Trophoblast Cell Surface Antigen 2 (TROP2) as a Predictive Bio-Marker for the Therapeutic Efficacy of Sacituzumab Govitecan in Adenocarcinoma of the Esophagus
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Material
2.1. Cell Lines
2.2. RNA Analysis
2.3. Chemosensitivity Assay
2.4. Immunocytochemistry
2.5. In Vivo Xenograft Treatment
2.6. Patient Cohort
2.7. Immunohistochemistry
2.8. Data Analysis and Statistics
3. Results
3.1. Patients’ Baseline Characteristics
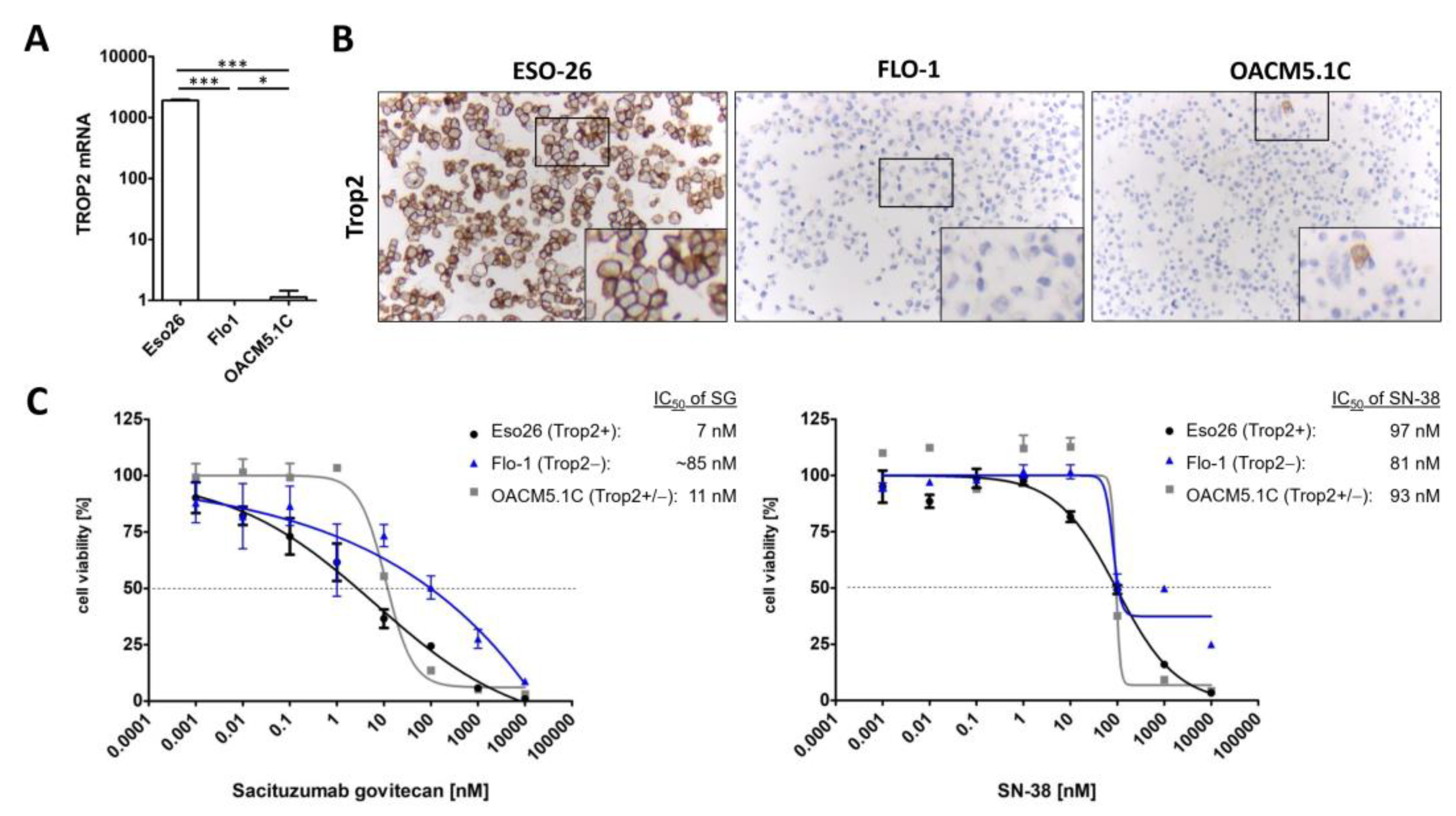
3.2. TROP2 Is Expressed by the Majority of EAC Tumors
3.3. Sacituzumab Govitecan Specifically Inhibits TROP2 Positive EAC Cells
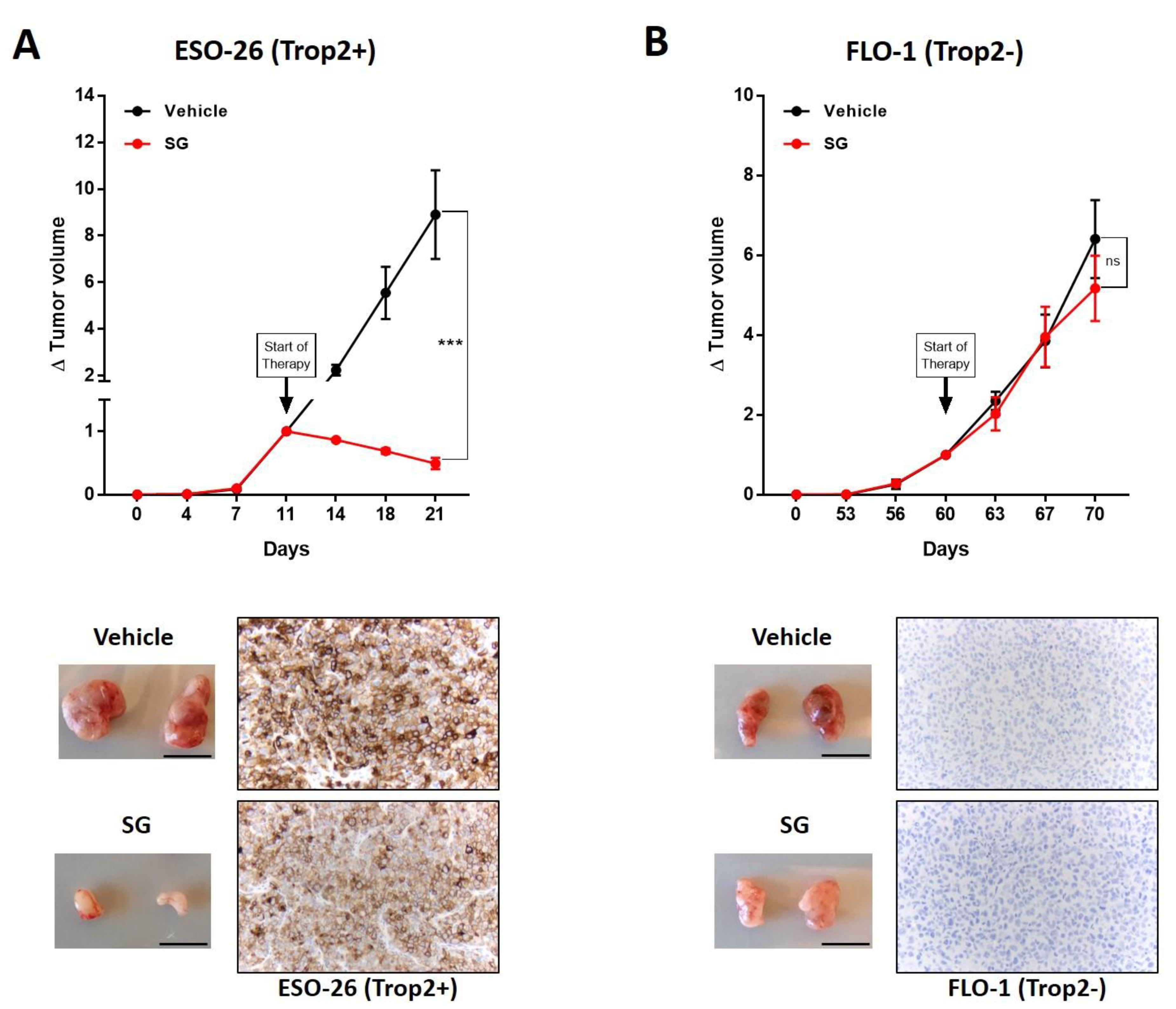
3.4. TROP2 Positive EAC Tumors Are Sensitive to Sacituzumab Govitecan
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ripani, E.; Sacchetti, A.; Corda, D.; Alberti, S. Human Trop-2 is a tumor-associated calcium signal transducer. Int. J. Cancer 1998, 76, 671–676. [Google Scholar] [CrossRef]
- Cubas, R.; Zhang, S.; Li, M.; Chen, C.; Yao, Q. Trop2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathway. Mol. Cancer 2010, 9, 253. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, X.; Zhang, W. TROP2 overexpression promotes proliferation and invasion of lung adenocarcinoma cells. Biochem. Biophys. Res. Commun. 2016, 470, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Chen, M.B.; Zhou, L.N.; Tang, M.; Liu, C.Y.; Lu, P.H. Impact of TROP2 expression on prognosis in solid tumors: A Systematic Review and Meta-analysis. Sci. Rep. 2016, 6, 33658. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, M.; Parks, D.R.; Rouse, R.V.; Herzenberg, L.A. Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proc. Natl. Acad. Sci. USA 1981, 78, 5147–5150. [Google Scholar] [CrossRef]
- Licini, C.; Avellini, C.; Picchiassi, E.; Mensà, E.; Fantone, S.; Ramini, D.; Tersigni, C.; Tossetta, G.; Castellucci, C.; Tarquini, F.; et al. Pre-eclampsia predictive ability of maternal miR-125b: A clinical and experimental study. Transl. Res. 2021, 228, 13–27. [Google Scholar] [CrossRef]
- McDougall, A.R.; Hooper, S.B.; Zahra, V.A.; Sozo, F.; Lo, C.Y.; Cole, T.J.; Doran, T.; Wallace, M.J. The oncogene Trop2 regulates fetal lung cell proliferation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L478–L489. [Google Scholar] [CrossRef]
- Bardia, A.; Messersmith, W.A.; Kio, E.A.; Berlin, J.D.; Vahdat, L.; Masters, G.A.; Moroose, R.; Santin, A.D.; Kalinsky, K.; Picozzi, V.; et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: Final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann. Oncol. 2021, 32, 746–756. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Bardia, A.; Tolaney, S.M.; Punie, K.; Loirat, D.; Oliveira, M.; Kalinsky, K.; Zelnak, A.; Aftimos, P.; Dalenc, F.; Sardesai, S.; et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 1148–1156. [Google Scholar] [CrossRef]
- Coates, J.T.; Sun, S.; Leshchiner, I.; Thimmiah, N.; Martin, E.E.; McLoughlin, D.; Danysh, B.P.; Slowik, K.; Jacobs, R.A.; Rhrissorrakrai, K.; et al. Parallel Genomic Alterations of Antigen and Payload Targets Mediate Polyclonal Acquired Clinical Resistance to Sacituzumab Govitecan in Triple-Negative Breast Cancer. Cancer Discov. 2021, 11, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, T.M.; Rossi, D.L.; Zalath, M.B.; Liu, D.; Arrojo, R.; Sharkey, R.M.; Chang, C.H.; Goldenberg, D.M. Predictive biomarkers for sacituzumab govitecan efficacy in Trop-2-expressing triple-negative breast cancer. Oncotarget 2020, 11, 3849–3862. [Google Scholar] [CrossRef]
- Hölscher, A.H.; Schneider, P.M.; Gutschow, C.; Schröder, W. Laparoscopic ischemic conditioning of the stomach for esophageal replacement. Ann. Surg. 2007, 245, 241–246. [Google Scholar] [CrossRef]
- Schneider, P.M.; Metzger, R.; Schaefer, H.; Baumgarten, F.; Vallbohmer, D.; Brabender, J.; Wolfgarten, E.; Bollschweiler, E.; Baldus, S.E.; Dienes, H.P.; et al. Response evaluation by endoscopy, rebiopsy, and endoscopic ultrasound does not accurately predict histopathologic regression after neoadjuvant chemoradiation for esophageal cancer. Ann. Surg. 2008, 248, 902–908. [Google Scholar] [CrossRef]
- Simon, R.; Mirlacher, M.; Sauter, G. Tissue microarrays. Methods Mol. Med. 2005, 114, 257–268. [Google Scholar] [CrossRef]
- Helbig, D.; Ihle, M.A.; Pütz, K.; Tantcheva-Poor, I.; Mauch, C.; Büttner, R.; Quaas, A. Oncogene and therapeutic target analyses in atypical fibroxanthomas and pleomorphic dermal sarcomas. Oncotarget 2016, 7, 21763–21774. [Google Scholar] [CrossRef]
- Liu, D.S.; Hoefnagel, S.J.; Fisher, O.M.; Krishnadath, K.K.; Montgomery, K.G.; Busuttil, R.A.; Colebatch, A.J.; Read, M.; Duong, C.P.; Phillips, W.A.; et al. Novel metastatic models of esophageal adenocarcinoma derived from FLO-1 cells highlight the importance of E-cadherin in cancer metastasis. Oncotarget 2016, 7, 83342–83358. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Sharkey, R.M. Antibody-drug conjugates targeting TROP-2 and incorporating SN-38: A case study of anti-TROP-2 sacituzumab govitecan. MAbs 2019, 11, 987–995. [Google Scholar] [CrossRef] [PubMed]
| TROP2 Expression | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | p Value | ||||||||
| total | 598 | 72 | 12.0% | 133 | 22.2% | 268 | 44.8% | 125 | 20.9% | |||
| sex | female | 67 | 11.2% | 13 | 19.4% | 19 | 28.4% | 23 | 34.3% | 12 | 17.9% | |
| male | 531 | 88.8% | 59 | 11.1% | 114 | 21.5% | 245 | 46.1% | 113 | 21.3% | 0.077 | |
| agegroup | <65 yrs | 310 | 51.9% | 33 | 10.6% | 74 | 23.9% | 142 | 45.7% | 62 | 19.8% | |
| >65 yrs | 288 | 48.1% | 38 | 13.2% | 58 | 20.2% | 128 | 44.5% | 63 | 22.1% | 0.554 | |
| Neoadjuvant | No | 247 | 41.3% | 34 | 13.8% | 53 | 21.5% | 104 | 42.1% | 56 | 22.7% | |
| treatment | Yes | 351 | 58.7% | 38 | 10.8% | 80 | 22.8% | 164 | 46.7% | 69 | 19.7% | 0.480 |
| Tumor stage | pT1 | 114 | 19.1% | 12 | 10.5% | 29 | 25.4% | 55 | 48.2% | 18 | 15.8% | |
| pT2 | 114 | 19.1% | 14 | 12.3% | 21 | 18.4% | 52 | 45.6% | 27 | 23.7% | ||
| pT3 | 349 | 58.6% | 43 | 12.3% | 78 | 22.3% | 154 | 44.1% | 74 | 21.2% | 0.828 | |
| pT4 | 19 | 3.2% | 2 | 10.5% | 4 | 21.1% | 7 | 36.8% | 6 | 31.6% | ||
| Lymph node | pN0 | 234 | 39.3% | 33 | 14.1% | 60 | 25.6% | 102 | 43.6% | 39 | 16.7% | |
| metastasis | pN1 | 197 | 33.1% | 22 | 11.2% | 44 | 22.3% | 90 | 45.7% | 41 | 20.8% | |
| pN2 | 86 | 14.4% | 9 | 10.5% | 10 | 11.6% | 42 | 48.8% | 25 | 29.1% | 0.173 | |
| pN3 | 79 | 13.3% | 8 | 10.1% | 19 | 24.1% | 33 | 41.8% | 19 | 24.1% | ||
| UICC stage | I | 85 | 14.3% | 9 | 10.6% | 22 | 25.9% | 39 | 45.9% | 15 | 17.6% | |
| II | 73 | 12.2% | 10 | 13.7% | 20 | 27.4% | 32 | 43.8% | 11 | 15.1% | ||
| III | 275 | 46.1% | 35 | 12.7% | 61 | 22.2% | 124 | 45.1% | 55 | 20.0% | 0.508 | |
| IV | 163 | 27.3% | 17 | 10.4% | 29 | 17.8% | 73 | 44.8% | 44 | 27.0% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoppe, S.; Meder, L.; Gebauer, F.; Ullrich, R.T.; Zander, T.; Hillmer, A.M.; Buettner, R.; Plum, P.; Puppe, J.; Malter, W.; et al. Trophoblast Cell Surface Antigen 2 (TROP2) as a Predictive Bio-Marker for the Therapeutic Efficacy of Sacituzumab Govitecan in Adenocarcinoma of the Esophagus. Cancers 2022, 14, 4789. https://doi.org/10.3390/cancers14194789
Hoppe S, Meder L, Gebauer F, Ullrich RT, Zander T, Hillmer AM, Buettner R, Plum P, Puppe J, Malter W, et al. Trophoblast Cell Surface Antigen 2 (TROP2) as a Predictive Bio-Marker for the Therapeutic Efficacy of Sacituzumab Govitecan in Adenocarcinoma of the Esophagus. Cancers. 2022; 14(19):4789. https://doi.org/10.3390/cancers14194789
Chicago/Turabian StyleHoppe, Sascha, Lydia Meder, Florian Gebauer, Roland T. Ullrich, Thomas Zander, Axel M. Hillmer, Reinhard Buettner, Patrick Plum, Julian Puppe, Wolfram Malter, and et al. 2022. "Trophoblast Cell Surface Antigen 2 (TROP2) as a Predictive Bio-Marker for the Therapeutic Efficacy of Sacituzumab Govitecan in Adenocarcinoma of the Esophagus" Cancers 14, no. 19: 4789. https://doi.org/10.3390/cancers14194789
APA StyleHoppe, S., Meder, L., Gebauer, F., Ullrich, R. T., Zander, T., Hillmer, A. M., Buettner, R., Plum, P., Puppe, J., Malter, W., & Quaas, A. (2022). Trophoblast Cell Surface Antigen 2 (TROP2) as a Predictive Bio-Marker for the Therapeutic Efficacy of Sacituzumab Govitecan in Adenocarcinoma of the Esophagus. Cancers, 14(19), 4789. https://doi.org/10.3390/cancers14194789






