesson-Welsh, L.; Kitajewski, J.K.; et al. Sphingosine-1-Phosphate Receptor 1 Activity Promotes Tumor Growth by Amplifying VEGF-VEGFR2 Angiogenic Signaling. Cell Rep. 2019, 29, 3472. [Google Scholar] [CrossRef]
- Lin, B.; Song, X.; Yang, D.; Bai, D.; Yao, Y.; Lu, N. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene 2018, 654, 77–86. [Google Scholar] [CrossRef]
- Scully, S.; Francescone, R.; Faibish, M.; Bentley, B.; Taylor, S.L.; Oh, D.; Schapiro, R.; Moral, L.; Yan, W.; Shao, R. Transdifferentiation of Glioblastoma Stem-Like Cells into Mural Cells Drives Vasculogenic Mimicry in Glioblastomas. J. Neurosci. 2012, 32, 12950–12960. [Google Scholar] [CrossRef] [PubMed]
- Plantamura, I.; Casalini, P.; Dugnani, E.; Sasso, M.; D'Ippolito, E.; Tortoreto, M.; Cacciatore, M.; Guarnotta, C.; Ghirelli, C.; Barajon, I.; et al. PDGFRβ and FGFR2 mediate endothelial cell differentiation capability of triple negative breast carcinoma cells. Mol. Oncol. 2014, 8, 968–981. [Google Scholar] [CrossRef]
- D’Ippolito, E.; Plantamura, I.; Bongiovanni, L.; Casalini, P.; Baroni, S.; Piovan, C.; Orlandi, R.; Gualeni, A.V.; Gloghini, A.; Rossini, A.; et al. miR-9 and miR-200 Regulate PDGFRβ-Mediated Endothelial Differentiation of Tumor Cells in Triple-Negative Breast Cancer. Cancer Res. 2016, 76, 5562–5572. [Google Scholar] [CrossRef]
- Lugassy, C.; Wadehra, M.; Li, X.; Corselli, M.; Akhavan, D.; Binder, S.W.; Péault, B.; Cochran, A.J.; Mischel, P.S.; Kleinman, H.K.; et al. Pilot Study on “Pericytic Mimicry” and Potential Embryonic/Stem Cell Properties of Angiotropic Melanoma Cells Interacting with the Abluminal Vascular Surface. Cancer Microenviron. 2013, 6, 19–29. [Google Scholar] [CrossRef][Green Version]
- Van Hensbergen, Y.; Broxterman, H.J.; Rana, S.; Van Diest, P.J.; Duyndam, M.C.; Hoekman, K.; Pinedo, H.M.; Boven, E. Reduced growth, increased vascular area, and reduced response to cisplatin in CD13-overexpressing human ovarian cancer xenografts. Clin. Cancer Res. 2004, 10, 1180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Francescone, R.A., 3rd; Faibish, M.; Shao, R. A Matrigel-based tube formation assay to assess the vasculogenic activity of tumor cells. J. Vis. Exp. 2011, 55, e3040. [Google Scholar] [CrossRef] [PubMed]
- Racordon, D.; Valdivia, A.; Mingo, G.; Erices, R.; Aravena, R.; Santoro, F.; Bravo, M.L.; Ramirez, C.; Gonzalez, P.; Sandoval, A.; et al. Structural and functional identification of vasculogenic mimicry in vitro. Sci. Rep. 2017, 7, 6985. [Google Scholar] [CrossRef] [PubMed]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehár, J.; Kryukov, G.V.; Sonkin, D.; et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 483, 603. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Goodloe, T.B., 3rd; Lloyd, N.D. Oncogenic functions of the FOXC2 transcription factor: A hallmarks of cancer perspective. Cancer Metastasis Rev. 2022. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Iwasaki, M.; Okazaki, T.; Tanaka, Y.; Yamaguchi, K.; Higuchi, T.; Yagi, H.; Takakura, K.; Minato, N.; et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 3360–3365. [Google Scholar] [CrossRef]
- Bi, Y.; Guo, S.; Xu, X.; Kong, P.; Cui, H.; Yan, T.; Ma, Y.; Cheng, Y.; Chen, Y.; Liu, X.; et al. Decreased ZNF750 promotes angiogenesis in a paracrine manner via activating DANCR/miR-4707-3p/FOXC2 axis in esophageal squamous cell carcinoma. Cell Death Dis. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Lin, Y.; McKinnon, K.E.; Ha, S.W.; Beck, G.R., Jr. Inorganic phosphate induces cancer cell mediated angiogenesis dependent on forkhead box protein C2 (FOXC2) regulated osteopontin expression. Mol. Carcinog. 2015, 54, 926–934. [Google Scholar] [CrossRef]
- Dai, J.; Peng, L.; Fan, K.; Wang, H.; Wei, R.; Ji, G.; Cai, J.; Lu, B.; Li, B.; Zhang, D.; et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 2009, 28, 3412. [Google Scholar] [CrossRef]
- Chakraborty, G.; Jain, S.; Kundu, G.C. Osteopontin Promotes Vascular Endothelial Growth Factor–Dependent Breast Tumor Growth and Angiogenesis via Autocrine and Paracrine Mechanisms. Cancer Res. 2008, 68, 152–161. [Google Scholar] [CrossRef]
- Xue, Y.; Cao, R.; Nilsson, D.; Chen, S.; Westergren, R.; Hedlund, E.-M.; Martijn, C.; Rondahl, L.; Krauli, P.; Walum, E.; et al. FOXC2 controls Ang-2 expression and modulates angiogenesis, vascular patterning, remodeling, and functions in adipose tissue. Proc. Natl. Acad. Sci. USA 2008, 105, 10167–10172. [Google Scholar] [CrossRef] [PubMed]
- Sphyris, N.; King, C.; Hoar, J.; Werden, S.J.; Vijay, G.V.; Miura, N.; Gaharwar, A.; Sarkar, T.R. Carcinoma cells that have undergone an epithelial-mesenchymal transition differentiate into endothelial cells and contribute to tumor growth. Oncotarget 2021, 12, 823–844. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, X.; Wen, H.; Zhu, B.; Yu, L. Expression and Clinical Significance of the NCAPH, AGGF1, and FOXC2 Proteins in Serous Ovarian Cancer. Cancer Manag. Res. 2021, 13, 7253–7262. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Gao, X.-L.; Liu, X.; Gao, S.-Y.; Fan, Y.-L.; Jiang, Y.-P.; Ma, X.-R.; Jiang, J.; Feng, H.; Chen, Q.-M.; et al. CD133+ cancer stem-like cells promote migration and invasion of salivary adenoid cystic carcinoma by inducing vasculogenic mimicry formation. Oncotarget 2016, 7, 29051–29062. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Recouvreux, M.S.; Miao, J.; Gozo, M.C.; Wu, J.; Walts, A.E.; Karlan, B.Y.; Orsulic, S. FOXC2 Promotes Vasculogenic Mimicry in Ovarian Cancer. Cancers 2022, 14, 4851. https://doi.org/10.3390/cancers14194851
Recouvreux MS, Miao J, Gozo MC, Wu J, Walts AE, Karlan BY, Orsulic S. FOXC2 Promotes Vasculogenic Mimicry in Ovarian Cancer. Cancers. 2022; 14(19):4851. https://doi.org/10.3390/cancers14194851
Chicago/Turabian StyleRecouvreux, Maria Sol, Jiangyong Miao, Maricel C. Gozo, Jingni Wu, Ann E. Walts, Beth Y. Karlan, and Sandra Orsulic. 2022. "FOXC2 Promotes Vasculogenic Mimicry in Ovarian Cancer" Cancers 14, no. 19: 4851. https://doi.org/10.3390/cancers14194851
APA StyleRecouvreux, M. S., Miao, J., Gozo, M. C., Wu, J., Walts, A. E., Karlan, B. Y., & Orsulic, S. (2022). FOXC2 Promotes Vasculogenic Mimicry in Ovarian Cancer. Cancers, 14(19), 4851. https://doi.org/10.3390/cancers14194851





