Heterogeneity and Functions of Tumor-Infiltrating Antibody Secreting Cells: Lessons from Breast, Ovarian, and Other Solid Cancers
Abstract
Simple Summary
Abstract
1. Introduction
2. Humoral Response Development and Prognosis Impact in Breast and Ovarian Cancers
2.1. ASCs Infiltrate Breast and Ovarian Tumors
2.2. Antibodies in Breast and Ovarian Cancer Patients
2.3. Origin of Tumor-Infiltrating ASCs
2.4. ASC and Antibodies Influence Cancer Patient Survival and Response to Immunotherapies
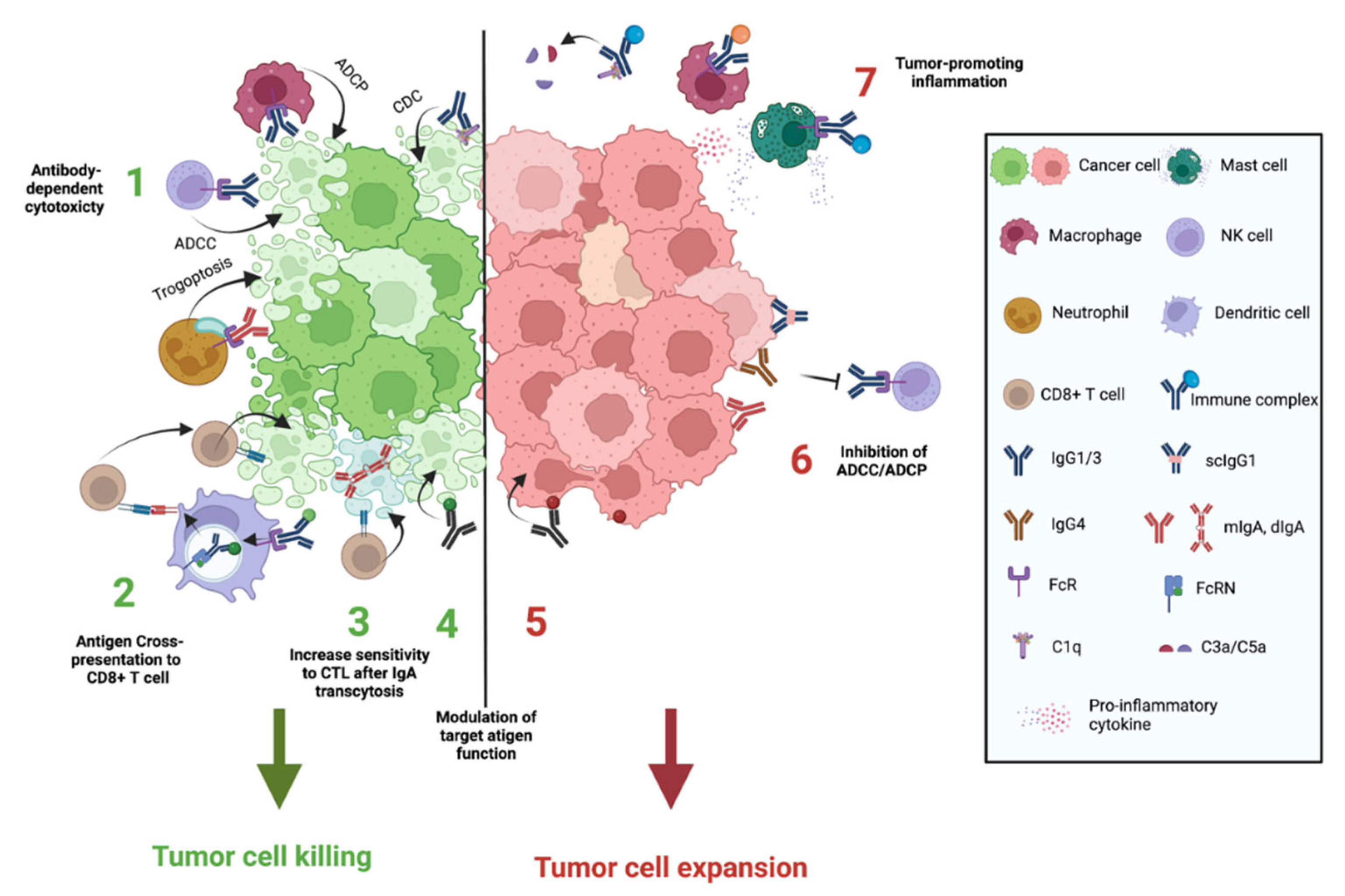
3. Antibodies Functions in Cancer
3.1. Antibody Isotypes and Isoforms Determine Their Functions
3.2. Antibody-Mediated Killing of Tumor Cells
3.3. Antibodies Can Promote Anti-Tumor Immunity by Favoring Antigen Presentation to T Cells
3.4. IgA Transcytosis in Tumor Cells May Increase Sensitivity to CD8+ T Lymphocytes Cytotoxicity
3.5. Antibodies Can Suppress Tumor Cell Killing
3.6. Antigen/Antibody Immune Complexes Tumor-Promoting Inflammation
3.7. Modulation of Tumor-Associated Antigens by Antibodies
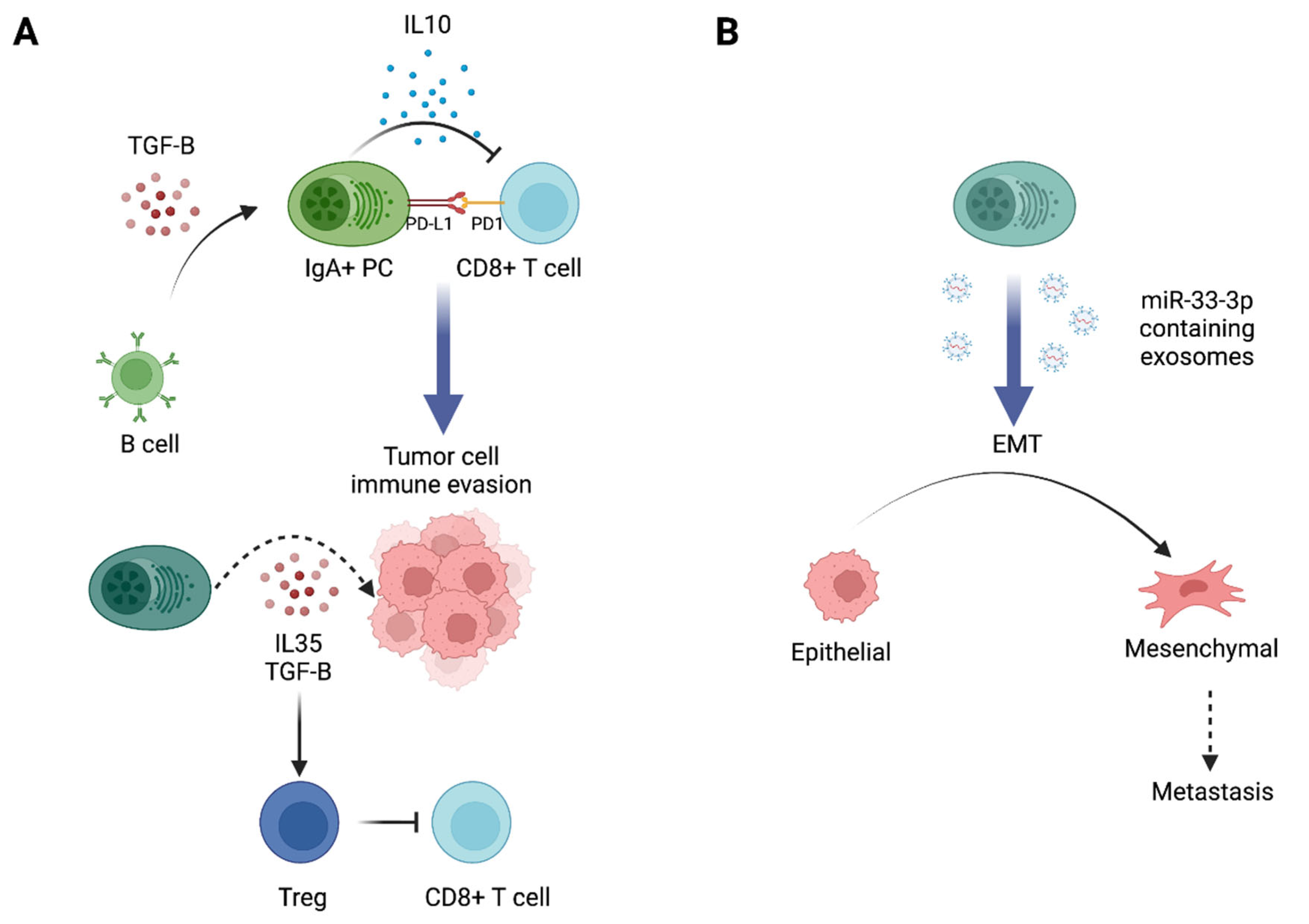
4. Antibody-Independent Pro- vs. Antitumor Functions of Antibody-Producing Cells
4.1. Cytokine Secretion
4.2. Other Functions of ASCs in the TME
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garaud, S.; Buisseret, L.; Solinas, C.; Gu-Trantien, C.; De Wind, A.; Van den Eynden, G.; Naveaux, C.; Lodewyckx, J.-N.; Boisson, A.; Duvillier, H.; et al. Tumor-infiltrating B cells signal functional humoral immune responses in breast cancer. JCI Insight 2019, 4, e129641. [Google Scholar] [CrossRef] [PubMed]
- Marsigliante, S.; Biscozzo, L.; Marra, A.; Nicolardi, G.; Leo, G.; Lobreglio, G.B.; Storelli, C. Computerised counting of tumour infiltrating lymphocytes in 90 breast cancer specimens. Cancer Lett. 1999, 9, 33–41. [Google Scholar] [CrossRef]
- Milne, K.; Köbel, M.; Kalloger, S.E.; Barnes, R.O.; Gao, D.; Gilks, C.B.; Watson, P.H.; Nelson, B.H. Systematic Analysis of Immune Infiltrates in High-Grade Serous Ovarian Cancer Reveals CD20, FoxP3 and TIA-1 as Positive Prognostic Factors. PLoS ONE 2009, 4, e6412. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.; Janseens, J.; Vandepitte, J.; Vandenbrande, J.; Opdebeek, L.; Raus, J. Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res. 1992, 12, 1463–1466. [Google Scholar]
- Buisseret, L.; Garaud, S.; de Wind, A.; Van den Eynden, G.; Boisson, A.; Solinas, C.; Gu-Trantien, C.; Naveaux, C.; Lodewyckx, J.-N.; Duvillier, H.; et al. Tumor-infiltrating lymphocyte composition, organization and PD-1/ PD-L1 expression are linked in breast cancer. Oncoimmunology 2017, 6, e1257452. [Google Scholar] [CrossRef]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020, 577, 549–555. [Google Scholar] [CrossRef]
- Kroeger, D.R.; Milne, K.; Nelson, B.H. Tumor-Infiltrating Plasma Cells Are Associated with Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer. Clin. Cancer Res. 2016, 22, 3005–3015. [Google Scholar] [CrossRef]
- Roumenina, L.T.; Daugan, M.V.; Petitprez, F.; Sautès-Fridman, C.; Fridman, W.H. Context-dependent roles of complement in cancer. Nat. Rev. Cancer 2019, 19, 698–715. [Google Scholar] [CrossRef]
- Shalapour, S.; Font-Burgada, J.; Di Caro, G.; Zhong, Z.; Sanchez-Lopez, E.; Dhar, D.; Willimsky, G.; Ammirante, M.; Strasner, A.; Hansel, D.E.; et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 2015, 521, 94–98. [Google Scholar] [CrossRef]
- Shalapour, S.; Lin, X.-J.; Bastian, I.N.; Brain, J.; Burt, A.D.; Aksenov, A.A.; Vrbanac, A.F.; Li, W.; Perkins, A.; Matsutani, T.; et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 2017, 551, 340–345. [Google Scholar] [CrossRef]
- Machado-Santos, J.; Saji, E.; Tröscher, A.R.; Paunovic, M.; Liblau, R.; Gabriely, G.; Bien, C.G.; Bauer, J.; Lassmann, H. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 2018, 141, 2066–2082. [Google Scholar] [CrossRef]
- Beissert, S.; Hosoi, J.; Grabbe, S.; Asahina, A.; Granstein, R.D. IL-10 inhibits tumor antigen presentation by epidermal antigen-presenting cells. J. Immunol. 1995, 154, 1280–1286. [Google Scholar] [PubMed]
- Yue, F.Y.; Dummer, R.; Geertsen, R.; Hofbauer, G.; Laine, E.; Manolio, S.; Burg, G. Interleukin-10 is a growth factor for human melanoma cells and down-regulates HLA class-I, HLA class-II and ICAM-1 molecules. Int. J. Cancer 1997, 71, 630–637. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, J.; Li, M.; Wu, Z.-J.; Song, K.H.; Zhan, T.W.; Wang, L.-H.; Sun, Y.-H. Interleukin 10-expressing B cells inhibit tumor-infiltrating T cell function and correlate with T cell Tim-3 expression in renal cell carcinoma. Tumor Biol. 2016, 37, 8209–8218. [Google Scholar] [CrossRef] [PubMed]
- Neves, P.; Lampropoulou, V.; Calderon-Gomez, E.; Roch, T.; Stervbo, U.; Shen, P.; Kühl, A.A.; Loddenkemper, C.; Haury, M.; Nedospasov, S.A.; et al. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity 2010, 33, 777–790. [Google Scholar] [CrossRef]
- Fillatreau, S.; Sweenie, C.H.; McGeachy, M.J.; Gray, D.; Anderton, S.M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 2002, 3, 944–950. [Google Scholar] [CrossRef]
- Mohammed, Z.M.A.; Going, J.J.; Edwards, J.; Elsberger, B.; Doughty, J.C.; McMillan, D.C. The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br. J. Cancer 2012, 107, 864–873. [Google Scholar] [CrossRef]
- Nielsen, J.S.; Sahota, R.A.; Milne, K.; Kost, S.E.; Nesslinger, N.J.; Watson, P.H.; Nelson, B.H. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin. Cancer Res. 2012, 18, 3281–3292. [Google Scholar] [CrossRef]
- Wang, Y.; Ylera, F.; Boston, M.; Kang, S.-G.; Kutok, J.L.; Klein-Szanto, A.J.P.; Junghans, R.P. Focused antibody response in plasma cell-infiltrated non-medullary (NOS) breast cancers. Breast Cancer Res. Treat. 2007, 104, 129–144. [Google Scholar] [CrossRef]
- Lundgren, S.; Berntsson, J.; Nodin, B.; Micke, P.; Jirström, K. Prognostic impact of tumour-associated B cells and plasma cells in epithelial ovarian cancer. J. Ovarian Res. 2016, 9, 21. [Google Scholar] [CrossRef]
- Pal, B.; Chen, Y.; Vaillant, F.; Capaldo, B.D.; Joyce, R.; Song, X.; Bryant, V.L.; Penington, J.S.; Di Stefano, L.; Ribera, N.T.; et al. A single-cell RNA expression atlas of normal, preneoplastic and tumorigenic states in the human breast. EMBO J. 2021, 40, e107333. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Hong, Y.; Qi, P.; Lu, G.; Mai, X.; Xu, S.; He, X.; Guo, Y.; Gao, L.; Jing, Z.; et al. Atlas of breast cancer infiltrated B-lymphocytes revealed by paired single-cell RNA-sequencing and antigen receptor profiling. Nat. Commun. 2021, 12, 2186. [Google Scholar] [CrossRef] [PubMed]
- Miligy, I.; Mohan, P.; Gaber, A.; Aleskandarany, M.A.; Nolan, C.C.; Diez-Rodriguez, M.; Mukherjee, A.; Chapman, C.; Ellis, I.O.; Green, A.R.; et al. Prognostic significance of tumour infiltrating B lymphocytes in breast ductal carcinoma in situ. Histopathology 2017, 71, 258–268. [Google Scholar] [CrossRef] [PubMed]
- deLeeuw, R.J.; Kroeger, D.R.; Kost, S.E.; Chang, P.-P.; Webb, J.R.; Nelson, B.H. CD25 Identifies a Subset of CD4+ FoxP3− TIL That Are Exhausted Yet Prognostically Favorable in Human Ovarian Cancer. Cancer Immunol. Res. 2015, 3, 245–253. [Google Scholar] [CrossRef]
- Yeong, J.; Lim, J.C.T.; Lee, B.; Li, H.; Chia, N.; Ong, C.C.H.; Lye, W.K.; Putti, T.C.; Dent, R.; Lim, E.; et al. High Densities of Tumor-Associated Plasma Cells Predict Improved Prognosis in Triple Negative Breast Cancer. Front. Immunol. 2018, 9, 1209. [Google Scholar] [CrossRef]
- Montfort, A.; Pearce, O.; Maniati, E.; Vincent, B.G.; Bixby, L.; Böhm, S.; Dowe, T.; Wilkes, E.H.; Chakravarty, P.; Thompson, R.; et al. A Strong B-cell Response Is Part of the Immune Landscape in Human High-Grade Serous Ovarian Metastases. Clin. Cancer Res. 2017, 23, 250–262. [Google Scholar] [CrossRef]
- Wieczorek, M.; Braicu, E.I.; Oliveira-Ferrer, L.; Sehouli, J.; Blanchard, V. Immunoglobulin G Subclass-Specific Glycosylation Changes in Primary Epithelial Ovarian Cancer. Front. Immunol. 2020, 11, 654. [Google Scholar] [CrossRef]
- Gerçel-Taylor, C.; Bazzett, L.B.; Taylor, D.D. Presence of aberrant tumor-reactive immunoglobulins in the circulation of patients with ovarian cancer. Gynecol. Oncol. 2001, 81, 71–76. [Google Scholar] [CrossRef]
- Ruhaak, L.R.; Kim, K.; Stroble, C.; Taylor, S.L.; Hong, Q.; Miyamoto, S.; Lebrilla, C.B.; Leiserowitz, G. Protein-Specific Differential Glycosylation of Immunoglobulins in Serum of Ovarian Cancer Patients. J. Proteome Res. 2016, 15, 1002–1010. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, Y.; Zhang, X.; Zhou, L.; Zhang, Z.; Xu, J.; Ruan, Y.; Ren, S.; Xu, C.; Gu, J. Quantitative Analysis of Serum IgG Galactosylation Assists Differential Diagnosis of Ovarian Cancer. J. Proteome Res. 2013, 12, 4046–4055. [Google Scholar] [CrossRef]
- Alley, W.R.; Vasseur, J.A.; Goetz, J.A.; Svoboda, M.; Mann, B.F.; Matei, D.E.; Menning, N.; Hussein, A.; Mechref, Y.; Novotny, M.V. N-linked Glycan Structures and Their Expressions Change in the Blood Sera of Ovarian Cancer Patients. J. Proteome Res. 2012, 11, 2282–2300. [Google Scholar] [CrossRef] [PubMed]
- Saldova, R.; Royle, L.; Radcliffe, C.M.; Abd Hamid, U.M.; Evans, R.; Arnold, J.N.; Banks, R.E.; Hutson, R.; Harvey, D.J.; Antrobus, R.; et al. Ovarian Cancer is Associated with Changes in Glycosylation in Both Acute-Phase Proteins and IgG. Glycobiology 2007, 17, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Gaffey, M.J.; Frierson, H.F.; Mills, S.E.; Boyd, J.C.; Zarbo, R.J.; Simpson, J.F.; Gross, L.K.; Weiss, L.M. Medullary carcinoma of the breast. Identification of lymphocyte subpopulations and their significance. Mod. Pathol. 1993, 6, 721–728. [Google Scholar] [PubMed]
- Ito, T.; Saga, S.; Nagayoshi, S.; Imai, M.; Aoyama, A.; Yokoi, T.; Hoshino, M. Class distribution of immunoglobulin-containing plasma cells in the stroma of medullary carcinoma of breast. Breast Cancer Res. Treat. 1986, 7, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Mandal, G.; Payne, K.K.; Anadon, C.M.; Gatenbee, C.D.; Chaurio, R.A.; Costich, T.L.; Moran, C.; Harro, C.M.; Rigolizzo, K.E.; et al. IgA transcytosis and antigen recognition govern ovarian cancer immunity. Nature 2021, 591, 464–470. [Google Scholar] [CrossRef]
- Sahin, U.; Türeci, O.; Schmitt, H.; Cochlovius, B.; Johannes, T.; Schmits, R.; Stenner, F.; Luo, G.; Schobert, I.; Pfreundschuh, M. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc. Natl. Acad. Sci. USA 1995, 92, 11810–11813. [Google Scholar] [CrossRef]
- Fosså, A.; Alsøe, L.; Crameri, R.; Funderud, S.; Gaudernack, G.; Smeland, E.B. Serological cloning of cancer/testis antigens expressed in prostate cancer using cDNA phage surface display. Cancer Immunol. Immunother. CII 2004, 53, 431–438. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Scanlan, M.J.; Sahin, U.; Türeci, Ö.; Gure, A.O.; Tsang, S.; Williamson, B.; Stockert, E.; Pfreundschuh, M.; Old, L.J. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc. Natl. Acad. Sci. USA 1997, 94, 1914–1918. [Google Scholar] [CrossRef]
- Gnjatic, S.; Ritter, E.; Büchler, M.W.; Giese, N.A.; Brors, B.; Frei, C.; Murray, A.; Halama, N.; Zörnig, I.; Chen, Y.-T.; et al. Seromic profiling of ovarian and pancreatic cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 5088–5093. [Google Scholar] [CrossRef]
- Blixt, O.; Bueti, D.; Burford, B.; Allen, D.; Julien, S.; Hollingsworth, M.; Gammerman, A.; Fentiman, I.; Taylor-Papadimitriou, J.; Burchell, J.M. Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis. Breast Cancer Res. 2011, 13, R25. [Google Scholar] [CrossRef]
- Richards, E.R.; Devine, P.L.; Quin, R.J.; Fontenot, J.D.; Ward, B.G.; McGuckin, M.A. Antibodies reactive with the protein core of MUC1 mucin are present in ovarian cancer patients and healthy women. Cancer Immunol. Immunother. 1998, 46, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Kulić, A.; Sirotković-Skerlev, M.; Jelisavac-Cosić, S.; Herceg, D.; Kovac, Z.; Vrbanec, D. Anti-p53 antibodies in serum: Relationship to tumor biology and prognosis of breast cancer patients. Med. Oncol. 2010, 27, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Lenner, P.; Wiklund, F.; Emdin, S.O.; Arnerlöv, C.; Eklund, C.; Hallmans, G.; Zentgraf, H.; Dillner, J. Serum antibodies against p53 in relation to cancer risk and prognosis in breast cancer: A population-based epidemiological study. Br. J. Cancer 1999, 79, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Erić-Nikolić, A.; Milovanović, Z.; Sánchez, D.; Pekáriková, A.; Džodić, R.; Matić, I.Z.; Tučková, L.; Jevrić, M.; Buta, M.; Rašković, S.; et al. Overexpression of calreticulin in malignant and benign breast tumors: Relationship with humoral immunity. Oncology 2012, 82, 48–55. [Google Scholar] [CrossRef]
- Desmetz, C.; Bascoul-Mollevi, C.; Rochaix, P.; Lamy, P.-J.; Kramar, A.; Rouanet, P.; Maudelonde, T.; Mangé, A.; Solassol, J. Identification of a New Panel of Serum Autoantibodies Associated with the Presence of In situ Carcinoma of the Breast in Younger Women. Clin. Cancer Res. 2009, 15, 4733–4741. [Google Scholar] [CrossRef]
- Honnorat, J.; Cartalat-Carel, S.; Ricard, D.; Camdessanche, J.P.; Carpentier, A.F.; Rogemond, V.; Chapuis, F.; Aguera, M.; Decullier, E.; Duchemin, A.M.; et al. Onco-neural antibodies and tumour type determine survival and neurological symptoms in paraneoplastic neurological syndromes with Hu or CV2/CRMP5 antibodies. J. Neurol. Neurosurg. Psychiatry 2009, 80, 412–416. [Google Scholar] [CrossRef]
- Eichler, T.W.; Totland, C.; Haugen, M.; Qvale, T.H.; Mazengia, K.; Storstein, A.; Haukanes, B.I.; Vedeler, C.A. CDR2L Antibodies: A New Player in Paraneoplastic Cerebellar Degeneration. PLoS ONE 2013, 8, e66002. [Google Scholar] [CrossRef]
- Jarius, S.; Wildemann, B. “Medusa head ataxia”: The expanding spectrum of Purkinje cell antibodies in autoimmune cerebellar ataxia. Part 2: Anti-PKC-gamma, anti-GluR-delta2, anti-Ca/ARHGAP26 and anti-VGCC. J. Neuroinflamm. 2015, 12, 167. [Google Scholar] [CrossRef]
- Germain, C.; Gnjatic, S.; Tamzalit, F.; Knockaert, S.; Remark, R.; Goc, J.; Lepelley, A.; Becht, E.; Katsahian, S.; Bizouard, G.; et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am. J. Respir. Crit. Care Med. 2014, 189, 832–844. [Google Scholar] [CrossRef]
- Garaud, S.; Zayakin, P.; Buisseret, L.; Rulle, U.; Silina, K.; de Wind, A.; Van den Eyden, G.; Larsimont, D.; Willard-Gallo, K.; Linē, A. Antigen Specificity and Clinical Significance of IgG and IgA Autoantibodies Produced in situ by Tumor-Infiltrating B Cells in Breast Cancer. Front. Immunol. 2018, 9, 2660. [Google Scholar] [CrossRef]
- Meylan, M.; Petitprez, F.; Becht, E.; Bougoüin, A.; Pupier, G.; Calvez, A.; Giglioli, I.; Verkarre, V.; Lacroix, G.; Verneau, J.; et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity 2022, 55, 527–541.e5. [Google Scholar] [CrossRef] [PubMed]
- Coronella, J.A.; Spier, C.; Welch, M.; Trevor, K.T.; Stopeck, A.T.; Villar, H.; Hersh, E.M. Antigen-driven oligoclonal expansion of tumor-infiltrating B cells in infiltrating ductal carcinoma of the breast. J. Immunol. 2002, 169, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Odendahl, M.; Mei, H.; Hoyer, B.F.; Jacobi, A.M.; Hansen, A.; Muehlinghaus, G.; Berek, C.; Hiepe, F.; Manz, R.; Radbruch, A.; et al. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood 2005, 105, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Martinet, L.; Garrido, I.; Filleron, T.; Le Guellec, S.; Bellard, E.; Fournie, J.-J.; Rochaix, P.; Girard, J.-P. Human solid tumors contain high endothelial venules: Association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011, 71, 5678–5687. [Google Scholar] [CrossRef]
- Truxova, I.; Kasikova, L.; Hensler, M.; Skapa, P.; Laco, J.; Pecen, L.; Belicova, L.; Praznovec, I.; Halaska, M.J.; Brtnicky, T.; et al. Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. J. Immunother. Cancer 2018, 6, 139. [Google Scholar] [CrossRef]
- Ukita, M.; Hamanishi, J.; Yoshitomi, H.; Yamanoi, K.; Takamatsu, S.; Ueda, A.; Suzuki, H.; Hosoe, Y.; Furutake, Y.; Taki, M.; et al. Tertiary lymphoid structures induced by CXCL13-producing CD4+ T cells increase tumor infiltrating CD8+ T cells and B cells in ovarian cancer. bioRxiv 2021. [Google Scholar] [CrossRef]
- Sautès-Fridman, C.; Verneau, J.; Sun, C.-M.; Moreira, M.; Chen, T.W.-W.; Meylan, M.; Petitprez, F.; Fridman, W.H. Tertiary Lymphoid Structures and B cells: Clinical impact and therapeutic modulation in cancer. Semin. Immunol. 2020, 48, 101406. [Google Scholar] [CrossRef]
- Griss, J.; Bauer, W.; Wagner, C.; Simon, M.; Chen, M.; Grabmeier-Pfistershammer, K.; Maurer-Granofszky, M.; Roka, F.; Penz, T.; Bock, C.; et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat. Commun. 2019, 10, 4186. [Google Scholar] [CrossRef]
- Wieland, A.; Patel, M.R.; Cardenas, M.A.; Eberhardt, C.S.; Hudson, W.H.; Obeng, R.C.; Griffith, C.C.; Wang, X.; Chen, Z.G.; Kissick, H.T.; et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature 2021, 597, 274–278. [Google Scholar] [CrossRef]
- Nzula, S.; Going, J.J.; Stott, D.I. Antigen-driven clonal proliferation, somatic hypermutation, and selection of B lymphocytes infiltrating human ductal breast carcinomas. Cancer Res. 2003, 63, 3275–3280. [Google Scholar]
- Mazor, R.D.; Nathan, N.; Gilboa, A.; Stoler-Barak, L.; Moss, L.; Solomonov, I.; Hanuna, A.; Divinsky, Y.; Shmueli, M.D.; Hezroni, H.; et al. Tumor-reactive antibodies evolve from non-binding and autoreactive precursors. Cell 2022, 185, 1208–1222.e21. [Google Scholar] [CrossRef] [PubMed]
- Small, M.; Treilleux, I.; Couillault, C.; Pissaloux, D.; Picard, G.; Paindavoine, S.; Attignon, V.; Wang, Q.; Rogemond, V.; Lay, S.; et al. Genetic alterations and tumor immune attack in Yo paraneoplastic cerebellar degeneration. Acta Neuropathol. 2018, 135, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Bosisio, F.M.; Wilmott, J.S.; Volders, N.; Mercier, M.; Wouters, J.; Stas, M.; Blokx, W.A.; Massi, D.; Thompson, J.F.; Scolyer, R.A.; et al. Plasma cells in primary melanoma. Prognostic significance and possible role of IgA. Mod. Pathol. 2016, 29, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Cipponi, A.; Mercier, M.; Seremet, T.; Baurain, J.-F.; Théate, I.; van den Oord, J.; Stas, M.; Boon, T.; Coulie, P.G.; van Baren, N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012, 72, 3997–4007. [Google Scholar] [CrossRef]
- Aversa, G.; Cocks, B.G.; Punnonen, J.; Carballido, J.M.; de Vries, J.E. Contact-mediated signals and cytokines involved in B-cell activation and isotype switching in pre-B and mature B cells. Res. Immunol. 1994, 145, 222–226, discussion 244–249. [Google Scholar] [CrossRef]
- Coffman, R.L.; Lebman, D.A.; Rothman, P. Mechanism and regulation of immunoglobulin isotype switching. Adv. Immunol. 1993, 54, 229–270. [Google Scholar] [CrossRef]
- Morita, R.; Schmitt, N.; Bentebibel, S.-E.; Ranganathan, R.; Bourdery, L.; Zurawski, G.; Foucat, E.; Dullaers, M.; Oh, S.; Sabzghabaei, N.; et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011, 34, 108–121. [Google Scholar] [CrossRef]
- Noël, G.; Fontsa, M.L.; Garaud, S.; De Silva, P.; de Wind, A.; Van den Eynden, G.G.; Salgado, R.; Boisson, A.; Locy, H.; Thomas, N.; et al. Functional Th1-oriented T follicular helper cells that infiltrate human breast cancer promote effective adaptive immunity. J. Clin. Investig. 2021, 131, e139905. [Google Scholar] [CrossRef]
- Karagiannis, P.; Gilbert, A.E.; Josephs, D.H.; Ali, N.; Dodev, T.; Saul, L.; Correa, I.; Roberts, L.; Beddowes, E.; Koers, A.; et al. IgG4 subclass antibodies impair antitumor immunity in melanoma. J. Clin. Investig. 2013, 123, 1457–1474. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Sad, S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 1996, 17, 138–146. [Google Scholar] [CrossRef]
- Kurte, M.; López, M.; Aguirre, A.; Escobar, A.; Aguillón, J.C.; Charo, J.; Larsen, C.G.; Kiessling, R.; Salazar-Onfray, F. A synthetic peptide homologous to functional domain of human IL-10 down-regulates expression of MHC class I and Transporter associated with Antigen Processing 1/2 in human melanoma cells. J. Immunol. 2004, 173, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Fu, P.; Yao, M.; Chen, Y.; Du, L. Breast cancer stem cells phenotype and plasma cell-predominant breast cancer independently indicate poor survival. Pathol. Res. Pract. 2016, 212, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, W.; Zhao, L.; Wang, X.; Gimple, R.C.; Xu, L.; Wang, Y.; Rich, J.N.; Zhou, S. Plasma cells shape the mesenchymal identity of ovarian cancers through transfer of exosome-derived microRNAs. Sci. Adv. 2021, 7, eabb0737. [Google Scholar] [CrossRef] [PubMed]
- Saqi, A.; Yun, S.S.; Yu, G.H.; Alexis, D.; Taub, R.N.; Powell, C.A.; Borczuk, A.C. Utility of CD138 (syndecan-1) in distinguishing carcinomas from mesotheliomas. Diagn. Cytopathol. 2005, 33, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lohr, M.; Edlund, K.; Botling, J.; Hammad, S.; Hellwig, B.; Othman, A.; Berglund, A.; Lambe, M.; Holmberg, L.; Ekman, S.; et al. The prognostic relevance of tumour-infiltrating plasma cells and immunoglobulin kappa C indicates an important role of the humoral immune response in non-small cell lung cancer. Cancer Lett. 2013, 333, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Hellwig, B.; Hammad, S.; Othman, A.; Lohr, M.; Chen, Z.; Boehm, D.; Gebhard, S.; Petry, I.; Lebrecht, A.; et al. A Comprehensive Analysis of Human Gene Expression Profiles Identifies Stromal Immunoglobulin κ C as a Compatible Prognostic Marker in Human Solid Tumors. Clin. Cancer Res. 2012, 18, 2695–2703. [Google Scholar] [CrossRef]
- McCarron, M.J.; Park, P.W.; Fooksman, D.R. CD138 mediates selection of mature plasma cells by regulating their survival. Blood 2017, 129, 2749–2759. [Google Scholar] [CrossRef]
- Bolotin, D.A.; Poslavsky, S.; Davydov, A.N.; Frenkel, F.E.; Fanchi, L.; Zolotareva, O.I.; Hemmers, S.; Putintseva, E.V.; Obraztsova, A.S.; Shugay, M.; et al. Antigen receptor repertoire profiling from RNA-seq data. Nat. Biotechnol. 2017, 35, 908–911. [Google Scholar] [CrossRef]
- Isaeva, O.I.; Sharonov, G.V.; Serebrovskaya, E.O.; Turchaninova, M.A.; Zaretsky, A.R.; Shugay, M.; Chudakov, D.M. Intratumoral immunoglobulin isotypes predict survival in lung adenocarcinoma subtypes. J. Immunother. Cancer 2019, 7, 279. [Google Scholar] [CrossRef]
- Welinder, C.; Jirström, K.; Lehn, S.; Nodin, B.; Marko-Varga, G.; Blixt, O.; Danielsson, L.; Jansson, B. Intra-tumour IgA1 is common in cancer and is correlated with poor prognosis in bladder cancer. Heliyon 2016, 2, e00143. [Google Scholar] [CrossRef]
- Wang, H.; Chen, D.; Wang, R.; Quan, W.; Xia, D.; Mei, J.; Xu, J.; Liu, C. NY-ESO-1 expression in solid tumors predicts prognosis. Medicine 2019, 98, e17990. [Google Scholar] [CrossRef]
- Patil, N.S.; Nabet, B.Y.; Müller, S.; Koeppen, H.; Zou, W.; Giltnane, J.; Au-Yeung, A.; Srivats, S.; Cheng, J.H.; Takahashi, C.; et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer. Cancer Cell 2022, 40, 289–300.e4. [Google Scholar] [CrossRef] [PubMed]
- Hollern, D.P.; Xu, N.; Thennavan, A.; Glodowski, C.; Garcia-Recio, S.; Mott, K.R.; He, X.; Garay, J.P.; Carey-Ewend, K.; Marron, D.; et al. B Cells and T Follicular Helper Cells Mediate Response to Checkpoint Inhibitors in High Mutation Burden Mouse Models of Breast Cancer. Cell 2019, 179, 1191–1206.e21. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alonso, S.; Setti-Jerez, G.; Arroyo, M.; Hernández, T.; Martos, M.I.; Sánchez-Torres, J.M.; Colomer, R.; Ramiro, A.R.; Alfranca, A. A new role for circulating T follicular helper cells in humoral response to anti-PD-1 therapy. J. Immunother. Cancer 2020, 8, e001187. [Google Scholar] [CrossRef] [PubMed]
- Iglesia, M.D.; Vincent, B.G.; Parker, J.S.; Hoadley, K.; Carey, L.A.; Perou, C.M.; Serody, J.S. Prognostic B-Cell Signatures using mRNA-Seq in Patients with Subtype-Specific Breast and Ovarian Cancer. Clin. Cancer Res. 2014, 20, 3818–3829. [Google Scholar] [CrossRef] [PubMed]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Ridolfi, R.L.; Rosen, P.P.; Port, A.; Kinne, D.; Miké, V. Medullary carcinoma of the breast. A clinicopathologic study with 10 year follow-up. Cancer 1977, 40, 1365–1385. [Google Scholar] [CrossRef]
- Kuroda, H.; Jamiyan, T.; Yamaguchi, R.; Kakumoto, A.; Abe, A.; Harada, O.; Enkhbat, B.; Masunaga, A. Prognostic value of tumor-infiltrating B lymphocytes and plasma cells in triple-negative breast cancer. Breast Cancer 2021, 28, 904–914. [Google Scholar] [CrossRef]
- Fan, C.; Prat, A.; Parker, J.S.; Liu, Y.; Carey, L.A.; Troester, M.A.; Perou, C.M. Building prognostic models for breast cancer patients using clinical variables and hundreds of gene expression signatures. BMC Med. Genom. 2011, 4, 3. [Google Scholar] [CrossRef]
- Harris, R.J.; Cheung, A.; Ng, J.C.F.; Laddach, R.; Chenoweth, A.M.; Crescioli, S.; Fittall, M.; Dominguez-Rodriguez, D.; Roberts, J.; Levi, D.; et al. Tumor-Infiltrating B Lymphocyte Profiling Identifies IgG-Biased, Clonally Expanded Prognostic Phenotypes In Triple-Negative Breast Cancer. Cancer Res. 2021, 81, 4290–4304. [Google Scholar] [CrossRef]
- Steplewski, Z.; Sun, L.K.; Shearman, C.W.; Ghrayeb, J.; Daddona, P.; Koprowski, H. Biological activity of human-mouse IgG1, IgG2, IgG3, and IgG4 chimeric monoclonal antibodies with antitumor specificity. Proc. Natl. Acad. Sci. USA 1988, 85, 4852–4856. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.C.; Kornblith, P.L.; Quindlen, E.A.; Pollock, L.A. Detection of humoral immune response to human brain tumors: Specificity and reliability of microcytotoxicity assay. Cancer 1979, 43, 86–90. [Google Scholar] [CrossRef]
- Gilbert, A.E.; Karagiannis, P.; Dodev, T.; Koers, A.; Lacy, K.; Josephs, D.H.; Takhar, P.; Geh, J.L.C.; Healy, C.; Harries, M.; et al. Monitoring the systemic human memory B cell compartment of melanoma patients for anti-tumor IgG antibodies. PLoS ONE 2011, 6, e19330. [Google Scholar] [CrossRef] [PubMed]
- DeFalco, J.; Harbell, M.; Manning-Bog, A.; Baia, G.; Scholz, A.; Millare, B.; Sumi, M.; Zhang, D.; Chu, F.; Dowd, C.; et al. Non-progressing cancer patients have persistent B cell responses expressing shared antibody paratopes that target public tumor antigens. Clin. Immunol. 2018, 187, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Bendtzen, K.; Hansen, M.B.; Ross, C.; Svenson, M. High-avidity autoantibodies to cytokines. Immunol. Today 1998, 19, 209–211. [Google Scholar] [CrossRef]
- Lopes-Carvalho, T.; Kearney, J.F. Development and selection of marginal zone B cells. Immunol. Rev. 2004, 197, 192–205. [Google Scholar] [CrossRef]
- Elkon, K.; Casali, P. Nature and functions of autoantibodies. Nat. Clin. Pract. Rheumatol. 2008, 4, 491–498. [Google Scholar] [CrossRef]
- Vollmers, H.P.; Brandlein, S. Death by stress: Natural IgM-induced apoptosis. Methods Find. Exp. Clin. Pharmacol. 2005, 27, 185–191. [Google Scholar] [CrossRef]
- Atif, S.M.; Gibbings, S.L.; Redente, E.F.; Camp, F.A.; Torres, R.M.; Kedl, R.M.; Henson, P.M.; Jakubzick, C.V. Immune Surveillance by Natural IgM Is Required for Early Neoantigen Recognition and Initiation of Adaptive Immunity. Am. J. Respir. Cell Mol. Biol. 2018, 59, 580–591. [Google Scholar] [CrossRef]
- Díaz-Zaragoza, M.; Hernández-Ávila, R.; Viedma-Rodríguez, R.; Arenas-Aranda, D.; Ostoa-Saloma, P. Natural and adaptive IgM antibodies in the recognition of tumor-associated antigens of breast cancer (Review). Oncol. Rep. 2015, 34, 1106–1114. [Google Scholar] [CrossRef]
- Musolino, A.; Naldi, N.; Bortesi, B.; Pezzuolo, D.; Capelletti, M.; Missale, G.; Laccabue, D.; Zerbini, A.; Camisa, R.; Bisagni, G.; et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J. Clin. Oncol. 2008, 26, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Banks, N.D.; Kinsey, N.; Clements, J.; Hildreth, J.E.K. Sustained antibody-dependent cell-mediated cytotoxicity (ADCC) in SIV-infected macaques correlates with delayed progression to AIDS. AIDS Res. Hum. Retrovir. 2002, 18, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Hatjiharissi, E.; Xu, L.; Santos, D.D.; Hunter, Z.R.; Ciccarelli, B.T.; Verselis, S.; Modica, M.; Cao, Y.; Manning, R.J.; Leleu, X.; et al. Increased natural killer cell expression of CD16, augmented binding and ADCC activity to rituximab among individuals expressing the Fc{gamma}RIIIa-158 V/V and V/F polymorphism. Blood 2007, 110, 2561–2564. [Google Scholar] [CrossRef] [PubMed]
- Clynes, R.A.; Towers, T.L.; Presta, L.G.; Ravetch, J.V. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat. Med. 2000, 6, 443–446. [Google Scholar] [CrossRef]
- Hubert, P.; Heitzmann, A.; Viel, S.; Nicolas, A.; Sastre-Garau, X.; Oppezzo, P.; Pritsch, O.; Osinaga, E.; Amigorena, S. Antibody-dependent cell cytotoxicity synapses form in mice during tumor-specific antibody immunotherapy. Cancer Res. 2011, 71, 5134–5143. [Google Scholar] [CrossRef] [PubMed]
- Muntasell, A.; Cabo, M.; Servitja, S.; Tusquets, I.; Martínez-García, M.; Rovira, A.; Rojo, F.; Albanell, J.; López-Botet, M. Interplay between Natural Killer Cells and Anti-HER2 Antibodies: Perspectives for Breast Cancer Immunotherapy. Front. Immunol. 2017, 8, 1544. [Google Scholar] [CrossRef]
- Gül, N.; Babes, L.; Siegmund, K.; Korthouwer, R.; Bögels, M.; Braster, R.; Vidarsson, G.; ten Hagen, T.L.M.; Kubes, P.; van Egmond, M. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. J. Clin. Investig. 2014, 124, 812–823. [Google Scholar] [CrossRef]
- Albanesi, M.; Mancardi, D.A.; Jönsson, F.; Iannascoli, B.; Fiette, L.; Di Santo, J.P.; Lowell, C.A.; Bruhns, P. Neutrophils mediate antibody-induced antitumor effects in mice. Blood 2013, 122, 3160–3164. [Google Scholar] [CrossRef] [PubMed]
- Matlung, H.L.; Szilagyi, K.; Barclay, N.A.; van den Berg, T.K. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol. Rev. 2017, 276, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Merle, N.S.; Noe, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part II: Role in Immunity. Front. Immunol. 2015, 6, 257. [Google Scholar] [CrossRef]
- Niculescu, F.; Rus, H.G.; Retegan, M.; Vlaicu, R. Persistent complement activation on tumor cells in breast cancer. Am. J. Pathol. 1992, 140, 1039–1043. [Google Scholar] [PubMed]
- Cho, M.S.; Vasquez, H.G.; Rupaimoole, R.; Pradeep, S.; Wu, S.; Zand, B.; Han, H.-D.; Rodriguez-Aguayo, C.; Bottsford-Miller, J.; Huang, J.; et al. Autocrine effects of tumor-derived complement. Cell Rep. 2014, 6, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.W.; Laskowski, J.; Li, H.Y.; McSharry, M.V.; Sippel, T.R.; Bullock, B.L.; Johnson, A.M.; Poczobutt, J.M.; Neuwelt, A.J.; Malkoski, S.P.; et al. Complement Activation via a C3a Receptor Pathway Alters CD4+ T Lymphocytes and Mediates Lung Cancer Progression. Cancer Res. 2018, 78, 143–156. [Google Scholar] [CrossRef]
- Taylor, R.P.; Lindorfer, M.A.; Cook, E.M.; Beurskens, F.J.; Schuurman, J.; Parren, P.W.H.I.; Zent, C.S.; VanDerMeid, K.R.; Burack, R.; Mizuno, M.; et al. Hexamerization-enhanced CD20 antibody mediates complement-dependent cytotoxicity in serum genetically deficient in C9. Clin. Immunol. 2017, 181, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Gaetano, N.D.; Cittera, E.; Nota, R.; Vecchi, A.; Grieco, V.; Scanziani, E.; Botto, M.; Introna, M.; Golay, J. Complement Activation Determines the Therapeutic Activity of Rituximab In Vivo. J. Immunol. 2003, 171, 1581–1587. [Google Scholar] [CrossRef]
- Liu, M.; Yang, Y.-J.; Zheng, H.; Zhong, X.-R.; Wang, Y.; Wang, Z.; Wang, Y.-G.; Wang, Y.-P. Membrane-bound complement regulatory proteins are prognostic factors of operable breast cancer treated with adjuvant trastuzumab: A retrospective study. Oncol. Rep. 2014, 32, 2619–2627. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.-J.; Wang, Z.; Liao, J.; Liu, M.; Zhong, X.-R.; Zheng, H.; Wang, Y.-P. CD55 and CD59 expression protects HER2-overexpressing breast cancer cells from trastuzumab-induced complement-dependent cytotoxicity. Oncol. Lett. 2017, 14, 2961–2969. [Google Scholar] [CrossRef]
- Gordan, S.; Albert, H.; Danzer, H.; Lux, A.; Biburger, M.; Nimmerjahn, F. The Immunological Organ Environment Dictates the Molecular and Cellular Pathways of Cytotoxic Antibody Activity. Cell Rep. 2019, 29, 3033–3046.e4. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Neovius, M.; Ye, W.; Hammarström, L. IgA deficiency and risk of cancer: A population-based matched cohort study. J. Clin. Immunol. 2015, 35, 182–188. [Google Scholar] [CrossRef]
- Woof, J.M.; Russell, M.W. Structure and function relationships in IgA. Mucosal Immunol. 2011, 4, 590–597. [Google Scholar] [CrossRef]
- Brandtzaeg, P.; Kvale, D.; Sollid, L.M.; Thrane, P.S. The Poly-Ig Receptor—Functional Aspects of Secretory Component Expression. In Histophysiology of the Immune System: The Life History, Organization, and Interactions of Its Cell Populations; Fossum, S., Rolstad, B., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1988; pp. 9–20. ISBN 978-1-4684-5535-9. [Google Scholar]
- Lu, J.; Marjon, K.D.; Marnell, L.L.; Wang, R.; Mold, C.; Du Clos, T.W.; Sun, P. Recognition and functional activation of the human IgA receptor (FcαRI) by C-reactive protein. Proc. Natl. Acad. Sci. USA 2011, 108, 4974–4979. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.C.; Van De Winkel, J.G.J. IgA Fc receptors. Annu. Rev. Immunol. 2003, 21, 177–204. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, A.M.; Jacobino, S.R.; Meyer, S.; ten Broeke, T.; Leusen, J.H.W. Fc receptor inside-out signaling and possible impact on antibody therapy. Immunol. Rev. 2015, 268, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Boross, P.; van Lent, P.L.; Martin-Ramirez, J.; van der Kaa, J.; Mulder, M.H.C.M.; Claassens, J.W.C.; van den Berg, W.B.; Arandhara, V.L.; Verbeek, J.S. Destructive arthritis in the absence of both FcgammaRI and FcgammaRIII. J. Immunol. 2008, 180, 5083–5091. [Google Scholar] [CrossRef]
- Borrok, M.J.; Luheshi, N.M.; Beyaz, N.; Davies, G.C.; Legg, J.W.; Wu, H.; Dall’Acqua, W.F.; Tsui, P. Enhancement of antibody-dependent cell-mediated cytotoxicity by endowing IgG with FcαRI (CD89) binding. mAbs 2015, 7, 743–751. [Google Scholar] [CrossRef]
- Lohse, S.; Loew, S.; Kretschmer, A.; Jansen, J.H.M.; Meyer, S.; Ten Broeke, T.; Rösner, T.; Dechant, M.; Derer, S.; Klausz, K.; et al. Effector mechanisms of IgA antibodies against CD20 include recruitment of myeloid cells for antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity. Br. J. Haematol. 2018, 181, 413–417. [Google Scholar] [CrossRef]
- Boross, P.; Lohse, S.; Nederend, M.; Jansen, J.H.M.; van Tetering, G.; Dechant, M.; Peipp, M.; Royle, L.; Liew, L.P.; Boon, L.; et al. IgA EGFR antibodies mediate tumour killing in vivo. EMBO Mol. Med. 2013, 5, 1213–1226. [Google Scholar] [CrossRef]
- Treffers, L.W.; Ten Broeke, T.; Rösner, T.; Jansen, J.H.M.; van Houdt, M.; Kahle, S.; Schornagel, K.; Verkuijlen, P.J.J.H.; Prins, J.M.; Franke, K.; et al. IgA-Mediated Killing of Tumor Cells by Neutrophils Is Enhanced by CD47-SIRPα Checkpoint Inhibition. Cancer Immunol. Res. 2020, 8, 120–130. [Google Scholar] [CrossRef]
- Strunk, R.C.; Bloomberg, G.R. Omalizumab for asthma. N. Engl. J. Med. 2006, 354, 2689–2695. [Google Scholar] [CrossRef]
- Crawford, G.; Hayes, M.D.; Seoane, R.C.; Ward, S.; Dalessandri, T.; Lai, C.; Healy, E.; Kipling, D.; Proby, C.; Moyes, C.; et al. Epithelial damage and tissue γδ T cells promote a unique tumor-protective IgE response. Nat. Immunol. 2018, 19, 859–870. [Google Scholar] [CrossRef]
- Josephs, D.H.; Bax, H.J.; Dodev, T.; Georgouli, M.; Nakamura, M.; Pellizzari, G.; Saul, L.; Karagiannis, P.; Cheung, A.; Herraiz, C.; et al. Anti-Folate Receptor-α IgE but not IgG Recruits Macrophages to Attack Tumors via TNFα/MCP-1 Signaling. Cancer Res. 2017, 77, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Ly, D.; Zhu, C.-Q.; Cabanero, M.; Tsao, M.-S.; Zhang, L. Role for High-Affinity IgE Receptor in Prognosis of Lung Adenocarcinoma Patients. Cancer Immunol. Res. 2017, 5, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Amigorena, S.; Lankar, D.; Briken, V.; Gapin, L.; Viguier, M.; Bonnerot, C. Type II and III receptors for immunoglobulin G (IgG) control the presentation of different T cell epitopes from single IgG-complexed antigens. J. Exp. Med. 1998, 187, 505–515. [Google Scholar] [CrossRef]
- Baker, K.; Rath, T.; Flak, M.B.; Arthur, J.C.; Chen, Z.; Glickman, J.N.; Zlobec, I.; Karamitopoulou, E.; Stachler, M.D.; Odze, R.D.; et al. Neonatal Fc Receptor Expression in Dendritic Cells Mediates Protective Immunity against Colorectal Cancer. Immunity 2013, 39, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, K.; Bergtold, A.; Clynes, R. Immune complex–mediated antigen presentation induces tumor immunity. J. Clin. Investig. 2002, 110, 71–79. [Google Scholar] [CrossRef]
- Platzer, B.; Elpek, K.G.; Cremasco, V.; Baker, K.; Stout, M.M.; Schultz, C.; Dehlink, E.; Shade, K.-T.C.; Anthony, R.M.; Blumberg, R.S.; et al. IgE/FcεRI-Mediated Antigen Cross-Presentation by Dendritic Cells Enhances Anti-Tumor Immune Responses. Cell Rep. 2015, 10, 1487–1495. [Google Scholar] [CrossRef]
- Otten, M.A.; Groenveld, I.; van de Winkel, J.G.J.; van Egmond, M. Inefficient antigen presentation via the IgA Fc receptor (FcalphaRI) on dendritic cells. Immunobiology 2006, 211, 503–510. [Google Scholar] [CrossRef]
- Gayet, R.; Michaud, E.; Nicoli, F.; Chanut, B.; Paul, M.; Rochereau, N.; Guillon, C.; He, Z.; Papagno, L.; Bioley, G.; et al. Impact of IgA isoforms on their ability to activate dendritic cells and to prime T cells. Eur. J. Immunol. 2020, 50, 1295–1306. [Google Scholar] [CrossRef]
- Mandal, G.; Biswas, S.; Anadon, C.M.; Yu, X.; Gatenbee, C.D.; Prabhakaran, S.; Payne, K.K.; Chaurio, R.A.; Martin, A.; Innamarato, P.; et al. IgA-Dominated Humoral Immune Responses Govern Patients’ Outcome in Endometrial Cancer. Cancer Res. 2022, 82, 859–871. [Google Scholar] [CrossRef]
- Cianga, P.; Cianga, C.; Cozma, L.; Ward, E.S.; Carasevici, E. The MHC class I related Fc receptor, FcRn, is expressed in the epithelial cells of the human mammary gland. Hum. Immunol. 2003, 64, 1152–1159. [Google Scholar] [CrossRef]
- Aalberse, R.C.; Stapel, S.O.; Schuurman, J.; Rispens, T. Immunoglobulin G4: An odd antibody. Clin. Exp. Allergy 2009, 39, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Oaks, M.; Taylor, S.; Shaffer, J. Autoantibodies targeting tumor-associated antigens in metastatic cancer: Sialylated IgGs as candidate anti-inflammatory antibodies. Oncoimmunology 2013, 2, e24841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Deng, H.; Fan, X.; Gonzalez, A.; Zhang, S.; Brezski, R.J.; Choi, B.-K.; Rycyzyn, M.; Strohl, W.; Jordan, R.; et al. Dysfunctional Antibodies in the Tumor Microenvironment Associate with Impaired Anticancer Immunity. Clin. Cancer Res. 2015, 21, 5380–5390. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Brezski, R.J.; Fa, M.; Deng, H.; Oberholtzer, A.; Gonzalez, A.; Dubinsky, W.P.; Strohl, W.R.; Jordan, R.E.; Zhang, N.; et al. A single proteolytic cleavage within the lower hinge of trastuzumab reduces immune effector function and in vivo efficacy. Breast Cancer Res. 2012, 14, R116. [Google Scholar] [CrossRef]
- Tomaras, G.D.; Ferrari, G.; Shen, X.; Alam, S.M.; Liao, H.-X.; Pollara, J.; Bonsignori, M.; Moody, M.A.; Fong, Y.; Chen, X.; et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc. Natl. Acad. Sci. USA 2013, 110, 9019–9024. [Google Scholar] [CrossRef]
- Monteiro, R.C. Role of IgA and IgA fc receptors in inflammation. J. Clin. Immunol. 2010, 30, 1–9. [Google Scholar] [CrossRef]
- Pasquier, B.; Launay, P.; Kanamaru, Y.; Moura, I.C.; Pfirsch, S.; Ruffié, C.; Hénin, D.; Benhamou, M.; Pretolani, M.; Blank, U.; et al. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: Dual role of FcRgamma ITAM. Immunity 2005, 22, 31–42. [Google Scholar] [CrossRef]
- Wehrli, M.; Cortinas-Elizondo, F.; Hlushchuk, R.; Daudel, F.; Villiger, P.M.; Miescher, S.; Zuercher, A.W.; Djonov, V.; Simon, H.-U.; von Gunten, S. Human IgA Fc receptor FcαRI (CD89) triggers different forms of neutrophil death depending on the inflammatory microenvironment. J. Immunol. 2014, 193, 5649–5659. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Dass, T.K.; Aziz, M.; Rattan, A.; Tyagi, S.P. Clinical utility and monitoring of breast cancer by circulating immune complexes. Indian J. Pathol. Microbiol. 1992, 35, 298–307. [Google Scholar]
- Andreu, P.; Johansson, M.; Affara, N.I.; Pucci, F.; Tan, T.; Junankar, S.; Korets, L.; Lam, J.; Tawfik, D.; DeNardo, D.G.; et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell 2010, 17, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Gunn, L.; Ding, C.; Liu, M.; Ma, Y.; Qi, C.; Cai, Y.; Hu, X.; Aggarwal, D.; Zhang, H.-G.; Yan, J. Opposing roles for complement component C5a in tumor progression and the tumor microenvironment. J. Immunol. 2012, 189, 2985–2994. [Google Scholar] [CrossRef] [PubMed]
- Vollmers, H.P.; Brändlein, S. Natural human immunoglobulins in cancer immunotherapy. Immunotherapy 2009, 1, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Pohle, T.; Brändlein, S.; Ruoff, N.; Müller-Hermelink, H.K.; Vollmers, H.P. Lipoptosis: Tumor-specific cell death by antibody-induced intracellular lipid accumulation. Cancer Res. 2004, 64, 3900–3906. [Google Scholar] [CrossRef] [PubMed]
- Gazit, G.; Hung, G.; Chen, X.; Anderson, W.F.; Lee, A.S. Use of the glucose starvation-inducible glucose-regulated protein 78 promoter in suicide gene therapy of murine fibrosarcoma. Cancer Res. 1999, 59, 3100–3106. [Google Scholar]
- Dalmau, J.; Tüzün, E.; Wu, H.; Masjuan, J.; Rossi, J.E.; Voloschin, A.; Baehring, J.M.; Shimazaki, H.; Koide, R.; King, D.; et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann. Neurol. 2007, 61, 25–36. [Google Scholar] [CrossRef]
- North, W.G.; Liu, F.; Tian, R.; Abbasi, H.; Akerman, B. NMDA receptors are expressed in human ovarian cancer tissues and human ovarian cancer cell lines. Clin. Pharmacol. 2015, 7, 111–117. [Google Scholar] [CrossRef]
- Hughes, E.G.; Peng, X.; Gleichman, A.J.; Lai, M.; Zhou, L.; Tsou, R.; Parsons, T.D.; Lynch, D.R.; Dalmau, J.; Balice-Gordon, R.J. Cellular and Synaptic Mechanisms of Anti-NMDA Receptor Encephalitis. J. Neurosci. 2010, 30, 5866–5875. [Google Scholar] [CrossRef]
- Lynch, D.R.; Rattelle, A.; Dong, Y.N.; Roslin, K.; Gleichman, A.J.; Panzer, J.A. Anti-NMDA Receptor Encephalitis: Clinical Features and Basic Mechanisms. Adv. Pharmacol. 2018, 82, 235–260. [Google Scholar] [CrossRef]
- Lancaster, E.; Lai, M.; Peng, X.; Hughes, E.; Constantinescu, R.; Raizer, J.; Friedman, D.; Skeen, M.B.; Grisold, W.; Kimura, A.; et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: Case series and characterisation of the antigen. Lancet Neurol. 2010, 9, 67–76. [Google Scholar] [CrossRef]
- Zhu, F.; Shan, W.; Lv, R.; Li, Z.; Wang, Q. Clinical Characteristics of Anti-GABA-B Receptor Encephalitis. Front. Neurol. 2020, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Fillatreau, S. Antibody-independent functions of B cells: A focus on cytokines. Nat. Rev. Immunol. 2015, 15, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Narayanan, P.; Kang, N.; Clayton, S.; Ohne, Y.; Shi, P.; Herve, M.-C.; Balderas, R.; Picard, C.; Casanova, J.-L.; et al. Human plasma cells express granzyme B. Eur. J. Immunol. 2014, 44, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Cupi, M.L.; Sarra, M.; Marafini, I.; Monteleone, I.; Franzè, E.; Ortenzi, A.; Colantoni, A.; Sica, G.; Sileri, P.; Rosado, M.M.; et al. Plasma Cells in the Mucosa of Patients with Inflammatory Bowel Disease Produce Granzyme B and Possess Cytotoxic Activities. J. Immunol. 2014, 192, 6083–6091. [Google Scholar] [CrossRef] [PubMed]
- Adrain, C.; Murphy, B.M.; Martin, S.J. Molecular ordering of the caspase activation cascade initiated by the cytotoxic T lymphocyte/natural killer (CTL/NK) protease granzyme B. J. Biol. Chem. 2005, 280, 4663–4673. [Google Scholar] [CrossRef] [PubMed]
- Sharif-Askari, E.; Alam, A.; Rhéaume, E.; Beresford, P.J.; Scotto, C.; Sharma, K.; Lee, D.; DeWolf, W.E.; Nuttall, M.E.; Lieberman, J.; et al. Direct cleavage of the human DNA fragmentation factor-45 by granzyme B induces caspase-activated DNase release and DNA fragmentation. EMBO J. 2001, 20, 3101–3113. [Google Scholar] [CrossRef] [PubMed]
- Neumann, L.; Mueller, M.; Moos, V.; Heller, F.; Meyer, T.F.; Loddenkemper, C.; Bojarski, C.; Fehlings, M.; Doerner, T.; Allers, K.; et al. Mucosal Inducible NO Synthase–Producing IgA+ Plasma Cells in Helicobacter pylori–Infected Patients. J. Immunol. 2016, 197, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Glynn, S.A.; Boersma, B.J.; Dorsey, T.H.; Yi, M.; Yfantis, H.G.; Ridnour, L.A.; Martin, D.N.; Switzer, C.H.; Hudson, R.S.; Wink, D.A.; et al. Increased NOS2 predicts poor survival in estrogen receptor–negative breast cancer patients. J. Clin. Investig. 2010, 120, 3843–3854. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, M.; Martin, J.H.J. Nitric oxide decreases motility and increases adhesion in human breast cancer cells. Oncol. Rep. 2009, 21, 275–281. [Google Scholar]
- Jing, L.; Kim, S.; Sun, L.; Wang, L.; Mildner, E.; Divaris, K.; Jiao, Y.; Offenbacher, S. IL-37- and IL-35/IL-37-Producing Plasma Cells in Chronic Periodontitis. J. Dent. Res. 2019, 98, 813–821. [Google Scholar] [CrossRef]
- Liu, X.; Ren, H.; Guo, H.; Wang, W.; Zhao, N. Interleukin-35 has a tumor-promoting role in hepatocellular carcinoma. Clin. Exp. Immunol. 2021, 203, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Kurebayashi, Y.; Emoto, K.; Hayashi, Y.; Kamiyama, I.; Ohtsuka, T.; Asamura, H.; Sakamoto, M. Comprehensive Immune Profiling of Lung Adenocarcinomas Reveals Four Immunosubtypes with Plasma Cell Subtype a Negative Indicator. Cancer Immunol. Res. 2016, 4, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ullrich, S.E.; Bar-Eli, M. Regulation of tumor growth and metastasis by interleukin-10: The melanoma experience. J. Interferon Cytokine Res. 1999, 19, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, A.; Horimoto, Y.; Onagi, H.; Ikarashi, D.; Nakayama, T.; Nakatsura, T.; Shimizu, H.; Kojima, K.; Yao, T.; Matsumoto, T.; et al. Plasma cell infiltration and treatment effect in breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res. 2021, 23, 99. [Google Scholar] [CrossRef] [PubMed]
- Lino, A.C.; Dang, V.D.; Lampropoulou, V.; Welle, A.; Joedicke, J.; Pohar, J.; Simon, Q.; Thalmensi, J.; Baures, A.; Flühler, V.; et al. LAG-3 Inhibitory Receptor Expression Identifies Immunosuppressive Natural Regulatory Plasma Cells. Immunity 2018, 49, 120–133. [Google Scholar] [CrossRef]

| Author/Year | Histological Tumor Type | Number of Patients | Identification of ASC | Prognosis | Reference |
|---|---|---|---|---|---|
| Kroeger et al., 2016 | HGSOC | 30 | CD20−CD38+CD138+cytosolicCD79a+ IHC CD19+IgD−CD38+ Flow Cytometry TNFRSF17/IGJ PC gene signature | Good | [7] |
| Lundgren et al., 2016 | OC | 209 | CD138 IHC IGKC gene expression | Poor Neutral | [20] |
| Yang et al., 2021 | HGSOC | 351 | Gene signature (CIBERSORT) | Poor | [73] |
| Biswas et al., 2021 | HGSOC | 534 | CD19+CD138+ (multiplex IHC) Internalized IgA in tumor cells | Good (total area and epithelial tumor islets) Good | [35] |
| Schmidt et al., 2012 | BC OC | 1810 426 | IGKC expression | Good Neutral | [76] |
| Iglesia et al., 2014 | BC OC | 728 266 | IgG cluster | Good (nonluminal BC) Good (mesenchymal and immunoreactive molecular subtypes) | [85] |
| Gentles et al., 2015 | Pan-cancer | 796 BC 1127 OC | Plasma cell gene signature (Cibersort) | Good (BC) Neutral (OC) | [86] |
| Ridolfi et al., 1977 | Infiltrating ductal carcinoma | 192 | Morphological identification on hematoxylin and eosin-stained slides | Neutral (Medullary carcinoma) Good (others) | [87] |
| Yeong et al., 2018 | Triple-negative BC | 269 | intratumoral CD38+ IHC stromal CD38+ IHC | Good Neutral | [25] |
| Mohammed et al., 2012 | Invasive ductal breast cancer | 468 | CD138+ IHC and morphological identification (H&E) | Poor | [17] |
| Miligy et al., 2017 | Invasive BC | 44 | CD138+ IHC | Neutral | [23] |
| Kuroda et al., 2021 | TNBC | 114 | Stromal CD38+ IHC Intratumoral CD38+ IHC, stromal and intratumoral CD138+ IHC | Good Neutral | [88] |
| Fan et al., 2011 | BC | 550 | IGG gene cluster expression | Good | [89] |
| Harris et al., 2021 | TNBC | 69 | Plasma cell signature (Cibersort) | Neutral | [90] |
| Wei et al., 2016 | BC | 92 | Morphological identification (typical “cartwheel” nucleus) | Poor | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lounici, Y.; Le Saux, O.; Chemin, G.; Wajda, P.; Barrin, S.; Berthet, J.; Caux, C.; Dubois, B. Heterogeneity and Functions of Tumor-Infiltrating Antibody Secreting Cells: Lessons from Breast, Ovarian, and Other Solid Cancers. Cancers 2022, 14, 4800. https://doi.org/10.3390/cancers14194800
Lounici Y, Le Saux O, Chemin G, Wajda P, Barrin S, Berthet J, Caux C, Dubois B. Heterogeneity and Functions of Tumor-Infiltrating Antibody Secreting Cells: Lessons from Breast, Ovarian, and Other Solid Cancers. Cancers. 2022; 14(19):4800. https://doi.org/10.3390/cancers14194800
Chicago/Turabian StyleLounici, Yasmine, Olivia Le Saux, Gabriel Chemin, Pauline Wajda, Sarah Barrin, Justine Berthet, Christophe Caux, and Bertrand Dubois. 2022. "Heterogeneity and Functions of Tumor-Infiltrating Antibody Secreting Cells: Lessons from Breast, Ovarian, and Other Solid Cancers" Cancers 14, no. 19: 4800. https://doi.org/10.3390/cancers14194800
APA StyleLounici, Y., Le Saux, O., Chemin, G., Wajda, P., Barrin, S., Berthet, J., Caux, C., & Dubois, B. (2022). Heterogeneity and Functions of Tumor-Infiltrating Antibody Secreting Cells: Lessons from Breast, Ovarian, and Other Solid Cancers. Cancers, 14(19), 4800. https://doi.org/10.3390/cancers14194800








