Impact of the Coronavirus Disease 2019 (COVID-19) Pandemic on Cervical Cancer Screening in Gynecological Practices in Germany
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Database
2.2. Study Population
2.3. Statistical Analyses
3. Results
3.1. Decreased Number of Women with Cervical Cancer Screening per Practice
3.2. Age-Stratified Differences in the Number of Women with Cervical Cancer Screening per Practice
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjose, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Bujan Rivera, J.; Klug, S.J. Gebärmutterhalskrebsscreening in Deutschland. Bundesgesundheitsblatt—Gesundh.—Gesundh. 2018, 61, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.; Kraywinkel, K.; Nowossadeck, E.; Schönfeld, I.; Starker, A.; Wienecke, A.; Wolf, U. Bericht zum Krebsgeschehen in Deutschland 2016; Robert Koch-Institut: Berlin, Germany, 2016. [CrossRef]
- Siebert, U.; Sroczynski, G.; Hillemanns, P.; Engel, J.; Stabenow, R.; Stegmaier, C.; Voigt, K.; Gibis, B.; Holzel, D.; Goldie, S.J. The German cervical cancer screening model: Development and validation of a decision-analytic model for cervical cancer screening in Germany. Eur. J. Public Health 2006, 16, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Geyer, S.; Jaunzeme, J.; Hillemanns, P. Cervical cancer screening in Germany: Group-specific participation rates in the state of Niedersachsen (Lower Saxony). A study with health insurance data. Arch. Gynecol. Obstet. 2015, 291, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Hrgovic, Z.; Fures, R.; Jaska, S. Implementation of the Program for Early Detection of Cervical Cancer in the Federal Republic of Germany. Mater. SocioMed. 2020, 32, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Ronco, G.; Dillner, J.; Elfstrom, K.M.; Tunesi, S.; Snijders, P.J.; Arbyn, M.; Kitchener, H.; Segnan, N.; Gilham, C.; Giorgi-Rossi, P.; et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet 2014, 383, 524–532. [Google Scholar] [CrossRef]
- Gage, J.C.; Schiffman, M.; Katki, H.A.; Castle, P.E.; Fetterman, B.; Wentzensen, N.; Poitras, N.E.; Lorey, T.; Cheung, L.C.; Kinney, W.K. Reassurance against future risk of precancer and cancer conferred by a negative human papillomavirus test. J. Natl. Cancer Inst. 2014, 106, dju153. [Google Scholar] [CrossRef]
- Lozar, T.; Nagvekar, R.; Rohrer, C.; Dube Mandishora, R.S.; Ivanus, U.; Fitzpatrick, M.B. Cervical Cancer Screening Postpandemic: Self-Sampling Opportunities to Accelerate the Elimination of Cervical Cancer. Int. J. Womens Health 2021, 13, 841–859. [Google Scholar] [CrossRef]
- Chan, C.K.; Aimagambetova, G.; Ukybassova, T.; Kongrtay, K.; Azizan, A. Human Papillomavirus Infection and Cervical Cancer: Epidemiology, Screening, and Vaccination-Review of Current Perspectives. J. Oncol. 2019, 2019, 3257939. [Google Scholar] [CrossRef]
- Bos, A.B.; Rebolj, M.; Habbema, J.D.; van Ballegooijen, M. Nonattendance is still the main limitation for the effectiveness of screening for cervical cancer in the Netherlands. Int. J. Cancer 2006, 119, 2372–2375. [Google Scholar] [CrossRef] [PubMed]
- Andrae, B.; Kemetli, L.; Sparen, P.; Silfverdal, L.; Strander, B.; Ryd, W.; Dillner, J.; Tornberg, S. Screening-preventable cervical cancer risks: Evidence from a nationwide audit in Sweden. J. Natl. Cancer Inst. 2008, 100, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Spayne, J.; Ackerman, I.; Milosevic, M.; Seidenfeld, A.; Covens, A.; Paszat, L. Invasive cervical cancer: A failure of screening. Eur. J. Public Health 2008, 18, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, C.; Gage, A.; Kim, M.K.; Kapoor, N.R.; Akweongo, P.; Amponsah, F.; Aryal, A.; Asai, D.; Awoonor-Williams, J.K.; Ayele, W.; et al. COVID-19 and resilience of healthcare systems in ten countries. Nat. Med. 2022, 28, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Haldane, V.; De Foo, C.; Abdalla, S.M.; Jung, A.S.; Tan, M.; Wu, S.; Chua, A.; Verma, M.; Shrestha, P.; Singh, S.; et al. Health systems resilience in managing the COVID-19 pandemic: Lessons from 28 countries. Nat. Med. 2021, 27, 964–980. [Google Scholar] [CrossRef] [PubMed]
- Jacob, L.; Loosen, S.H.; Kalder, M.; Luedde, T.; Roderburg, C.; Kostev, K. Impact of the COVID-19 Pandemic on Cancer Diagnoses in General and Specialized Practices in Germany. Cancers 2021, 13, 408. [Google Scholar] [CrossRef]
- Castanon, A.; Rebolj, M.; Burger, E.A.; de Kok, I.; Smith, M.A.; Hanley, S.J.B.; Carozzi, F.M.; Peacock, S.; O’Mahony, J.F. Cervical screening during the COVID-19 pandemic: Optimising recovery strategies. Lancet Public Health 2021, 6, e522–e527. [Google Scholar] [CrossRef]
- Yong, J.H.; Mainprize, J.G.; Yaffe, M.J.; Ruan, Y.; Poirier, A.E.; Coldman, A.; Nadeau, C.; Iragorri, N.; Hilsden, R.J.; Brenner, D.R. The impact of episodic screening interruption: COVID-19 and population-based cancer screening in Canada. J. Med. Screen. 2021, 28, 100–107. [Google Scholar] [CrossRef]
- Maringe, C.; Spicer, J.; Morris, M.; Purushotham, A.; Nolte, E.; Sullivan, R.; Rachet, B.; Aggarwal, A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol. 2020, 21, 1023–1034. [Google Scholar] [CrossRef]
- Teglia, F.; Angelini, M.; Astolfi, L.; Casolari, G.; Boffetta, P. Global Association of COVID-19 Pandemic Measures With Cancer Screening: A Systematic Review and Meta-analysis. JAMA Oncol. 2022, 8, 1287–1293. [Google Scholar] [CrossRef]
- Rathmann, W.; Bongaerts, B.; Carius, H.J.; Kruppert, S.; Kostev, K. Basic characteristics and representativeness of the German Disease Analyzer database. Int. J. Clin. Pharmacol. Ther. 2018, 56, 459–466. [Google Scholar] [CrossRef] [PubMed]
- de Pelsemaeker, M.C.; Guiot, Y.; Vanderveken, J.; Galant, C.; Van Bockstal, M.R. The Impact of the COVID-19 Pandemic and the Associated Belgian Governmental Measures on Cancer Screening, Surgical Pathology and Cytopathology. Pathobiology 2021, 88, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Gorin, S.N.S.; Jimbo, M.; Heizelman, R.; Harmes, K.M.; Harper, D.M. The future of cancer screening after COVID-19 may be at home. Cancer 2021, 127, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Cancino, R.S.; Su, Z.; Mesa, R.; Tomlinson, G.E.; Wang, J. The Impact of COVID-19 on Cancer Screening: Challenges and Opportunities. JMIR Cancer 2020, 6, e21697. [Google Scholar] [CrossRef] [PubMed]
- Nardi, C.; Sandhu, P.; Selix, N. Cervical Cancer Screening Among Minorities in the United States. J. Nurse Pract. 2016, 12, 675–682. [Google Scholar] [CrossRef]
- Arbyn, M.; Smith, S.B.; Temin, S.; Sultana, F.; Castle, P.; Collaboration on, S.-S.; Testing, H.P.V. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: Updated meta-analyses. BMJ 2018, 363, k4823. [Google Scholar] [CrossRef]
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.B.; Nayar, R.; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low Genit Tract Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef]
- Monaghesh, E.; Hajizadeh, A. The role of telehealth during COVID-19 outbreak: A systematic review based on current evidence. BMC Public Health 2020, 20, 1193. [Google Scholar] [CrossRef]
- Wentzensen, N.; Clarke, M.A.; Perkins, R.B. Impact of COVID-19 on cervical cancer screening: Challenges and opportunities to improving resilience and reduce disparities. Prev. Med. 2021, 151, 106596. [Google Scholar] [CrossRef]
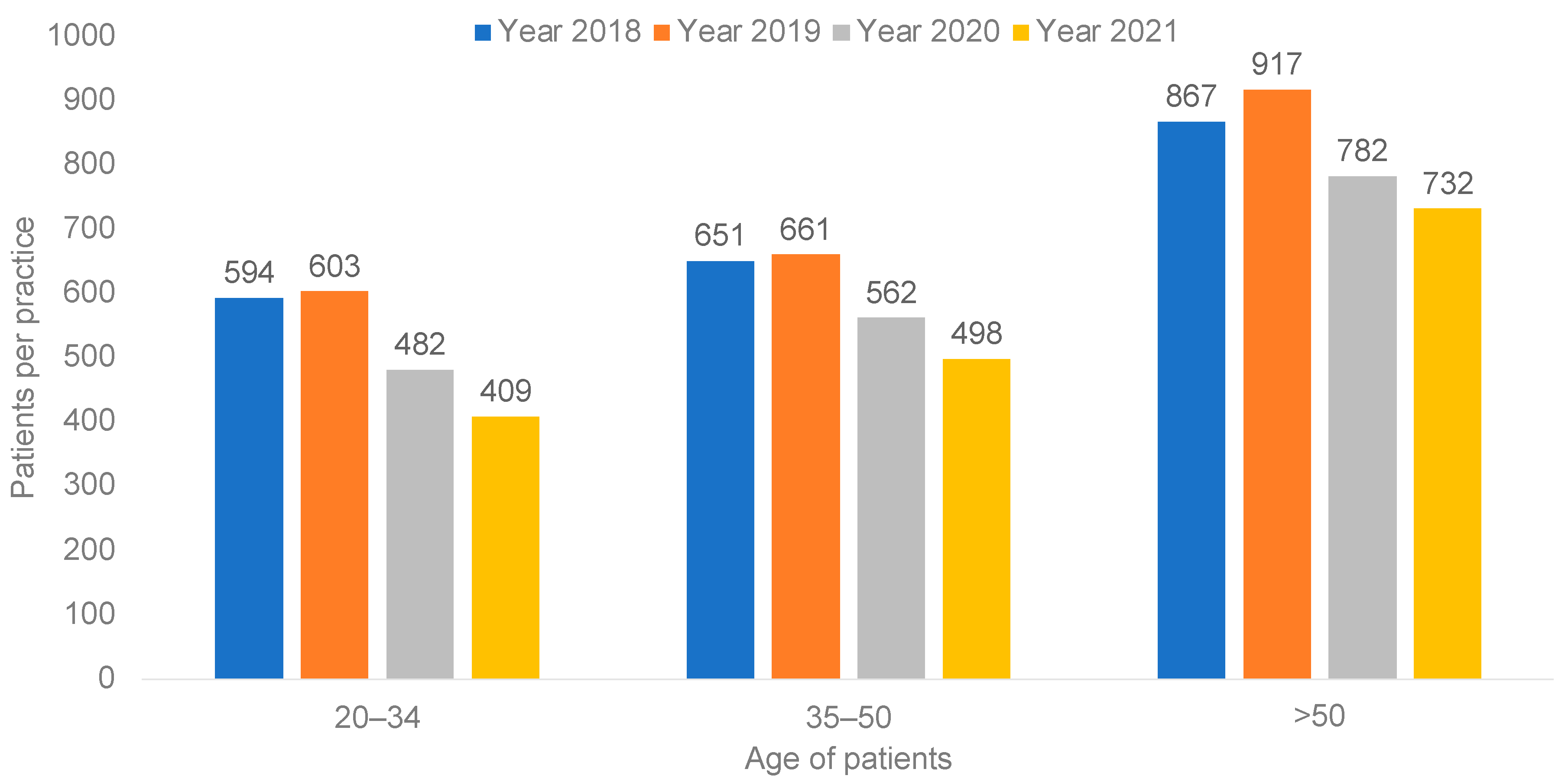
| Examination | Age Group | 2018, 2019 Mean Number of Women per Year | 2020, 2021 Mean Number of Women per Year | Difference | p-Value |
|---|---|---|---|---|---|
| Cervical cancer screening | Total | 2147 (1509) | 1732 (1308) | −19.3% | <0.001 |
| Cervical cancer screening | 20–34 | 599 (468) | 445 (389) | −25.6% | <0.001 |
| Cervical cancer screening | 35–50 | 656 (474) | 530 (412) | −19.2% | <0.001 |
| Cervical cancer screening | >50 | 892 (648) | 757 (566) | −15.2% | <0.001 |
| Clinical examination | Total | 2051 (1263) | 1687 (1204) | −17.8% | <0.001 |
| Clinical examination | 20–34 | 575 (416) | 431 (353) | −25.0% | <0.001 |
| Clinical examination | 35–50 | 627 (411) | 518 (390) | −17.5% | <0.001 |
| Clinical examination | >50 | 849 (527) | 738 (523) | −13.1% | <0.001 |
| Cytological examination | Total | 236 (1134) | 101 (644) | −57.3% | 0.003 |
| Cytological examination | 20–34 | 62 (298) | 32 (213) | −47.5% | 0.003 |
| Cytological examination | 35–50 | 70 (333) | 28 (176) | −59.3% | 0.001 |
| Cytological examination | >50 | 104 (508) | 40 (258) | −61.8% | 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gremke, N.; Griewing, S.; Felgentreff, M.; Kostev, K.; Kalder, M. Impact of the Coronavirus Disease 2019 (COVID-19) Pandemic on Cervical Cancer Screening in Gynecological Practices in Germany. Cancers 2022, 14, 4820. https://doi.org/10.3390/cancers14194820
Gremke N, Griewing S, Felgentreff M, Kostev K, Kalder M. Impact of the Coronavirus Disease 2019 (COVID-19) Pandemic on Cervical Cancer Screening in Gynecological Practices in Germany. Cancers. 2022; 14(19):4820. https://doi.org/10.3390/cancers14194820
Chicago/Turabian StyleGremke, Niklas, Sebastian Griewing, Markus Felgentreff, Karel Kostev, and Matthias Kalder. 2022. "Impact of the Coronavirus Disease 2019 (COVID-19) Pandemic on Cervical Cancer Screening in Gynecological Practices in Germany" Cancers 14, no. 19: 4820. https://doi.org/10.3390/cancers14194820
APA StyleGremke, N., Griewing, S., Felgentreff, M., Kostev, K., & Kalder, M. (2022). Impact of the Coronavirus Disease 2019 (COVID-19) Pandemic on Cervical Cancer Screening in Gynecological Practices in Germany. Cancers, 14(19), 4820. https://doi.org/10.3390/cancers14194820








