Intestinal Microbiota: The Driving Force behind Advances in Cancer Immunotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. The Amazing Therapeutic Potential of Cancer Immunotherapy
3. Combination Immunotherapy
4. Limitations of CAR T-Cell Therapy in Solid Tumors
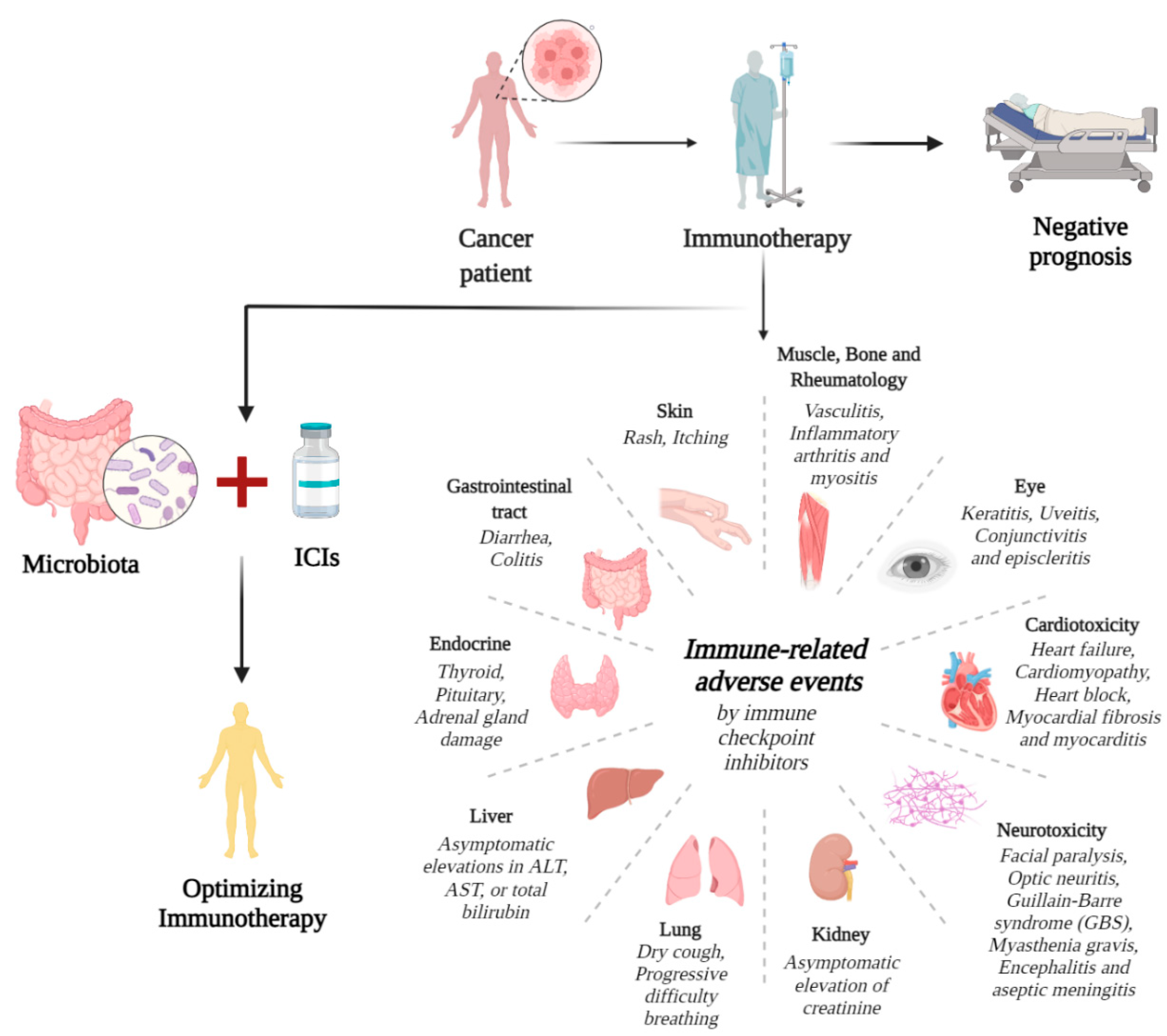
5. Immune-Related Adverse Events
6. Intestinal Microbiota Regulates Pharmacokinetics
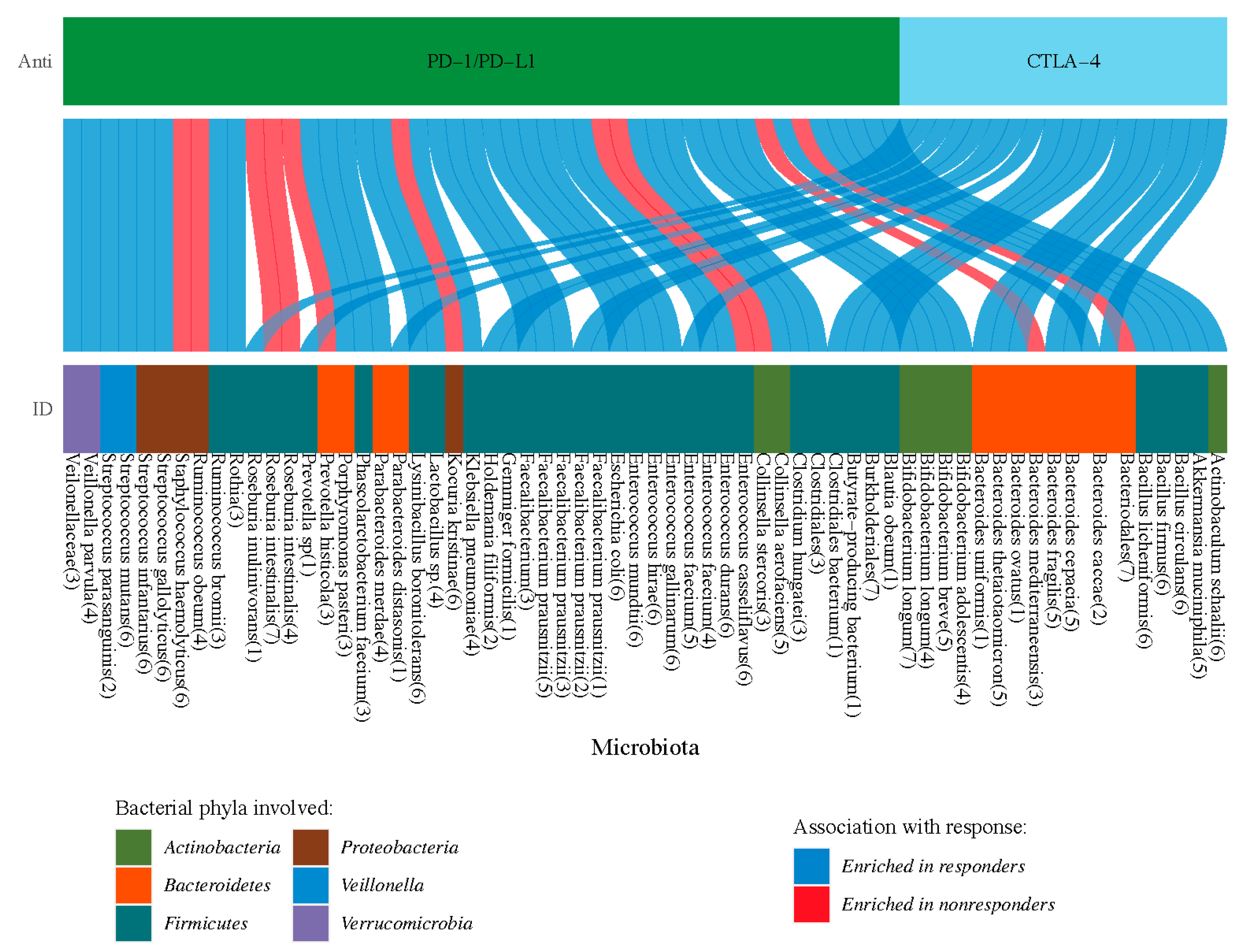
7. Microbiota Correlates with Cancer Immunotherapy
8. Gut Microbiome Expands Path for Cancer Immunotherapy
| Microbial Species | Immune Optimization | Anti-PD-1/L1 | Anti-CTLA-4 | Tumors | References |
|---|---|---|---|---|---|
| Alistipes putredinis | Memory CD8+ T cells ↑ NK cells(peripheral) ↑ | ↑ | NSCLC RCC | [100] | |
| Akkermansia muciniphila | CXCR3+CCR9+CD4+ T cells ↑ DCs ↑ IL-12 ↑ | ↑ | NSCLC RCC | [100] | |
| Bacteroides spp. | MDSCs and Tregs ↑ Immune-related adverse events ↑ IL-12 ↓ DCs ↓ | ↓ | ↓ | MM | [87] |
| Bacteroides fragilis | Th1 cells ↑ Foxp3+ Tregs ↑ DCs ↑ | ↑ | MM NSCLC | [107] | |
| Bifidobacterium spp. | DCs ↑ Lymphocytes ↑ IFN-γ ↑ Pro-inflammatory cytokine ↑ Tumor-specific CD8+ T cells ↑ | ↑ | MM | [94] [85] | |
| Enterococcus faecium | T cell responses ↑ | ↑ | MM | [10] | |
| Escherichia Clostridium | Differentiation of Tregs ↑ Inflammation ↓ | ↑ | MM | [7] | |
| Faecalibacterium. spp. | CD4+/CD8+ T cells ↑ Tregs ↑ ICOS expression of T cells ↑ | ↑ | ↑ | MM | [87] |
| Ruminococcaceae spp. | Antigen presentation ↑ T cells ↑ IFN-γ CD8+ T cells ↑ | ↓ | MM | [94] | |
| Microbial-derived SCFAs (butyrate, propionate) | Differentiation of Tregs ↑ | ↑ | CRC | [108] |
9. The Clinical Application of Intestinal Microbiota
10. The Combination of FMT and Immunotherapy
11. Probiotics and Prebiotics
12. Antibiotic and Immunotherapy Efficacy
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Nakajima, H.; Nakatsura, T. Towards the era of immune checkpoint inhibitors and personalized cancer immunotherapy. Immunol. Med. 2021, 44, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, Z.; Zhang, W.; Zhang, W.; Buzdin, A.; Mu, X.; Yan, Q.; Zhao, X.; Chang, H.H.; Duhon, M.; et al. FDA-Approved and Emerging Next Generation Predictive Biomarkers for Immune Checkpoint Inhibitors in Cancer Patients. Front. Oncol. 2021, 11, 683419. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Soon, Y.Y.; Aminkeng, F.; Tay, S.H.; Ang, Y.; Kee, A.C.L.; Goh, B.C.; Wong, A.S.C.; Soo, R.A. Risk factors for immune-related adverse events from anti-PD-1 or anti-PD-L1 treatment in an Asian cohort of nonsmall cell lung cancer patients. Int. J. Cancer 2021, 150, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Derakhshani, A.; Rostami, Z.; Safarpour, H.; Shadbad, M.A.; Nourbakhsh, N.S.; Argentiero, A.; Taefehshokr, S.; Tabrizi, N.J.; Kooshkaki, O.; Astamal, R.V.; et al. From Oncogenic Signaling Pathways to Single-Cell Sequencing of Immune Cells: Changing the Landscape of Cancer Immunotherapy. Molecules 2021, 26, 2278. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.M.; Doyle, R.M.; Temisak, S.; Redshaw, N.; Whale, A.S.; Logan, G.; Huang, J.; Fischer, N.; Amos, G.C.A.; Preston, M.D.; et al. An inter-laboratory study to investigate the impact of the bioinformatics component on microbiome analysis using mock communities. Sci. Rep. 2021, 11, 10590. [Google Scholar] [CrossRef]
- Tourlousse, D.M.; Narita, K.; Miura, T.; Sakamoto, M.; Ohashi, A.; Shiina, K.; Matsuda, M.; Miura, D.; Shimamura, M.; Ohyama, Y.; et al. Validation and standardization of DNA extraction and library construction methods for metagenomics-based human fecal microbiome measurements. Microbiome 2021, 9, 95. [Google Scholar] [CrossRef]
- Frankel, A.E.; Deshmukh, S.; Reddy, A.; Lightcap, J.; Hayes, M.; McClellan, S.; Singh, S.; Rabideau, B.; Glover, T.G.; Roberts, B.; et al. Cancer Immune Checkpoint Inhibitor Therapy and the Gut Microbiota. Integr. Cancer 2019, 18, 1534735419846379. [Google Scholar] [CrossRef]
- Fessler, J.; Matson, V.; Gajewski, T.F. Exploring the emerging role of the microbiome in cancer immunotherapy. J. Immunother. Cancer 2019, 7, 108. [Google Scholar] [CrossRef]
- Vetizou, M.; Pitt, J.M.; Daillere, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, M.; Tamiya, A.; Hosoya, K.; Taniguchi, Y.; Yokoyama, T.; Fukuda, Y.; Hirano, K.; Matsumoto, H.; Kominami, R.; Suzuki, H.; et al. Efficacy and safety of pembrolizumab as first-line therapy in advanced non-small cell lung cancer with at least 50% PD-L1 positivity: A multicenter retrospective cohort study (HOPE-001). Investig. New Drugs 2019, 37, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Dudnik, E.; Moskovitz, M.; Rottenberg, Y.; Lobachov, A.; Mandelboim, R.; Shochat, T.; Urban, D.; Wollner, M.; Nechushtan, H.; Rotem, O.; et al. Pembrolizumab as a monotherapy or in combination with platinum-based chemotherapy in advanced non-small cell lung cancer with PD-L1 tumor proportion score (TPS) >/=50%: Real-world data. Oncoimmunology 2021, 10, 1865653. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Elez, E.; Baraibar, I. Immunotherapy in colorectal cancer: An unmet need deserving of change. Lancet Oncol. 2022, 23, 830–831. [Google Scholar] [CrossRef]
- Lee, D.W.; Han, S.W.; Bae, J.M.; Jang, H.; Han, H.; Kim, H.; Bang, D.; Jeong, S.Y.; Park, K.J.; Kang, G.H.; et al. Tumor Mutation Burden and Prognosis in Patients with Colorectal Cancer Treated with Adjuvant Fluoropyrimidine and Oxaliplatin. Clin. Cancer Res. 2019, 25, 6141–6147. [Google Scholar] [CrossRef]
- Picard, E.; Verschoor, C.P.; Ma, G.W.; Pawelec, G. Relationships Between Immune Landscapes, Genetic Subtypes and Responses to Immunotherapy in Colorectal Cancer. Front. Immunol. 2020, 11, 369. [Google Scholar] [CrossRef]
- Andre, T.; Amonkar, M.; Norquist, J.M.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.A.; Smith, D.; Garcia-Carbonero, R.; et al. Health-related quality of life in patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer treated with first-line pembrolizumab versus chemotherapy (KEYNOTE-177): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 665–677. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, X.; Li, Y.; Zhang, Q.; Li, Q.; Zhang, X.; Zhang, M.; Yu, Q.; Gao, D. How to overcome tumor resistance to anti-PD-1/PD-L1 therapy by immunotherapy modifying the tumor microenvironment in MSS CRC. Clin. Immunol. 2022, 237, 108962. [Google Scholar] [CrossRef]
- Xiao, J.; Li, W.; Huang, Y.; Huang, M.; Li, S.; Zhai, X.; Zhao, J.; Gao, C.; Xie, W.; Qin, H.; et al. A next-generation sequencing-based strategy combining microsatellite instability and tumor mutation burden for comprehensive molecular diagnosis of advanced colorectal cancer. BMC Cancer 2021, 21, 282. [Google Scholar] [CrossRef] [PubMed]
- Maio, M.; Amonkar, M.M.; Norquist, J.M.; Ascierto, P.A.; Manzyuk, L.; Motola-Kuba, D.; Penel, N.; Cassier, P.A.; Bariani, G.M.; De Jesus Acosta, A.; et al. Health-related quality of life in patients treated with pembrolizumab for microsatellite instability-high/mismatch repair-deficient advanced solid tumours: Results from the KEYNOTE-158 study. Eur. J. Cancer 2022, 169, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Santin, A.D.; Deng, W.; Frumovitz, M.; Buza, N.; Bellone, S.; Huh, W.; Khleif, S.; Lankes, H.A.; Ratner, E.S.; O’Cearbhaill, R.E.; et al. Phase II evaluation of nivolumab in the treatment of persistent or recurrent cervical cancer (NCT02257528/NRG-GY002). Gynecol. Oncol. 2020, 157, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Janne, P.A.; Opyrchal, M.; Hafez, N.; Raez, L.E.; Gabrilovich, D.I.; Wang, F.; Trepel, J.B.; Lee, M.J.; Yuno, A.; et al. Entinostat plus Pembrolizumab in Patients with Metastatic NSCLC Previously Treated with Anti-PD-(L)1 Therapy. Clin. Cancer Res. 2021, 27, 1019–1028. [Google Scholar] [CrossRef]
- Tewari, K.S.; Monk, B.J.; Vergote, I.; Miller, A.; de Melo, A.C.; Kim, H.S.; Kim, Y.M.; Lisyanskaya, A.; Samouelian, V.; Lorusso, D.; et al. Survival with Cemiplimab in Recurrent Cervical Cancer. N. Engl. J. Med. 2022, 386, 544–555. [Google Scholar] [CrossRef]
- Adams, S.; Diamond, J.R.; Hamilton, E.; Pohlmann, P.R.; Tolaney, S.M.; Chang, C.W.; Zhang, W.; Iizuka, K.; Foster, P.G.; Molinero, L.; et al. Atezolizumab Plus nab-Paclitaxel in the Treatment of Metastatic Triple-Negative Breast Cancer With 2-Year Survival Follow-up: A Phase 1b Clinical Trial. JAMA Oncol. 2019, 5, 334–342. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Orlova, K.; Grignani, G.; Dudzisz-Sledz, M.; Fenig, E.; Chiarion Sileni, V.; Fazio, N.; Samimi, M.; Mortier, L.; Gebhardt, C.; et al. Avelumab expanded access program in metastatic Merkel cell carcinoma: Efficacy and safety findings from patients in Europe and the Middle East. Int. J. Cancer 2021, 149, 1926–1934. [Google Scholar] [CrossRef]
- Riudavets, M.; Auclin, E.; Mosteiro, M.; Dempsey, N.; Majem, M.; Lobefaro, R.; Lopez-Castro, R.; Bosch-Barrera, J.; Pilotto, S.; Escalera, E.; et al. Durvalumab consolidation in patients with unresectable stage III non-small cell lung cancer with driver genomic alterations. Eur. J. Cancer 2022, 167, 142–148. [Google Scholar] [CrossRef]
- Eggermont, A.M. Adjuvant ipilimumab in stage III melanoma: New landscape, new questions. Eur. J. Cancer 2016, 69, 39–42. [Google Scholar] [CrossRef]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit with Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Huo, X.; Shen, G.; Liu, Z.; Liang, Y.; Li, J.; Zhao, F.; Ren, D.; Zhao, J. Addition of immunotherapy to chemotherapy for metastatic triple-negative breast cancer: A systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Oncol. Hematol. 2021, 168, 103530. [Google Scholar] [CrossRef]
- Huang, W.; Chen, J.J.; Xing, R.; Zeng, Y.C. Combination therapy: Future directions of immunotherapy in small cell lung cancer. Transl. Oncol. 2021, 14, 100889. [Google Scholar] [CrossRef]
- Kaur, J.; Elms, J.; Munn, A.L.; Good, D.; Wei, M.Q. Immunotherapy for non-small cell lung cancer (NSCLC), as a stand-alone and in combination therapy. Crit. Rev. Oncol. Hematol. 2021, 164, 103417. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Guo, G.; Cai, X.; Yu, H.; Cai, Y.; Zhang, B.; Hong, S.; Zhang, L. Nivolumab plus ipilimumab versus pembrolizumab as chemotherapy-free, first-line treatment for PD-L1-positive non-small cell lung cancer. Clin. Transl. Med. 2020, 10, 107–115. [Google Scholar] [CrossRef]
- Pires da Silva, I.; Ahmed, T.; Reijers, I.L.M.; Weppler, A.M.; Betof Warner, A.; Patrinely, J.R.; Serra-Bellver, P.; Allayous, C.; Mangana, J.; Nguyen, K.; et al. Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: A multicentre, retrospective, cohort study. Lancet Oncol. 2021, 22, 836–847. [Google Scholar] [CrossRef]
- Amini, L.; Silbert, S.K.; Maude, S.L.; Nastoupil, L.J.; Ramos, C.A.; Brentjens, R.J.; Sauter, C.S.; Shah, N.N.; Abou-El-Enein, M. Preparing for CAR T cell therapy: Patient selection, bridging therapies and lymphodepletion. Nat. Rev. Clin. Oncol. 2022, 19, 342–355. [Google Scholar] [CrossRef]
- Kankeu Fonkoua, L.A.; Sirpilla, O.; Sakemura, R.; Siegler, E.L.; Kenderian, S.S. CAR T cell therapy and the tumor microenvironment: Current challenges and opportunities. Mol. Oncolytics 2022, 25, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Globerson Levin, A.; Riviere, I.; Eshhar, Z.; Sadelain, M. CAR T cells: Building on the CD19 paradigm. Eur. J. Immunol. 2021, 51, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, V.; Yazdanpanah, N.; Rezaei, N. The immunologic aspects of cytokine release syndrome and graft versus host disease following CAR T cell therapy. Int. Rev. Immunol. 2021, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Marofi, F.; Achmad, H.; Bokov, D.; Abdelbasset, W.K.; Alsadoon, Z.; Chupradit, S.; Suksatan, W.; Shariatzadeh, S.; Hasanpoor, Z.; Yazdanifar, M.; et al. Hurdles to breakthrough in CAR T cell therapy of solid tumors. Stem. Cell Res. 2022, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh Kozani, P.; Safarzadeh Kozani, P.; Ahmadi Najafabadi, M.; Yousefi, F.; Mirarefin, S.M.J.; Rahbarizadeh, F. Recent Advances in Solid Tumor CAR-T Cell Therapy: Driving Tumor Cells from Hero to Zero? Front. Immunol. 2022, 13, 795164. [Google Scholar] [CrossRef] [PubMed]
- Murad, J.P.; Tilakawardane, D.; Park, A.K.; Lopez, L.S.; Young, C.A.; Gibson, J.; Yamaguchi, Y.; Lee, H.J.; Kennewick, K.T.; Gittins, B.J.; et al. Pre-conditioning modifies the TME to enhance solid tumor CAR T cell efficacy and endogenous protective immunity. Mol. Ther. 2021, 29, 2335–2349. [Google Scholar] [CrossRef]
- Huang, M.; Xiong, D.; Pan, J.; Zhang, Q.; Sei, S.; Shoemaker, R.H.; Lubet, R.A.; Montuenga, L.M.; Wang, Y.; Slusher, B.S.; et al. Targeting Glutamine Metabolism to Enhance Immunoprevention of EGFR-Driven Lung Cancer. Adv. Sci. 2022, 9, e2105885. [Google Scholar] [CrossRef]
- Cha, S.E.; Kujawski, M.; Yazaki, P.J.; Brown, C.; Shively, J.E. Tumor regression and immunity in combination therapy with anti-CEA chimeric antigen receptor T cells and anti-CEA-IL2 immunocytokine. Oncoimmunology 2021, 10, 1899469. [Google Scholar] [CrossRef]
- Feng, Z.; He, X.; Zhang, X.; Wu, Y.; Xing, B.; Knowles, A.; Shan, Q.; Miller, S.; Hojnacki, T.; Ma, J.; et al. Potent suppression of neuroendocrine tumors and gastrointestinal cancers by CDH17CAR T cells without toxicity to normal tissues. Nat. Cancer 2022, 3, 581–594. [Google Scholar] [CrossRef]
- Andrea, A.E.; Chiron, A.; Bessoles, S.; Hacein-Bey-Abina, S. Engineering Next-Generation CAR-T Cells for Better Toxicity Management. Int. J. Mol. Sci. 2020, 21, 8620. [Google Scholar] [CrossRef]
- Xu, J.; Meng, Q.; Sun, H.; Zhang, X.; Yun, J.; Li, B.; Wu, S.; Li, X.; Yang, H.; Zhu, H.; et al. HER2-specific chimeric antigen receptor-T cells for targeted therapy of metastatic colorectal cancer. Cell Death Dis. 2021, 12, 1109. [Google Scholar] [CrossRef] [PubMed]
- Povoski, S.P.; Hatzaras, I.S.; Mojzisik, C.M.; Martin, E.W., Jr. Oncologic theranostics: Recognition of this concept in antigen-directed cancer therapy for colorectal cancer with anti-TAG-72 monoclonal antibodies. Expert. Rev. Mol. Diagn. 2011, 11, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Magee, M.S.; Abraham, T.S.; Baybutt, T.R.; Flickinger, J.C., Jr.; Ridge, N.A.; Marszalowicz, G.P.; Prajapati, P.; Hersperger, A.R.; Waldman, S.A.; Snook, A.E. Human GUCY2C-Targeted Chimeric Antigen Receptor (CAR)-Expressing T Cells Eliminate Colorectal Cancer Metastases. Cancer Immunol. Res. 2018, 6, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Gao, F.; Li, N.; Li, Q.; Zhou, Y.; Yang, T.; Cai, Z.; Du, P.; Chen, F.; Cai, J. Antitumor activity of NKG2D CAR-T cells against human colorectal cancer cells in vitro and in vivo. Am. J. Cancer Res. 2019, 9, 945–958. [Google Scholar]
- Kim, J.H.; Park, S.Y.; Jeon, S.E.; Choi, J.H.; Lee, C.J.; Jang, T.Y.; Yun, H.J.; Lee, Y.; Kim, P.; Cho, S.H.; et al. DCLK1 promotes colorectal cancer stemness and aggressiveness via the XRCC5/COX2 axis. Theranostics 2022, 12, 5258–5271. [Google Scholar] [CrossRef]
- Sureban, S.M.; Berahovich, R.; Zhou, H.; Xu, S.; Wu, L.; Ding, K.; May, R.; Qu, D.; Bannerman-Menson, E.; Golubovskaya, V.; et al. DCLK1 Monoclonal Antibody-Based CAR-T Cells as a Novel Treatment Strategy against Human Colorectal Cancers. Cancers 2019, 12, 54. [Google Scholar] [CrossRef]
- Zhao, W.; Jia, L.; Zhang, M.; Huang, X.; Qian, P.; Tang, Q.; Zhu, J.; Feng, Z. The killing effect of novel bi-specific Trop2/PD-L1 CAR-T cell targeted gastric cancer. Am. J. Cancer Res. 2019, 9, 1846–1856. [Google Scholar]
- Jin, L.; Tao, H.; Karachi, A.; Long, Y.; Hou, A.Y.; Na, M.; Dyson, K.A.; Grippin, A.J.; Deleyrolle, L.P.; Zhang, W.; et al. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat. Commun. 2019, 10, 4016. [Google Scholar] [CrossRef]
- Gonzalez-Mazon, I.; Sanchez-Bilbao, L.; Martin-Varillas, J.L.; Garcia-Castano, A.; Delgado-Ruiz, M.; Bernat Pina, I.; Hernandez, J.L.; Castaneda, S.; Llorca, J.; Gonzalez-Gay, M.A.; et al. Immune-related adverse events in patients with solid-organ tumours treated with immunotherapy: A 3-year study of 102 cases from a single centre. Clin. Exp. Rheumatol. 2021, 39, 612–620. [Google Scholar] [CrossRef]
- Kartolo, A.; Holstead, R.; Khalid, S.; Emack, J.; Hopman, W.; Baetz, T. Safety of Immunotherapy Rechallenge After Immune-related Adverse Events in Patients with Advanced Cancer. J. Immunother. 2021, 44, 41–48. [Google Scholar] [CrossRef]
- Presotto, E.M.; Rastrelli, G.; Desideri, I.; Scotti, V.; Gunnella, S.; Pimpinelli, N.; Vaccher, E.; Bearz, A.; Di Costanzo, F.; Bruggia, M.; et al. Endocrine toxicity in cancer patients treated with nivolumab or pembrolizumab: Results of a large multicentre study. J. Endocrinol. Investig. 2020, 43, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Zhang, X.C.; Wang, D.Y.; Zhang, H.R.; Zhu, C.; Hou, Y.L.; Liu, J.L.; Gao, Z.H. The risk of immune-related endocrine disorders associated with anti-PD-1 inhibitors therapy for solid tumors: A systematic review and meta-analysis. Int. Immunopharmacol. 2018, 59, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Khadaroo, P.A.; Su, H.; Kong, L.; Chen, L.; Wang, X.; Li, X.; Zhu, H.; Zhong, X.; Pan, J.; et al. The safety and tolerability of combined immune checkpoint inhibitors (anti-PD-1/PD-L1 plus anti-CTLA-4): A systematic review and meta-analysis. BMC Cancer 2019, 19, 559. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, F.; Mangana, J.; Micaletto, S.; Braun, R.P.; Dummer, R. Cytokine release syndrome as an important differential diagnosis of severe skin toxicity with organ damage during switch from immunotherapy to targeted therapy in metastatic melanoma. Melanoma Res. 2019, 29, 107–108. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Shi, C.; Zhou, X.; Xu, K.; Jiao, D.; Sun, Z.; Han, X. A novel immune classification reveals distinct immune escape mechanism and genomic alterations: Implications for immunotherapy in hepatocellular carcinoma. J. Transl. Med. 2021, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhou, J.; Chen, H.; Li, J.; Zhang, C.; Jiang, X.; Ni, C. The immunomodulatory effects of endocrine therapy in breast cancer. J. Exp. Clin. Cancer Res. 2021, 40, 19. [Google Scholar] [CrossRef] [PubMed]
- Siwicki, M.; Gort-Freitas, N.A.; Messemaker, M.; Bill, R.; Gungabeesoon, J.; Engblom, C.; Zilionis, R.; Garris, C.; Gerhard, G.M.; Kohl, A.; et al. Resident Kupffer cells and neutrophils drive liver toxicity in cancer immunotherapy. Sci. Immunol. 2021, 6, eabi7083. [Google Scholar] [CrossRef]
- Mitropoulou, G.; Daccord, C.; Sauty, A.; Pasche, A.; Egger, B.; Aedo Lopez, V.; Letovanec, I.; Beigelman-Aubry, C.; Nicod, L.P.; Lazor, R. Immunotherapy-Induced Airway Disease: A New Pattern of Lung Toxicity of Immune Checkpoint Inhibitors. Respiration 2020, 99, 181–186. [Google Scholar] [CrossRef]
- Haeuser, L.; Marchese, M.; Cone, E.B.; Noldus, J.; Bayliss, G.; Kilbridge, K.L.; Trinh, Q.D. Nephrotoxicity in immune checkpoint inhibitor therapy: A pharmacovigilance study. Nephrol. Dial. Transpl. 2021, 37, 1310–1316. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, C.; Zhou, L.; Dong, P.; Shi, L. Immune Checkpoint Inhibitors and Neurotoxicity. Curr. Neuropharmacol. 2021, 19, 1246–1263. [Google Scholar] [CrossRef]
- Stower, H. Immunotherapy for heart injury. Nat. Med. 2019, 25, 1799. [Google Scholar] [CrossRef] [PubMed]
- Maloney, J.; Durham, S.; Skoner, D.; Dahl, R.; Bufe, A.; Bernstein, D.; Murphy, K.; Waserman, S.; Berman, G.; White, M.; et al. Safety of sublingual immunotherapy Timothy grass tablet in subjects with allergic rhinitis with or without conjunctivitis and history of asthma. Allergy 2015, 70, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Kostine, M.; Finckh, A.; Bingham, C.O.; Visser, K.; Leipe, J.; Schulze-Koops, H.; Choy, E.H.; Benesova, K.; Radstake, T.; Cope, A.P.; et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann. Rheum. Dis. 2021, 80, 36–48. [Google Scholar] [CrossRef]
- Flowers, S.A.; Bhat, S.; Lee, J.C. Potential Implications of Gut Microbiota in Drug Pharmacokinetics and Bioavailability. Pharmacotherapy 2020, 40, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Wang, R. Gut microbiota modulates drug pharmacokinetics. Drug Metab. Rev. 2018, 50, 357–368. [Google Scholar] [CrossRef]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science 2019, 363, eaat9931. [Google Scholar] [CrossRef]
- Ciccolini, J.; Mercier, C.; Blachon, M.F.; Favre, R.; Durand, A.; Lacarelle, B. A simple and rapid high-performance liquid chromatographic (HPLC) method for 5-fluorouracil (5-FU) assay in plasma and possible detection of patients with impaired dihydropyrimidine dehydrogenase (DPD) activity. J. Clin. Pharm. 2004, 29, 307–315. [Google Scholar] [CrossRef]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef]
- Liu, J.R.; Miao, H.; Deng, D.Q.; Vaziri, N.D.; Li, P.; Zhao, Y.Y. Gut microbiota-derived tryptophan metabolism mediates renal fibrosis by aryl hydrocarbon receptor signaling activation. Cell Mol. Life Sci. 2021, 78, 909–922. [Google Scholar] [CrossRef]
- Blasco, T.; Perez-Burillo, S.; Balzerani, F.; Hinojosa-Nogueira, D.; Lerma-Aguilera, A.; Pastoriza, S.; Cendoya, X.; Rubio, A.; Gosalbes, M.J.; Jimenez-Hernandez, N.; et al. An extended reconstruction of human gut microbiota metabolism of dietary compounds. Nat. Commun. 2021, 12, 4728. [Google Scholar] [CrossRef]
- Qiu, T.; Liu, L.; Zhou, H.; Sheng, H.; He, Y.; Liu, M.; Cai, H. Analysis of endometrial microbiota in intrauterine adhesion by high-throughput sequencing. Ann. Transl. Med. 2021, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Galyean, S.; Sawant, D.; Shin, A.C. Immunometabolism, Micronutrients, and Bariatric Surgery: The Use of Transcriptomics and Microbiota-Targeted Therapies. Mediat. Inflamm. 2020, 2020, 8862034. [Google Scholar] [CrossRef] [PubMed]
- Mokkala, K.; Paulin, N.; Houttu, N.; Koivuniemi, E.; Pellonpera, O.; Khan, S.; Pietila, S.; Tertti, K.; Elo, L.L.; Laitinen, K. Metagenomics analysis of gut microbiota in response to diet intervention and gestational diabetes in overweight and obese women: A randomised, double-blind, placebo-controlled clinical trial. Gut 2021, 70, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hauptman, R.; Kelly, L. Engineering the Microbiome to Prevent Adverse Events: Challenges and Opportunities. Annu. Rev. Pharm. Toxicol. 2021, 61, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Yu, S.; Qin, S.; Liu, Q.; Xu, H.; Zhao, W.; Chu, Q.; Wu, K. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 47. [Google Scholar] [CrossRef]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Kroemer, G.; Zitvogel, L. The intestinal microbiota determines the clinical efficacy of immune checkpoint blockers targeting PD-1/PD-L1. Oncoimmunology 2018, 7, e1434468. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillere, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Sears, C.L.; Pardoll, D.M. The intestinal microbiome influences checkpoint blockade. Nat. Med. 2018, 24, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Frankel, A.E.; Coughlin, L.A.; Kim, J.; Froehlich, T.W.; Xie, Y.; Frenkel, E.P.; Koh, A.Y. Metagenomic Shotgun Sequencing and Unbiased Metabolomic Profiling Identify Specific Human Gut Microbiota and Metabolites Associated with Immune Checkpoint Therapy Efficacy in Melanoma Patients. Neoplasia 2017, 19, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Han, Y.; Zhao, X.; Sun, Y. Characteristics of pathogenic microbes in lung microenvironment of lung cancer patients without respiratory infection. J BUON 2021, 26, 1862–1870. [Google Scholar] [PubMed]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Ekmekciu, I.; von Klitzing, E.; Fiebiger, U.; Escher, U.; Neumann, C.; Bacher, P.; Scheffold, A.; Kuhl, A.A.; Bereswill, S.; Heimesaat, M.M. Immune Responses to Broad-Spectrum Antibiotic Treatment and Fecal Microbiota Transplantation in Mice. Front. Immunol. 2017, 8, 397. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Unutmaz, D. Immune cells for microbiota surveillance. Science 2019, 366, 419–420. [Google Scholar] [CrossRef]
- Bachem, A.; Makhlouf, C.; Binger, K.J.; de Souza, D.P.; Tull, D.; Hochheiser, K.; Whitney, P.G.; Fernandez-Ruiz, D.; Dahling, S.; Kastenmuller, W.; et al. Microbiota-Derived Short-Chain Fatty Acids Promote the Memory Potential of Antigen-Activated CD8(+) T Cells. Immunity 2019, 51, 285–297.e5. [Google Scholar] [CrossRef]
- Teng, L.; Wang, K.; Chen, W.; Wang, Y.S.; Bi, L. HYR-2 plays an anti-lung cancer role by regulating PD-L1 and Akkermansia muciniphila. Pharm. Res 2020, 160, 105086. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Fidelle, M.; Iebba, V.; Alla, L.; Pasolli, E.; Segata, N.; Desnoyer, A.; Pietrantonio, F.; Ferrere, G.; et al. Gut Bacteria Composition Drives Primary Resistance to Cancer Immunotherapy in Renal Cell Carcinoma Patients. Eur. Urol. 2020, 78, 195–206. [Google Scholar] [CrossRef]
- Routy, B.; Gopalakrishnan, V.; Daillere, R.; Zitvogel, L.; Wargo, J.A.; Kroemer, G. The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 2018, 15, 382–396. [Google Scholar] [CrossRef]
- Ishikawa, D.; Sasaki, T.; Takahashi, M.; Kuwahara-Arai, K.; Haga, K.; Ito, S.; Okahara, K.; Nakajima, A.; Shibuya, T.; Osada, T.; et al. The Microbial Composition of Bacteroidetes Species in Ulcerative Colitis Is Effectively Improved by Combination Therapy With Fecal Microbiota Transplantation and Antibiotics. Inflamm. Bowel Dis. 2018, 24, 2590–2598. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Hudson, M.; Batist, G. Tofacitinib for Refractory Immune-Related Colitis from PD-1 Therapy. N. Engl. J. Med. 2020, 382, 2374–2375. [Google Scholar] [CrossRef] [PubMed]
- Sakellariou, S.; Zouki, D.N.; Ziogas, D.C.; Pouloudi, D.; Gogas, H.; Delladetsima, I. Granulomatous colitis in a patient with metastatic melanoma under immunotherapy: A case report and literature review. BMC Gastroenterol. 2021, 21, 227. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Song, M.; Wang, A.; Zhao, Y.; Wei, Z.; Lu, Y. Microbiome Crosstalk in Immunotherapy and Antiangiogenesis Therapy. Front. Immunol. 2021, 12, 747914. [Google Scholar] [CrossRef]
- Sakurai, T.; De Velasco, M.A.; Sakai, K.; Nagai, T.; Nishiyama, H.; Hashimoto, K.; Uemura, H.; Kawakami, H.; Nakagawa, K.; Ogata, H.; et al. Integrative analysis of gut microbiome and host transcriptomes reveals associations between treatment outcomes and immunotherapy-induced colitis. Mol. Oncol. 2021, 16, 1493–1507. [Google Scholar] [CrossRef]
- Zhou, C.B.; Zhou, Y.L.; Fang, J.Y. Gut Microbiota in Cancer Immune Response and Immunotherapy. Trends Cancer 2021, 7, 647–660. [Google Scholar] [CrossRef]
- Vetizou, M.; Daillere, R.; Zitvogel, L. The role of intestinal microbiota in the response to anti-tumor therapies. Med. Sci. 2016, 32, 974–982. [Google Scholar] [CrossRef]
- Wang, G.; Yu, Y.; Wang, Y.Z.; Wang, J.J.; Guan, R.; Sun, Y.; Shi, F.; Gao, J.; Fu, X.L. Role of SCFAs in gut microbiome and glycolysis for colorectal cancer therapy. J. Cell Physiol. 2019, 234, 17023–17049. [Google Scholar] [CrossRef]
- Temraz, S.; Nassar, F.; Nasr, R.; Charafeddine, M.; Mukherji, D.; Shamseddine, A. Gut Microbiome: A Promising Biomarker for Immunotherapy in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 4155. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, J.; Wu, Q.; Fang, H.; Shi, C.; Li, Z.; Lin, C.; Tang, D.; Wang, D. Intestinal microbiota: A new force in cancer immunotherapy. Cell Commun. Signal 2020, 18, 90. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, N.; Luo, Q.; Jiang, L.; He, B.; Yuan, X.; Shen, L. Probiotics Lactobacillus reuteri Abrogates Immune Checkpoint Blockade-Associated Colitis by Inhibiting Group 3 Innate Lymphoid Cells. Front. Immunol. 2019, 10, 1235. [Google Scholar] [CrossRef]
- Sun, M.C.; Zhang, F.C.; Yin, X.; Cheng, B.J.; Zhao, C.H.; Wang, Y.L.; Zhang, Z.Z.; Hao, H.W.; Zhang, T.H.; Ye, H.Q. Lactobacillus reuteri F-9-35 Prevents DSS-Induced Colitis by Inhibiting Proinflammatory Gene Expression and Restoring the Gut Microbiota in Mice. J. Food Sci. 2018, 83, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Brachhold, K. Molecular Nutrition-From Gut Microbiota to Metabolomics and Inter-Individual Nutrition. Mol. Nutr. Food Res. 2019, 63, e1970005. [Google Scholar] [CrossRef] [PubMed]
- Ferrere, G.; Tidjani Alou, M.; Liu, P.; Goubet, A.G.; Fidelle, M.; Kepp, O.; Durand, S.; Iebba, V.; Fluckiger, A.; Daillere, R.; et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight 2021, 6, e145207. [Google Scholar] [CrossRef] [PubMed]
- McQuade, J.L.; Daniel, C.R.; Helmink, B.A.; Wargo, J.A. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019, 20, e77–e91. [Google Scholar] [CrossRef]
- Benech, N.; Sokol, H. Fecal microbiota transplantation in gastrointestinal disorders: Time for precision medicine. Genome Med. 2020, 12, 58. [Google Scholar] [CrossRef]
- Baunwall, S.M.D.; Lee, M.M.; Eriksen, M.K.; Mullish, B.H.; Marchesi, J.R.; Dahlerup, J.F.; Hvas, C.L. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: An updated systematic review and meta-analysis. EClinicalMedicine 2020, 29-30, 100642. [Google Scholar] [CrossRef]
- Stojek, M.; Jablonska, A.; Adrych, K. The Role of Fecal Microbiota Transplantation in the Treatment of Inflammatory Bowel Disease. J. Clin. Med. 2021, 10, 4055. [Google Scholar] [CrossRef]
- He, Y.; Xu, R.; Wang, W.; Zhang, J.; Hu, X. Probiotics, prebiotics, antibiotic, Chinese herbal medicine, and fecal microbiota transplantation in irritable bowel syndrome: Protocol for a systematic review and network meta-analysis. Medicine 2020, 99, e21502. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Shamsaddini, A.; Fagan, A.; Sterling, R.K.; Gavis, E.; Khoruts, A.; Fuchs, M.; Lee, H.; Sikaroodi, M.; Gillevet, P.M. Fecal Microbiota Transplant in Cirrhosis Reduces Gut Microbial Antibiotic Resistance Genes: Analysis of Two Trials. Hepatol. Commun. 2021, 5, 258–271. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Baruch, K.; Deczkowska, A.; Rosenzweig, N.; Tsitsou-Kampeli, A.; Sharif, A.M.; Matcovitch-Natan, O.; Kertser, A.; David, E.; Amit, I.; Schwartz, M. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat. Med. 2016, 22, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Baruch, E.N.; Gaglani, T.; Wargo, J.A. Fecal microbiota transplantation as a mean of overcoming immunotherapy-resistant cancers-hype or hope? Adv. Med. Oncol. 2021, 13, 17588359211045853. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Morrow, C.D. Incongruence between dominant commensal donor microbes in recipient feces post fecal transplant and response to anti-PD-1 immunotherapy. BMC Microbiol. 2021, 21, 251. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef]
- Ni, J.; Shen, T.D.; Chen, E.Z.; Bittinger, K.; Bailey, A.; Roggiani, M.; Sirota-Madi, A.; Friedman, E.S.; Chau, L.; Lin, A.; et al. A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci. Transl. Med. 2017, 9, eaah6888. [Google Scholar] [CrossRef]
- Torres-Maravilla, E.; Boucard, A.S.; Mohseni, A.H.; Taghinezhad, S.S.; Cortes-Perez, N.G.; Bermudez-Humaran, L.G. Role of Gut Microbiota and Probiotics in Colorectal Cancer: Onset and Progression. Microorganisms 2021, 9, 1021. [Google Scholar] [CrossRef]
- Walker, A. Intestinal colonization and programming of the intestinal immune response. J. Clin. Gastroenterol. 2014, 48 (Suppl. 1), S8–S11. [Google Scholar] [CrossRef]
- Zitvogel, L.; Derosa, L.; Kroemer, G. Modulation of cancer immunotherapy by dietary fibers and over-the-counter probiotics. Cell Metab. 2022, 34, 350–352. [Google Scholar] [CrossRef]
- Vale, G.C.; Mota, B.I.S.; Ando-Suguimoto, E.S.; Mayer, M.P.A. Effect of Probiotics Lactobacillus acidophilus and Lacticaseibacillus rhamnosus on Antibacterial Response Gene Transcription of Human Peripheral Monocytes. Probiotics Antimicrob. Proteins 2021. [Google Scholar] [CrossRef]
- Hibberd, A.A.; Lyra, A.; Ouwehand, A.C.; Rolny, P.; Lindegren, H.; Cedgard, L.; Wettergren, Y. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017, 4, e000145. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Tian, Y.; Song, C.; Li, J.; Liu, T.; Chen, Z.; Chen, C.; Huang, Y.; Zhang, Y. Urinary microbiota-a potential biomarker and therapeutic target for bladder cancer. J. Med. Microbiol. 2019, 68, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Tawfick, M.M.; Xie, H.; Zhao, C.; Shao, P.; Farag, M.A. Inulin fructans in diet: Role in gut homeostasis, immunity, health outcomes and potential therapeutics. Int. J. Biol. Macromol. 2022, 208, 948–961. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Hamano, H.; Ochi, H.; Abe, F.; Masuda, K.; Iino, H. Lactulose ingestion causes an increase in the abundance of gut-resident bifidobacteria in Japanese women: A randomised, double-blind, placebo-controlled crossover trial. Benef Microbes 2021, 12, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Athamneh, A.I.M.; Deshpande, R.; Ramirez, J.A.R.; Adu, K.T.; Muthuirulan, P.; Pawar, S.; Biazzo, M.; Apidianakis, Y.; Sundekilde, U.K.; et al. Probiotics: Insights and new opportunities for Clostridioides difficile intervention. Crit. Rev. Microbiol. 2022, 1–21. [Google Scholar] [CrossRef]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef]
- Lamberte, L.E.; van Schaik, W. Antibiotic resistance in the commensal human gut microbiota. Curr. Opin. Microbiol. 2022, 68, 102150. [Google Scholar] [CrossRef]
- Blaser, M.J. Antibiotic use and its consequences for the normal microbiome. Science 2016, 352, 544–545. [Google Scholar] [CrossRef]
- Pinato, D.J.; Howlett, S.; Ottaviani, D.; Urus, H.; Patel, A.; Mineo, T.; Brock, C.; Power, D.; Hatcher, O.; Falconer, A.; et al. Association of Prior Antibiotic Treatment with Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients With Cancer. JAMA Oncol. 2019, 5, 1774–1778. [Google Scholar] [CrossRef]
- Kaderbhai, C.; Richard, C.; Fumet, J.D.; Aarnink, A.; Foucher, P.; Coudert, B.; Favier, L.; Lagrange, A.; Limagne, E.; Boidot, R.; et al. Antibiotic Use Does Not Appear to Influence Response to Nivolumab. Anticancer Res. 2017, 37, 3195–3200. [Google Scholar] [CrossRef]
| ICIs | Cancer | Results | References | |
|---|---|---|---|---|
| Anti-PD-1 | Nivolumab | Advanced cervical cancer | 36% patients had stable disease (9/25; 90% CI, 20.2–54.4%) for a median of 5.7 months. Estimated PFS and OS at 6 months were 16% and 78.4%. | [23] |
| Pembrolizumab | NSCLC | Half-year PFS:22%; median PFS:2.8 months (95% CI: 1.5–4.1); median OS: 11.7 months (95% CI: 7.6–13.4). | [24] | |
| Cemiplimab | Advanced squamous cell carcinomas | PD-L1 expression was ≥1% in 18% patients and <1% in 11% of patients. | [25] | |
| Anti-PD-L1 | Atezolizumab | Advanced triple-negative breast cancer | Median survival to progression and overall survival were 5.5 months (95% CI, 5.1–7.7 months) and 14.7 months (95% CI, 10.1, not evaluable). | [26] |
| Avelumab | Advanced Merkel cell cancer | ORR:48.0%; median duration of treatment:7.4 months (1.0–41.7 months). | [27] | |
| Durvalumab | NSCLC | Median PFS:17.5 months (95% CI, 13.2–24.9); median OS:47 months (95%CI, 47 [NR]). | [28] | |
| Anti-CTLA4 | Ipilimumab | Metastatic melanoma | Survival rates at 5 years in patients were OS 11%. | [29] |
| Combination | Ipilimumab + nivolumab | Metastatic CRC | PFS:76% (9 months) and 71% (12 months); respective OS: 87% and 85%. | [30] |
| Systemic or Tissue Toxicity | Clinical Manifestation | Treatment Measures | References |
|---|---|---|---|
| Skin | Rash, itching | Symptomatic treatment (topical corticosteroids and oral antihistamines). | [64] |
| Gastrointestinal tract | Diarrhea, colitis | Rehydrate, rule out infection, and administer oral or intravenous corticosteroids. Colonoscopy or sigmoidoscopy. | [65] |
| Endocrine | Thyroid, pituitary, or adrenal gland damage | During ICIs, thyroid function is regularly monitored. | [66] |
| Liver | Asymptomatic elevations in ALT, AST, or total bilirubin | With oral corticosteroids, immune-mediated hepatitis usually resolves within 4–6 weeks. | [67] |
| Lung | Dry cough, progressive difficulty breathing | Nearly 75% of patients may require discontinuation of ICIs. | [68] |
| Kidney | Asymptomatic elevation of creatinine | Corticosteroid therapy and sparing immunotherapy are recommended. Renal biopsy is necessary for higher-grade events. | [69] |
| Neurotoxicity | Facial paralysis, optic neuritis, Guillain-Barre syndrome, myasthenia gravis, encephalitis, and aseptic meningitis | Steroid therapy is used to relieve mild symptoms, but severe toxicity requires high doses or other therapies. | [70] |
| Cardiotoxicity | Heart failure, cardiomyopathy, heart block, myocardial fibrosis, and myocarditis | ICIs were discontinued, and steroid therapy was initiated. | [71] |
| Eye | Keratitis, uveitis, conjunctivitis, and episcleritis | Topical or systemic corticosteroid therapy. | [72] |
| Muscle, Bone and Rheumatology | Vasculitis, inflammatory arthritis, and myositis | Low-dose steroids have some effects. | [73] |
| NCT Number | Title | Status | Conditions | Interventions | Phases |
|---|---|---|---|---|---|
| NCT05008861 | Gut Microbiota Reconstruction for NSCLC Immunotherapy | Not yet recruiting | Non-Small Cell Lung Cancer | Procedure: Capsulized Fecal Microbiota Transplant Drug: Anti-PD-1/PD-L1 Drug: Platinum-based chemotherapy | Phase 1 |
| NCT04924374 | Microbiota Transplant in Advanced Lung Cancer Treated with Immunotherapy | Recruiting | Lung Cancer | Dietary Supplement: Microbiota Transplant plus anti-PD-1 therapy Drug: anti-PD-1 therapy | Not Applicable |
| NCT04729322 | Fecal Microbiota Transplant and Re-introduction of Anti-PD-1 Therapy (Pembrolizumab or Nivolumab) for the Treatment of Metastatic Colorectal Cancer in Anti-PD-1 Non-responders | Recruiting | Metastatic Colorectal Adenocarcinoma Metastatic Small Intestinal Adenocarcinoma Stage IV Colorectal Cancer | Procedure: Fecal Microbiota Transplantation Drug: Metronidazole Biological: Nivolumab/Pembrolizumab | Early Phase 1 |
| NCT03353402 | Fecal Microbiota Transplantation (FMT) in Metastatic Melanoma Patients Who Failed Immunotherapy | Recruiting | Melanoma Stage Iv Unresectable Stage III Melanoma | Procedure: Fecal Microbiota Transplant (FMT) | Phase 1 |
| NCT03772899 | Fecal Microbial Transplantation in Combination with Immunotherapy in Melanoma Patients (MIMic) | Recruiting | Melanoma | Drug: Fecal Microbial Transplantation | Phase 1 |
| NCT04758507 | Fecal Microbiota Transplantation to Improve Efficacy of Immune Checkpoint Inhibitors in Renal Cell Carcinoma | Recruiting | Renal Cell Carcinoma | Biological: donor FMT Other: Placebo FMT | Phase 1 Phase 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Z.; Fu, J.; Peng, X.; Tang, D.; Song, J. Intestinal Microbiota: The Driving Force behind Advances in Cancer Immunotherapy. Cancers 2022, 14, 4796. https://doi.org/10.3390/cancers14194796
Dai Z, Fu J, Peng X, Tang D, Song J. Intestinal Microbiota: The Driving Force behind Advances in Cancer Immunotherapy. Cancers. 2022; 14(19):4796. https://doi.org/10.3390/cancers14194796
Chicago/Turabian StyleDai, Zhujiang, Jihong Fu, Xiang Peng, Dong Tang, and Jinglue Song. 2022. "Intestinal Microbiota: The Driving Force behind Advances in Cancer Immunotherapy" Cancers 14, no. 19: 4796. https://doi.org/10.3390/cancers14194796
APA StyleDai, Z., Fu, J., Peng, X., Tang, D., & Song, J. (2022). Intestinal Microbiota: The Driving Force behind Advances in Cancer Immunotherapy. Cancers, 14(19), 4796. https://doi.org/10.3390/cancers14194796





