Advances in Exosomes as Diagnostic and Therapeutic Biomarkers for Gynaecological Malignancies
Abstract
:Simple Summary
Abstract
1. Introduction
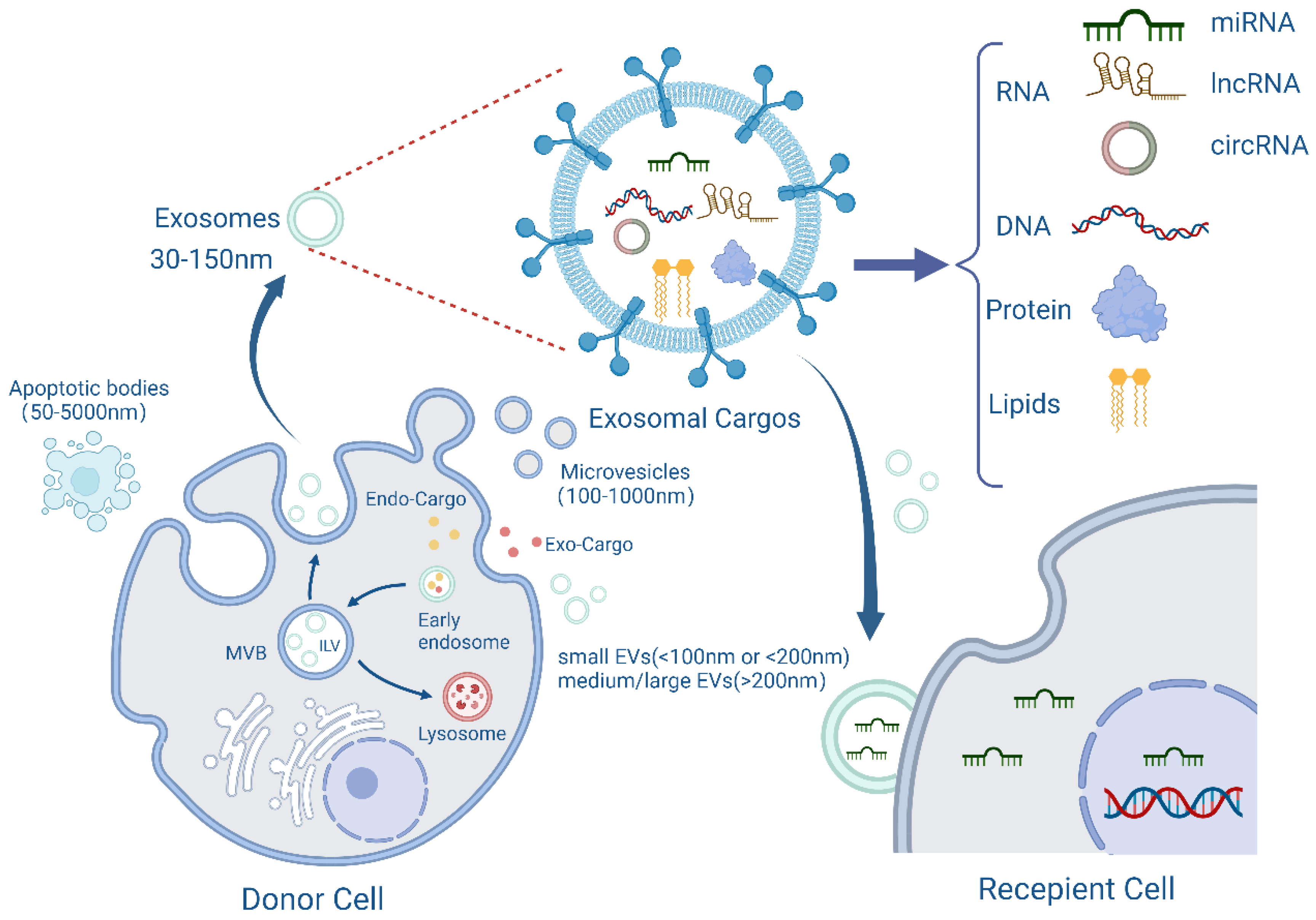
2. Structure and Function of Exosomes
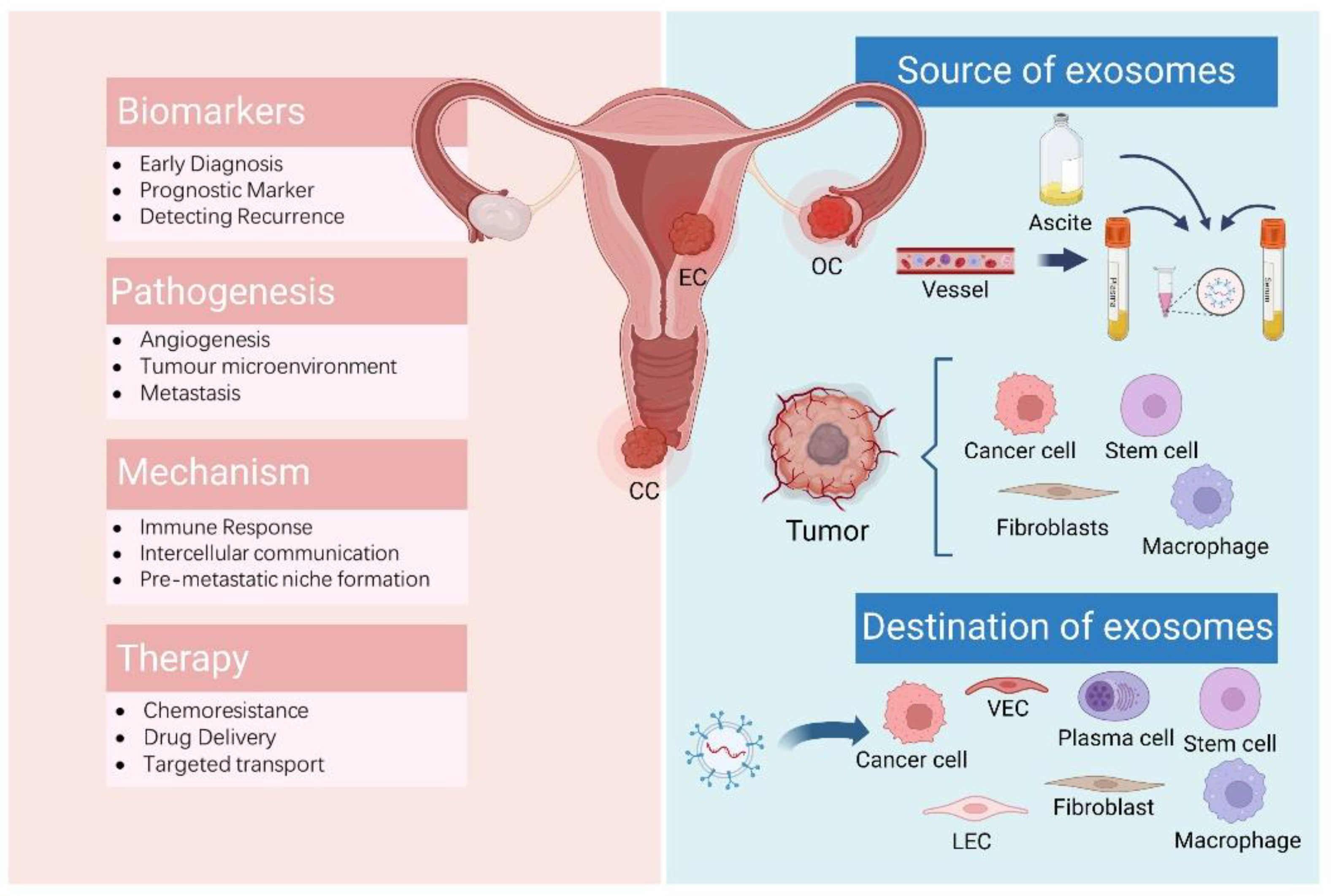
3. Exosomes and Gynaecologic Malignancies
3.1. Exosomes in Ovarian Cancer
3.1.1. Exosomes Derived from Body Fluids
3.1.2. Exosomes Derived from Cells
3.2. Exosomes in Cervical Cancer
3.2.1. Exosomes Derived from Body Fluids
3.2.2. Exosomes Derived from Cells
3.3. Exosomes in Endometrial Cancer
3.3.1. Exosomes Derived from Body Fluids
3.3.2. Exosomes Derived from Cells
4. Clinical Diagnosis and Therapeutic Applications of Exosomes in Gynaecologic Malignancies
4.1. Diagnostic Technology
4.2. Therapeutic Advances
4.3. Overcome Chemoresistance
5. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bowtell, D.D.; Böhm, S.; Ahmed, A.A.; Aspuria, P.-J.; Bast, R.C., Jr.; Beral, V.; Berek, J.S.; Birrer, M.J.; Blagden, S.; Bookman, M.A.; et al. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat. Rev. Cancer 2015, 15, 668–679. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef]
- Bazzan, E.; Tinè, M.; Casara, A.; Biondini, D.; Semenzato, U.; Cocconcelli, E.; Balestro, E.; Damin, M.; Radu, C.; Turato, G.; et al. Critical Review of the Evolution of Extracellular Vesicles’ Knowledge: From 1946 to Today. Int. J. Mol. Sci. 2021, 22, 6417. [Google Scholar] [CrossRef]
- Bonucci, E. Fine structure of early cartilage calcification. J. Ultrastruct. Res. 1967, 20, 33–50. [Google Scholar] [CrossRef]
- Anderson, H.C. Vesicles Associated with Calcification in the Matrix of Epiphyseal Cartilage. J. Cell Biol. 1969, 41, 59–72. [Google Scholar] [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Ludwig, A.-K.; Giebel, B. Exosomes: Small vesicles participating in intercellular communication. Int. J. Biochem. Cell Biol. 2012, 44, 11–15. [Google Scholar] [CrossRef]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Esfandyari, S.; Elkafas, H.; Chugh, R.M.; Park, H.-S.; Navarro, A.; Al-Hendy, A. Exosomes as Biomarkers for Female Reproductive Diseases Diagnosis and Therapy. Int. J. Mol. Sci. 2021, 22, 2165. [Google Scholar] [CrossRef] [PubMed]
- Preethi, K.A.; Selvakumar, S.C.; Ross, K.; Jayaraman, S.; Tusubira, D.; Sekar, D. Liquid biopsy: Exosomal microRNAs as novel diagnostic and prognostic biomarkers in cancer. Mol. Cancer 2022, 21, 54. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef]
- Kok, V.C.; Yu, C.-C. Cancer-Derived Exosomes: Their Role in Cancer Biology and Biomarker Development. Int. J. Nanomed. 2020, 15, 8019–8036. [Google Scholar] [CrossRef]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Z.; Wang, M.; Zhang, M.; Chen, Y.; Yang, X.; Zhou, C.; Liu, Y.; Hong, L.; Zhang, L. Detection of plasma exosomal miRNA-205 as a biomarker for early diagnosis and an adjuvant indicator of ovarian cancer staging. J. Ovarian Res. 2022, 15, 27. [Google Scholar] [CrossRef]
- Hu, R.; Chen, X.; Zhang, S.; Liu, B.; Pei, H.; Tu, F.; Liu, J.; Yu, H. Plasma exosome-derived fragile site-associated tumor suppressor as a powerful prognostic predictor for patients with ovarian cancer. Bosn. J. Basic Med. Sci. 2022, 22, 453–459. [Google Scholar]
- Xiong, J.; He, X.; Xu, Y.; Zhang, W.; Fu, F. MiR-200b is upregulated in plasma-derived exosomes and functions as an oncogene by promoting macrophage M2 polarization in ovarian cancer. J. Ovarian Res. 2021, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yoo, J.; Ho, J.Y.; Jung, Y.; Lee, S.; Hur, S.Y.; Choi, Y.J. Plasma-derived exosomal miR-4732-5p is a promising noninvasive diagnostic biomarker for epithelial ovarian cancer. J. Ovarian Res. 2021, 14, 59. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Dean, D.C.; Hornicek, F.J.; Shi, H.; Duan, Z. Exosomes promote pre-metastatic niche formation in ovarian cancer. Mol. Cancer 2019, 18, 124. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Nie, X.; Gou, R.; Hu, Y.; Dong, H.; Li, X.; Lin, B. Exosomal ANXA2 derived from ovarian cancer cells regulates epithelial-mesenchymal plasticity of human peritoneal mesothelial cells. J. Cell Mol. Med. 2021, 25, 10916–10929. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, Y.; Mu, J.; Yang, D.; Gu, X.; Zhang, J. Exosomal miR-21-5p contributes to ovarian cancer progression by regulating CDK6. Hum. Cell 2021, 34, 1185–1196. [Google Scholar] [CrossRef]
- Lai, Y.; Dong, L.; Jin, H.; Li, H.; Sun, M.; Li, J. Exosome long non-coding RNA SOX2-OT contributes to ovarian cancer malignant progression by miR-181b-5p/SCD1 signaling. Aging 2021, 13, 23726–23738. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Lu, C. Circular RNA Foxo3 enhances progression of ovarian carcinoma cells. Aging 2021, 13, 22432–22443. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Mu, W.; Barry, J.; Han, A.; Carpenter, R.L.; Jiang, B.-H.; Peiper, S.C.; Mahoney, M.G.; Aplin, A.E.; et al. Reactive oxygen species reprogram macrophages to suppress antitumor immune response through the exosomal miR-155-5p/PD-L1 pathway. J. Exp. Clin. Cancer Res. 2022, 41, 41. [Google Scholar] [CrossRef]
- Shimizu, A.; Sawada, K.; Kobayashi, M.; Yamamoto, M.; Yagi, T.; Kinose, Y.; Kodama, M.; Hashimoto, K.; Kimura, T. Exosomal CD47 Plays an Essential Role in Immune Evasion in Ovarian Cancer. Mol. Cancer Res. 2021, 19, 1583–1595. [Google Scholar] [CrossRef]
- Lu, L.; Ling, W.; Ruan, Z. TAM-derived extracellular vesicles containing microRNA-29a-3p explain the deterioration of ovarian cancer. Mol. Ther.-Nucleic Acids 2021, 25, 468–482. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, W.; Zhao, L.; Wang, X.; Gimple, R.C.; Xu, L.; Wang, Y.; Rich, J.N.; Zhou, S. Plasma cells shape the mesenchymal identity of ovarian cancers through transfer of exosome-derived microRNAs. Sci. Adv. 2021, 7, eabb0737. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Ye, X.; Cheng, H.; Cui, H.; Chang, X. Tumor-derived exosomal circRNA051239 promotes proliferation and migration of epithelial ovarian cancer. Am. J. Transl. Res. 2021, 13, 1125–1139. [Google Scholar] [PubMed]
- Shen, X.; Wang, C.; Zhu, H.; Wang, Y.; Wang, X.; Cheng, X.; Ge, W.; Lu, W. Exosome-mediated transfer of CD44 from high-metastatic ovarian cancer cells promotes migration and invasion of low-metastatic ovarian cancer cells. J. Ovarian Res. 2021, 14, 38. [Google Scholar] [CrossRef]
- Cai, J.; Gong, L.; Li, G.; Guo, J.; Yi, X.; Wang, Z. Exosomes in ovarian cancer ascites promote epithelial–mesenchymal transition of ovarian cancer cells by delivery of miR-6780b-5p. Cell Death Dis. 2021, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.; Lai, A.; Guanzon, D.; Palma, C.; Zuñiga, F.; Perrin, L.; He, Y.; Hooper, J.D.; Salomon, C. Ovarian cancer-derived exosomes promote tumour metastasis in vivo: An effect modulated by the invasiveness capacity of their originating cells. Clin. Sci. 2019, 133, 1401–1419. [Google Scholar] [CrossRef]
- Li, Z.; Yan-Qing, W.; Xiao, Y.; Shi-Yi, L.; Meng-Qin, Y.; Shu, X.; Dong-Yong, Y.; Ya-Jing, Z.; Yan-Xiang, C. Exosomes secreted by chemoresistant ovarian cancer cells promote angiogenesis. J. Ovarian Res. 2021, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Guo, T.; Zhu, D.; Ge, H.; Zhao, Y.; Huang, A.; Wang, X.; Cao, X.; He, C.; Qian, H.; et al. Exosomal lncRNA ATB Derived from Ovarian Cancer Cells Promotes Angiogenesis via Regulating miR-204-3p/TGFβR2 Axis. Cancer Manag. Res. 2022, 14, 327–337. [Google Scholar] [CrossRef]
- Zhang, X.; Sheng, Y.; Li, B.; Wang, Q.; Liu, X.; Han, J. Ovarian cancer derived PKR1 positive exosomes promote angiogenesis by promoting migration and tube formation in vitro. Cell Biochem. Funct. 2021, 39, 308–316. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Li, Y.; Li, M.; Zhu, T.; Shen, Z.; Wang, H.; Lv, W.; Wang, X.; Cheng, X.; et al. Potential of peptide-engineered exosomes with overexpressed miR-92b-3p in anti-angiogenic therapy of ovarian cancer. Clin. Transl. Med. 2021, 11, e425. [Google Scholar] [CrossRef]
- Li, T.; Lin, L.; Liu, Q.; Gao, W.; Chen, L.; Sha, C.; Chen, Q.; Xu, W.; Li, Y.; Zhu, X. Exosomal transfer of miR-429 confers chemoresistance in epithelial ovarian cancer. Am. J. Cancer Res. 2021, 11, 2124–2141. [Google Scholar]
- Zhuang, L.; Zhang, B.; Liu, X.; Lin, L.; Wang, L.; Hong, Z.; Chen, J. Exosomal miR-21-5p derived from cisplatin-resistant SKOV3 ovarian cancer cells promotes glycolysis and inhibits chemosensitivity of its progenitor SKOV3 cells by targeting PDHA1. Cell Biol. Int. 2021, 45, 2140–2149. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Yang, X.; Li, S.; Zhao, H.; Gao, Y.; Zhao, S.; Lv, X.; Zhang, X.; Li, L.; Zhai, L.; et al. Reduced O-GlcNAcylation of SNAP-23 promotes cisplatin resistance by inducing exosome secretion in ovarian cancer. Cell Death Discov. 2021, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.; Lai, A.; Sharma, S.; Kalita-de Croft, P.; Godbole, N.; Campos, A.; Guanzon, D.; Salas-Burgos, A.; Carrion, F.; Zuñiga, F.A.; et al. Extracellular Vesicle Transmission of Chemoresistance to Ovarian Cancer Cells Is Associated with Hypoxia-Induced Expression of Glycolytic Pathway Proteins, and Prediction of Epithelial Ovarian Cancer Disease Recurrence. Cancers 2021, 13, 3388. [Google Scholar] [CrossRef] [PubMed]
- Calo, C.A.; Smith, B.Q.; Dorayappan, K.D.P.; Saini, U.; Lightfoot, M.; Wagner, V.; Kalaiyarasan, D.; Cosgrove, C.; Wang, Q.E.; Maxwell, G.L.; et al. Aberrant expression of TMEM205 signaling promotes platinum resistance in ovarian cancer: An implication for the antitumor potential of DAP compound. Gynecol. Oncol. 2022, 164, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Parashar, D.; Geethadevi, A.; McAllister, D.; Ebben, J.; Peterson, F.C.; Jensen, D.R.; Bishop, E.; Pradeep, S.; Volkman, B.F.; Dwinell, M.B.; et al. Targeted biologic inhibition of both tumor cell-intrinsic and intercellular CLPTM1L/CRR9-mediated chemotherapeutic drug resistance. NPJ Precis. Oncol. 2021, 5, 16. [Google Scholar] [CrossRef]
- Zhao, Z.; Shuang, T.; Gao, Y.; Lu, F.; Zhang, J.; He, W.; Qu, L.; Chen, B.; Hao, Q. Targeted delivery of exosomal miR-484 reprograms tumor vasculature for chemotherapy sensitization. Cancer Lett. 2022, 530, 45–58. [Google Scholar] [CrossRef]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef]
- van der Watt, P.J.; Okpara, M.O.; Wishart, A.; Parker, M.I.; Soares, N.C.; Blackburn, J.M.; Leaner, V.D. Nuclear transport proteins are secreted by cancer cells and identified as potential novel cancer biomarkers. Int. J. Cancer 2022, 150, 347–361. [Google Scholar] [CrossRef]
- Ding, X.-Z.; Zhang, S.-Q.; Deng, X.-L.; Qiang, J.-H. Serum Exosomal lncRNA DLX6-AS1 Is a Promising Biomarker for Prognosis Prediction of Cervical Cancer. Technol. Cancer Res. Treat. 2021, 20, 1533033821990060. [Google Scholar] [CrossRef]
- Lv, A.; Tu, Z.; Huang, Y.; Lu, W.; Xie, B. Circulating exosomal miR-125a-5p as a novel biomarker for cervical cancer. Oncol. Lett. 2021, 21, 54. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Qian, J.; Tan, S.; Li, W.; Liu, P.; Zhao, J.; Zeng, Y.; Xu, L.; Wang, Z.; Cai, J. Tumor cell-derived exosomes deliver TIE2 protein to macrophages to promote angiogenesis in cervical cancer. Cancer Lett. 2022, 529, 168–179. [Google Scholar] [CrossRef]
- Bhat, A.; Yadav, J.; Thakur, K.; Aggarwal, N.; Tripathi, T.; Chhokar, A.; Singh, T.; Jadli, M.; Bharti, A.C. Exosomes from cervical cancer cells facilitate pro-angiogenic endothelial reconditioning through transfer of Hedgehog–GLI signaling components. Cancer Cell Int. 2021, 21, 319. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Sun, W.; Wang, Y.; Liu, X.; Wang, A.; Liu, L.; Han, S.; Sun, Y.; Zhang, J.; Guo, L.; et al. Cervical cancer-derived exosomal miR-663b promotes angiogenesis by inhibiting vinculin expression in vascular endothelial cells. Cancer Cell Int. 2021, 21, 684. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Guo, C.; Zheng, W.; Wang, Q.; Zhou, L. Exosome-Mediated Transfer of miR-1323 from Cancer-Associated Fibroblasts Confers Radioresistance of C33A Cells by Targeting PABPN1 and Activating Wnt/β-Catenin Signaling Pathway in Cervical Cancer. Reprod. Sci. 2022, 29, 1809–1821. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wei, W.; Ma, J.; Yang, Y.; Liang, L.; Zhang, Y.; Wang, Z.; Chen, X.; Huang, L.; Wang, W.; et al. Cancer-secreted exosomal miR-1468-5p promotes tumor immune escape via the immunosuppressive reprogramming of lymphatic vessels. Mol. Ther. 2021, 29, 1512–1528. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Yan, R.; Huang, L.; Mellor, A.L.; Yang, Y.; Chen, X.; Wei, W.; Wu, X.; Yu, L.; et al. Exosome-derived miR-142-5p remodels lymphatic vessels and induces IDO to promote immune privilege in the tumour microenvironment. Cell Death Differ. 2021, 28, 715–729. [Google Scholar] [CrossRef]
- You, X.; Wang, Y.; Meng, J.; Han, S.; Liu, L.; Sun, Y.; Zhang, J.; Sun, S.; Li, X.; Sun, W.; et al. Exosomal miR-663b exposed to TGF-β1 promotes cervical cancer metastasis and epithelial-mesenchymal transition by targeting MGAT3. Oncol. Rep. 2021, 45, 12. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, Q.; Ji, M.; Guo, X.; Li, L.; Su, X. Exosomal lncRNA UCA1 modulates cervical cancer stem cell self-renewal and differentiation through microRNA-122-5p/SOX2 axis. J. Transl. Med. 2021, 19, 229. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Ma, H.; Gao, L.; Zhao, K.; Huang, T. Extracellular Vesicles Long Non-Coding RNA AGAP2-AS1 Contributes to Cervical Cancer Cell Proliferation Through Regulating the miR-3064-5p/SIRT1 Axis. Front. Oncol. 2021, 11, 684477. [Google Scholar] [CrossRef]
- Huang, X.; Liu, X.; Du, B.; Liu, X.; Xue, M.; Yan, Q.; Wang, X.; Wang, Q. LncRNA LINC01305 promotes cervical cancer progression through KHSRP and exosome-mediated transfer. Aging 2021, 13, 19230–19242. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.-J.; Yang, Y.; Wei, W.-F.; Wu, X.-G.; Yan, R.-M.; Zhou, C.-F.; Chen, X.-J.; Wu, S.; Wang, W.; Fan, L.-S. Tumor-secreted exosomal Wnt2B activates fibroblasts to promote cervical cancer progression. Oncogenesis 2021, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Yadav, J.; Thakur, K.; Aggarwal, N.; Chhokar, A.; Tripathi, T.; Singh, T.; Jadli, M.; Veerapandian, V.; Bharti, A.C. Transcriptome analysis of cervical cancer exosomes and detection of HPVE6*I transcripts in exosomal RNA. BMC Cancer 2022, 22, 164. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Wang, F.; Yang, S.; Hu, J.; Lu, B.; Pan, Z.; Ma, Y.; Zheng, M.; Zhou, L.; et al. Plasma-derived exosomal miR-15a-5p as a promising diagnostic biomarker for early detection of endometrial carcinoma. Mol. Cancer 2021, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, M.; Tong, H.; Tan, Y.; Hu, X.; Wang, K.; Wan, X. Plasma exosomes from endometrial cancer patients contain LGALS3BP to promote endometrial cancer progression. Oncogene 2021, 40, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zou, X.; Liu, C.; Cheng, W.; Zhang, S.; Geng, X.; Zhu, W. MicroRNA expression profile in serum reveals novel diagnostic biomarkers for endometrial cancer. Biosci. Rep. 2021, 41, BSR20210111. [Google Scholar] [CrossRef]
- Fan, X.; Cao, M.; Liu, C.; Zhang, C.; Li, C.; Cheng, W.; Zhang, S.; Zhang, H.; Zhu, W. Three plasma-based microRNAs as potent diagnostic biomarkers for endometrial cancer. Cancer Biomark. 2021, 31, 127–138. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, H.; Li, Y.; Su, R. MiR-192-5p-Modified Tumor-Associated Macrophages-Derived Exosome Suppressed Endometrial Cancer Progression Through Targeting IRAK1/NF-κB Signaling. Reprod. Sci. 2022, 29, 436–447. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, X.; Li, Y.; Yan, P.; Zhang, H. Human umbilical cord blood mesenchymal stem cells-derived exosomal microRNA-503-3p inhibits progression of human endometrial cancer cells through downregulating MEST. Cancer Gene Ther. 2022, 29, 1130–1139. [Google Scholar] [CrossRef]
- Zhou, W.J.; Zhang, J.; Xie, F.; Wu, J.N.; Ye, J.F.; Wang, J.; Wu, K.; Li, M.Q. CD45RO(-)CD8(+) T cell-derived exosomes restrict estrogen-driven endometrial cancer development via the ERβ/miR-765/PLP2/Notch axis. Theranostics 2021, 11, 5330–5345. [Google Scholar] [CrossRef]
- Fan, J.T.; Zhou, Z.Y.; Luo, Y.L.; Luo, Q.; Chen, S.B.; Zhao, J.C.; Chen, Q.R. Exosomal lncRNA NEAT1 from cancer-associated fibroblasts facilitates endometrial cancer progression via miR-26a/b-5p-mediated STAT3/YKL-40 signaling pathway. Neoplasia 2021, 23, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Shi, Y.; Dong, M.; Jiang, L.; Yang, J.; Liu, Z. Exosomal transfer of tumor-associated macrophage-derived hsa_circ_0001610 reduces radiosensitivity in endometrial cancer. Cell Death Dis. 2021, 12, 818. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gong, X.; Yang, L.; Li, L.; Gao, X.; Ni, T.; Yang, X.; Fan, Q.; Sun, X.; Wang, Y. Loss of exosomal miR-26a-5p contributes to endometrial cancer lymphangiogenesis and lymphatic metastasis. Clin. Transl. Med. 2022, 12, e846. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Di He, D.; Guo, Q.; Zhang, Z.; Ru, D.; Wang, L.; Gong, K.; Liu, F.; Duan, Y.; Li, H. Exosome-liposome hybrid nanoparticle codelivery of TP and miR497 conspicuously overcomes chemoresistant ovarian cancer. J. Nanobiotechnol. 2022, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Hurley, J.; Roberts, D.; Chakrabortty, S.; Enderle, D.; Noerholm, M.; Breakefield, X.; Skog, J. Exosome-based liquid biopsies in cancer: Opportunities and challenges. Ann. Oncol. 2021, 32, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Kehoe, S.T.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. 2), 59–78. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Dalirfardouei, R.; Dias, T.; Lötvall, J.; Lässer, C. Tetraspanins distinguish separate extracellular vesicle subpopulations in human serum and plasma–Contributions of platelet extracellular vesicles in plasma samples. J. Extracell. Vesicles 2022, 11, e12213. [Google Scholar] [CrossRef]
- Vergauwen, G.; Tulkens, J.; Pinheiro, C.; Cobos, F.A.; Dedeyne, S.; De Scheerder, M.; Vandekerckhove, L.; Impens, F.; Miinalainen, I.; Braems, G.; et al. Robust sequential biophysical fractionation of blood plasma to study variations in the biomolecular landscape of systemically circulating extracellular vesicles across clinical conditions. J. Extracell. Vesicles 2021, 10, e12122. [Google Scholar] [CrossRef]
- Cho, O.; Kim, D.-W.; Cheong, J.-Y. Screening Plasma Exosomal RNAs as Diagnostic Markers for Cervical Cancer: An Analysis of Patients Who Underwent Primary Chemoradiotherapy. Biomolecules 2021, 11, 1691. [Google Scholar] [CrossRef]
- Krishnan, V.; Pandey, G.R.; Babu, K.A.; Paramasivam, S.; Kumar, S.S.; Balasubramanian, S.; Ravichandiran, V.; Pazhani, G.P.; Veerapandian, M. Chitosan grafted butein: A metal-free transducer for electrochemical genosensing of exosomal CD24. Carbohydr. Polym. 2021, 269, 118333. [Google Scholar] [CrossRef]
- Lee, E.; Cha, B.; Kim, S.; Park, K. Synthesis of Exosome-Based Fluorescent Gold Nanoclusters for Cellular Imaging Applications. Int. J. Mol. Sci. 2021, 22, 4433. [Google Scholar] [CrossRef] [PubMed]
- Abbasifarid, E.; Bolhassani, A.; Irani, S.; Sotoodehnejadnematalahi, F. Synergistic effects of exosomal crocin or curcumin compounds and HPV L1-E7 polypeptide vaccine construct on tumor eradication in C57BL/6 mouse model. PLoS ONE 2021, 16, e0258599. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, B.A.; Reddy, A.S.; Sangubotla, R.; Hong, J.W.; Kim, S. Ruthenium(II)-curcumin liposome nanoparticles: Synthesis, characterization, and their effects against cervical cancer. Colloids Surf. B Biointerfaces 2021, 204, 111773. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, M.; Shenoy, G.N.; Loyall, J.L.; Gray, B.D.; Bapardekar, M.; Conway, A.; Minderman, H.; Jr, R.J.K.; Carreno, B.M.; Linette, G.; et al. Novel phosphatidylserine-binding molecule enhances antitumor T-cell responses by targeting immunosuppressive exosomes in human tumor microenvironments. J. Immunother. Cancer 2021, 9, e003148. [Google Scholar] [CrossRef]
- Wang, J.; Yeung, B.; Wientjes, M.; Cui, M.; Peer, C.; Lu, Z.; Figg, W.; Woo, S.; Au, J. A Quantitative Pharmacology Model of Exosome-Mediated Drug Efflux and Perturbation-Induced Synergy. Pharmaceutics 2021, 13, 997. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Parra, D.C.; Motallebnejad, P.; Brocchi, M.; Chen, H.J. Exosomes: Small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact. Mater. 2022, 10, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef]
- Logozzi, M.; Mizzoni, D.; Angelini, D.; Di Raimo, R.; Falchi, M.; Battistini, L.; Fais, S. Microenvironmental pH and Exosome Levels Interplay in Human Cancer Cell Lines of Different Histotypes. Cancers 2018, 10, 370. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, T.; Li, Y.; Huang, C.; Suo, M.; Xia, L.; Xu, Y.; Li, G.; Tang, B.Z. Tumor-derived exosomes co-delivering aggregation-induced emission luminogens and proton pump inhibitors for tumor glutamine starvation therapy and enhanced type-I photodynamic therapy. Biomaterials 2022, 283, 121462. [Google Scholar] [CrossRef]
- Thakur, A.; Qiu, G.; Xu, C.; Han, X.; Yang, T.; Ng, S.P.; Chan, K.W.Y.; Wu, C.M.L.; Lee, Y. Label-free sensing of exosomal MCT1 and CD147 for tracking metabolic reprogramming and malignant progression in glioma. Sci. Adv. 2020, 6, eaaz6119. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Gu, Y.; Zhu, Z.; Zhang, H.; Liu, W.; Xu, B.; Zhou, F.; Zhang, M.; Hua, K.; Wu, L.; et al. Exosome Mediated Cytosolic Cisplatin Delivery Through Clathrin-Independent Endocytosis and Enhanced Anti-cancer Effect via Avoiding Endosome Trapping in Cisplatin-Resistant Ovarian Cancer. Front. Med. 2022, 9, 810761. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Sawada, K.; Miyamoto, M.; Shimizu, A.; Yamamoto, M.; Kinose, Y.; Nakamura, K.; Kawano, M.; Kodama, M.; Hashimoto, K.; et al. Exploring the potential of engineered exosomes as delivery systems for tumor-suppressor microRNA replacement therapy in ovarian cancer. Biochem. Biophys. Res. Commun. 2020, 527, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.W.; Charkhchi, P.; Akbari, M.R. Potential clinical utility of liquid biopsies in ovarian cancer. Mol. Cancer 2022, 21, 114. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lv, M.; Fang, S.; Ye, W.; Gao, Y.; Xu, Y. Poly(I:C) enhanced anti-cervical cancer immunities induced by dendritic cells-derived exosomes. Int. J. Biol. Macromol. 2018, 113, 1182–1187. [Google Scholar] [CrossRef]
| Disease | Exosomal Cargo | Type | Exosome Derivation | Recipient Cells | Clinical Value | References |
|---|---|---|---|---|---|---|
| OC | miRNA-205 | miRNA | Serum | - | Diagnosis | [19] |
| miR-200b | miRNA | Serum | Macrophage | Diagnosis and therapeutic target | [21] | |
| miR-4732-5p | miRNA | Serum | - | Diagnosis and monitoring progress | [22] | |
| miR-21-5p | miRNA | OC cells | OC cells | Progression and therapeutic target | [25] | |
| lncRNA SOX2-OT | lncRNA | OC cells | OC cells | Progression and therapeutic target | [26] | |
| circRNA Foxo3 | circRNA | OC cells | OC cells | Progression | [27] | |
| miR-155-5p | miRNA | OC cells | Macrophages | Inhibiting progression | [28] | |
| miR-29a-3p | miRNA | Macrophages | OC cells | Progression | [30] | |
| miR-330-3p | miRNA | Plasma cells | OC cells | Therapeutic target | [31] | |
| circRNA051239 | circRNA | OC cells | OC cells | Metastasis | [32] | |
| miR-6780b-5p | miRNA | Ascites | OC cells | Metastasis | [34] | |
| miR-130a | miRNA | OC cells | HUVECs | Angiogenesis | [36] | |
| lncRNA ATB | lncRNA | OC cells | HUVECs | Therapeutic target | [37] | |
| miR-92b-3p | miRNA | OC cells | HUVECs | Antiangiogenic therapy | [39] | |
| miR-429 | miRNA | OC cells | OC cells | Chemoresistance and therapeutic target | [40] | |
| miR-21-5p | miRNA | OC cells | OC cells | Chemoresistance and therapeutic target | [41] | |
| miR-484 | miRNA | Engineered | OC cells and AECs | Chemotherapy sensitization | [46] | |
| miR-497 | miRNA | Engineered | OC cells | Overcoming chemoresistance | [74] | |
| CC | lncRNA DLX6-AS1 | lncRNA | Serum | - | Diagnosis | [49] |
| miR-125a-5p | miRNA | Plasma | - | Diagnosis | [50] | |
| Hedgehog-GLI | - | CC cells | HUVECs | Angiogenesis | [53] | |
| miR-663b | miRNA | CC cells | HUVECs | Angiogenesis | [54] | |
| miR-1323 | miRNA | CAFs | CC cells | Progression and therapeutic target | [55] | |
| miR-1468-5p | miRNA | CC cells | LECs | Prognostic markers and therapeutic target | [56] | |
| miR-142-5p | miRNA | CC cells | LECs | Diagnostic marker and therapeutic target | [57] | |
| miR-663b | miRNA | CC cells | CC cells | Metastasis | [58] | |
| lncRNA UCA1 | lncRNA | CC stem cells | CC stem cells | Progression | [59] | |
| lncRNA AGAP2-AS1 | lncRNA | CC cells | CC cells | Therapeutic target | [60] | |
| LINC01305 | lncRNA | CC cells | CC cells | Progression | [61] | |
| EC | miR-15a-5p | miRNA | Plasma | - | Diagnosis | [64] |
| miR-143-3p,miR-195-5p, miR-20b-5p,miR-204-5p, miR-423-3p,miR-484 | miRNA | Serum | - | Diagnosis | [66] | |
| miR-142-3p,miR-146a-5p,miR-151a-5p | miRNA | Plasma | - | Diagnosis | [67] | |
| lncRNA NEAT1 | lncRNA | CAFs | EC cells | Therapeutic target | [71] | |
| hsa_circ_0001610 | circRNA | TAMs | EC cells | Radioresistance | [72] | |
| miR-26a-5p | miRNA | EC cells | LECs | Metastasis | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, M.; Miao, Y.; Zhu, Y.; Wang, J.; Zhou, H. Advances in Exosomes as Diagnostic and Therapeutic Biomarkers for Gynaecological Malignancies. Cancers 2022, 14, 4743. https://doi.org/10.3390/cancers14194743
Miao M, Miao Y, Zhu Y, Wang J, Zhou H. Advances in Exosomes as Diagnostic and Therapeutic Biomarkers for Gynaecological Malignancies. Cancers. 2022; 14(19):4743. https://doi.org/10.3390/cancers14194743
Chicago/Turabian StyleMiao, Mengdan, Yifei Miao, Yanping Zhu, Junnan Wang, and Huaijun Zhou. 2022. "Advances in Exosomes as Diagnostic and Therapeutic Biomarkers for Gynaecological Malignancies" Cancers 14, no. 19: 4743. https://doi.org/10.3390/cancers14194743
APA StyleMiao, M., Miao, Y., Zhu, Y., Wang, J., & Zhou, H. (2022). Advances in Exosomes as Diagnostic and Therapeutic Biomarkers for Gynaecological Malignancies. Cancers, 14(19), 4743. https://doi.org/10.3390/cancers14194743







