The Role and Significance of Bioumoral Markers in Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. PSA and PSA Derivatives
2.1. Total PSA
2.2. Free PSA and Free/Total PSA Ratio
2.3. Complexed PSA (cPSA)
2.4. Age-Specific PSA
2.5. PSA Doubling Time (PSADT)
2.6. PSA Velocity (PSAV)
2.7. PSA Density (PSAD)
2.8. ProPSA (pPSA)
2.9. isoPSA
3. Alkaline Phosphatase (ALP)
4. Prostate Specific Membrane Antigen (PSMA)
5. Prostate Health Index (PHI)
6. Prostate Cancer Antigen 3 (PCA3)
7. Michigan Prostate Score (MiPS)
8. 4Kscore (Four Kalikrein Panel)
9. The Stockholm-3 Model, or STHLM3
10. Androgen Receptor Variant 7 (AR V-7)
11. Genetic Panels in Prostate Cancer Prognosis
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- World Health Organisation. International Agency for Research on Cancer—Cancer Today—Prostate Cancer. Available online: https://gco.iarc.fr/today/online-analysis-map?v=2020&mode (accessed on 13 November 2021).
- Platz, E.A.; Giovannucci, E. Prostate cancer. In Cancer Epidemiology and Prevention; Schottenfeld, D., Fraumeni, J.F., Eds.; Oxford University Press: New York, NY, USA, 2006; pp. 1128–1150. [Google Scholar]
- Giri, V.N.; Hegarty, S.E.; Hyatt, C.; O’Leary, E.; Garcia, J.; Knudsen, K.E.; Kelly, W.K.; Gomella, L.G. Germline genetic testing for inherited prostate cancer in practice: Implications for genetic testing, precision therapy, and cascade testing. Prostate 2019, 79, 333–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giri, V.N.; Knudsen, K.; Kelly, W.K.; Abida, W.; Andriole, G.L.; Bangma, C.H.; Bekelman, J.E.; Benson, M.C.; Blanco, A.; Burnett, A.; et al. Role of Genetic Testing for Inherited Prostate Cancer Risk: Philadelphia Prostate Cancer Consensus Conference 2017. J. Clin. Oncol. 2018, 36, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Leitzmann, M.F.; Rohrmann, S. Risk factors for the onset of prostatic cancer: Age, location, and behavioral correlates. Clin. Epidemiol. 2012, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kasper, J.S.; Giovannucci, E. A meta-analysis of diabetes mellitusand the risk of prostate cancer. Cancer Epidemiol. Prev. Biomark. 2006, 15, 2056–2062. [Google Scholar] [CrossRef] [Green Version]
- Preston, M.A.; Riis, A.H.; Ehrenstein, V.; Breau, R.; Batista, J.L.; Olumi, A.F.; Mucci, L.A.; Adami, H.-O.; Sørensen, H.T. Metformin Use and Prostate Cancer Risk. Eur. Urol. 2014, 66, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Ansbaugh, N.; Shannon, J.; Mori, M.; Farris, P.E.; Garzotto, M. Agent Orange as a risk factor for high-grade prostate cancer. Cancer 2013, 119, 2399–2404. [Google Scholar] [CrossRef] [Green Version]
- Huncharek, M.; Haddock, K.S.; Reid, R.; Kupelnick, B. Smoking as a risk factor for prostate cancer: A meta-analysis of 24 prospective cohort studies. Am. J. Public Health 2010, 100, 693–701. [Google Scholar] [CrossRef]
- Burns, J.A.; Weiner, A.B.; Catalona, W.J.; Li, E.V.; Schaeffer, E.M.; Hanauer, S.B.; Strong, S.; Burns, J.; Hussain, M.H.; Kundu, S.D. Inflammatory Bowel Disease and the Risk of Prostate Cancer. Eur. Urol. 2019, 75, 846–852. [Google Scholar] [CrossRef]
- Lian, W.Q.; Luo, F.; Song, X.L.; Lu, Y.J.; Zhao, S.C. Gonorrhea and Prostate Cancer Incidence: An Updated Meta-Analysis of 21 Epidemiologic Studies. Med Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 1895. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.R.; Motiwala, H.G.; Karim, O.M. The discovery of prostate-specific antigen. BJU Int. 2007, 101, 5–10. [Google Scholar] [CrossRef]
- Loeb, S.; Bjurlin, M.A.; Nicholson, J.; Tammela, T.L.; Penson, D.; Carter, H.B.; Carroll, P.; Etzioni, R. Overdiagnosis and Overtreatment of Prostate Cancer. Eur. Urol. 2014, 65, 1046–1055. [Google Scholar] [CrossRef] [Green Version]
- Matlaga, B.R.; Eskew, L.A.; McCullough, D.L. Prostate biopsy: Indications and technique. J. Urol. 2003, 169, 12–19. [Google Scholar] [CrossRef]
- Schröder, F.H. PSA screening—a review of recent studies. Eur. J. Cancer 2009, 45, 402–404. [Google Scholar] [CrossRef]
- Noguchi, M.; A Stamey, T.; McNeal, J.E.; Yemoto, C.M. Preoperative serum prostate specific antigen does not reflect biochemical failure rates after radical prostatectomy in men with large volume cancers. J. Urol. 2000, 164, 1596–1600. [Google Scholar] [CrossRef]
- Roehrborn, C.G.; Boyle, P.; Gould, A.; Waldstreicher, J. Serum prostate-specific antigen as a predictor of prostate volume in men with benign prostatic hyperplasia. Urol. 1999, 53, 581–589. [Google Scholar] [CrossRef]
- Roehrborn, C.G.; McConnell, J.; Bonilla, J.; Rosenblatt, S.; Hudson, P.B.; Malek, G.H.; Schellhammer, P.F.; Bruskewitz, R.; Matsumoto, A.M.; Harrison, L.H.; et al. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. PROSCAR long-term efficacy and safety study. J. Urol. 2000, 163, 13–20. [Google Scholar] [CrossRef]
- Shariat, S.F.; Karam, J.A.; Margulis, V.; Karakiewicz, P.I. New blood-based biomarkers for the diagnosis, staging and prognosis of prostate cancer. BJU Int. 2008, 101, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Bjartell, A.S. Next-generation Prostate-specific Antigen Test: Ready To Use? Eur. Urol. 2013, 64, 700–702. [Google Scholar] [CrossRef] [PubMed]
- Oesterling, J.E. Prostate Specific Antigen: A Critical Assessment of the Most Useful Tumor Marker for Adenocarcinoma of the Prostate. J. Urol. 1991, 145, 907–923. [Google Scholar] [CrossRef]
- Luderer, A.A.; Chen, Y.-T.; Soriano, T.F.; Kramp, W.J.; Carlson, G.; Cuny, C.; Sharp, T.; Smith, W.; Petteway, J.; Brawer, M.K.; et al. Measurement of the proportion of free to total prostate-specific antigen improves diagnostic performance of prostate-specific antigen in the diagnostic gray zone of total prostate-specific antigen. Urol. 1995, 46, 187–194. [Google Scholar] [CrossRef]
- European Association of Urology. Guidelines on Prostate Cancer. Available online: https://uroweb.org/guideline/prostate-cancer/ (accessed on 13 November 2021).
- The Romanian Ministry of Health. Guidelines on Prostate Cancer. Available online: http://old.ms.ro/index.php?pag=181&pg=5,1323anexa14 (accessed on 13 November 2021).
- Lilja, H.; Christensson, A.; Dahlén, U.; Matikainen, M.T.; Nilsson, O.; Pettersson, K.; Lövgren, T. Prostate-specific antigen in serum occurs predominantly in complex with alpha 1-antichymotrypsin. Clin. Chem. 1991, 37, 1618–1625. [Google Scholar] [CrossRef]
- Stenman, U.H.; Leinonen, J.; Alfthan, H.; Rannikko, S.; Tuhkanen, K.; Alfthan, O. A complex between prostate-specific antigen and alpha 1-antichymotrypsin is the major form of prostate-specific antigen in serum of patients with prostatic cancer: Assay of the complex improves clinical sensitivity for cancer. Cancer Res. 1991, 51, 222. [Google Scholar] [PubMed]
- Makarov, D.V.; Loeb, S.; Getzenberg, R.H.; Partin, A.W. Biomarkers for prostate cancer. Annu. Rev. Med. 2009, 60, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Partin, A.W.; Brawer, M.K.; Subong, E.N.P.; Kelley, C.A.; Cox, J.L.; Bruzek, D.J.; Pannek, J.; E Meyer, G.; Chan, D.W. Prospective evaluation of percent free-PSA and complexed-PSA for early detection of prostate cancer. Prostate Cancer Prostatic Dis. 1998, 1, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Arcangeli, C.G.; Humphrey, P.A.; Smith, D.S.; Harmon, T.J.; Shepherd, D.L.; Keetch, D.W.; Catalona, W.J. Percentage of free serum prostate-specific antigen as a predictor of patho-logical features of prostate cancer in a screening population. Urology 1998, 51, 558–564. [Google Scholar] [CrossRef]
- Catalona, W.; Partin, A.; Slawin, K.; Brawer, M.; Flanigan, R.; Patel, A.; Richie, J.; Dekernion, J.; Walsh, P.; Scardino, P.; et al. Use of the Percentage of Free Prostate-Specific Antigen to Enhance Differentiation of Prostate Cancer From Benign Prostatic Disease: A Prospective Multicenter Clinical Trial. J. Urol. 1999, 161, 353–354. [Google Scholar] [CrossRef]
- Sturgeon, C.M.; Duffy, M.J.; Stenman, U.H.; Lilja, H.; Brunner, N.; Chan, D.W.; Babaian, R.; Bast, R.C., Jr.; Dowell, B.; Esteva, F.J.; et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin. Chem. 2008, 54, e11–e79. [Google Scholar] [CrossRef] [Green Version]
- Björk, T.; Ljungberg, B.; Piironen, T.; Abrahamsson, P.-A.; Pettersson, K.; Cockett, A.T.; Lilja, H. Rapid Exponential Elimination of Free Prostate-Specific Antigen Contrasts the Slow, Capacity-Limited Elimination of PSA Complexed to Alpha1-Antichymotrypsin From Serum. Urology 1998, 51, 57–62. [Google Scholar] [CrossRef]
- Brawer, M.K.; Meyer, G.E.; Letran, J.L.; Bankson, D.D.; Morris, D.L.; Yeung, K.K.; Allard, W. Measurement of complexed PSA improves specificity for early detection of prostate cancer. Urology 1998, 52, 372–378. [Google Scholar] [CrossRef]
- Partin, A.W.; Brawer, M.K.; Bartsch, G.; Horninger, W.; Taneja, S.S.; Lepor, H.; Babaian, R.; Childs, S.J.; Stamey, T.; Fritsche, H.A.; et al. Complexed Prostate Specific Antigen Improves Specificity for Prostate Cancer Detection: Results of a Prospective Multicenter Clinical Trial. J. Urol. 2003, 170, 1787–1791. [Google Scholar] [CrossRef] [PubMed]
- DeAntoni, E.P. Age-specific reference ranges for PSA in the detection of prostate cancer. Oncology 1997, 11, 475–482. [Google Scholar] [PubMed]
- Lankford, S.P.; Peters, K.L.; Elser, R.C. Potential Effects of Age-Specific Reference Ranges for Serum Prostate-Specific Antigen. Eur. Urol. 1995, 27, 182–186. [Google Scholar] [CrossRef]
- Reissigl, A.; Pointner, J.; Horninger, W.; Ennemoser, O.; Strasser, H.; Klocker, H.; Bartsch, G. Comparison of different prostate-specific antigen cutpoints for early detection of prostate cancer: Results of a large screening study. Urology 1995, 46, 662–665. [Google Scholar] [CrossRef]
- Wieder, J.A.; Belldegrun, A.S. The Utility of PSA Doubling Time to Monitor Prostate Cancer Recurrence. Mayo Clin. Proc. 2001, 76, 571–572. [Google Scholar] [CrossRef]
- Carter, H.B.; Pearson, J.D.; Metter, E.J.; Brant, L.J.; Chan, D.W.; Andres, R.; Fozard, J.L.; Walsh, P.C. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. JAMA 1992, 267, 2215–2220. [Google Scholar] [CrossRef] [Green Version]
- Carter, H.B.; Coey, D.S. The prostate: An increasing medical problem. Prostate 1990, 16, 39–48. [Google Scholar] [CrossRef]
- Vickers, A.J.; Savage, C.; O’Brien, M.F.; Lilja, H. Systematic Review of Pretreatment Prostate-Specific Antigen Velocity and Doubling Time As Predictors for Prostate Cancer. J. Clin. Oncol. 2009, 27, 398–403. [Google Scholar] [CrossRef] [Green Version]
- Wolters, T.; Roobol, M.J.; Bangma, C.H.; Schröder, F.H. Is Prostate-Specific Antigen Velocity Selective for Clinically Significant Prostate Cancer in Screening? European Randomized Study of Screening for Prostate Cancer (Rotterdam). Eur. Urol. 2009, 55, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.C.; Whang, I.S.; Pantuck, A.; Ring, K.; Kaplan, S.A.; Olsson, C.A.; Cooner, W.H. Prostate Specific Antigen Density: A Means of Distinguishing Benign Prostatic Hypertrophy and Prostate Cancer. J. Urol. 1992, 147, 815–816. [Google Scholar] [CrossRef]
- Polascik, T.J.; Oesterling, J.E.; Partin, A.W. Prostate specific antigen: A decade of discovery-what we have learned and where we are going. J. Urol. 1999, 162, 293–306. [Google Scholar] [CrossRef]
- Kalish, J.; Cooner, W.H.; Graham, S.D., Jr. Serum PSA adjusted for volume of transition zone (PSAT) is more ac-curate than PSA adjusted for total gland volume (PSAD) in detecting adenocarcinoma of the prostate. Urology 1994, 43, 601. [Google Scholar] [CrossRef]
- Kikuchi, E.; Nakashima, J.; Ishibashi, M.; Ohigashi, T.; Asakura, H.; Tachibana, M.; Murai, M. Prostate specific antigen adjusted for transition zone volume: The most powerful method for detecting prostate carcinoma. Cancer 2000, 89, 842. [Google Scholar] [CrossRef]
- Yilmaz, S.N.; Yildiz, A.; Ayyıldız, S.N.; Ayyıldız, A. PSA, PSA derivatives, proPSA and prostate health index in the diagnosis of prostate cancer. Turk. J. Urol. 2014, 40, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Bangma, C.; Wildhagen, M.; Yurdakul, G.; Schroder, F.; Blijenberg, B. The value of (−7, −5)pro-prostate-specific antigen and human kallikrein-2 as serum markers for grading prostate cancer. BJU Int. 2004, 93, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.Y.; Mikolajczyk, S.D.; Lecksell, K.; Shue, M.J.; Rittenhouse, H.G.; Partin, A.W.; Epstein, J.I. Immunohistochemical staining of prostate cancer with monoclonal antibodies to the precursor of prostate-specific antigen. Urology 2003, 62, 177–181. [Google Scholar] [CrossRef]
- Sokoll, L.J.; Sanda, M.G.; Feng, Z.; Kagan, J.; Mizrahi, I.A.; Broyles, D.L.; Partin, A.W.; Srivastava, S.; Thompson, I.M.; Wei, J.T.; et al. A Prospective, Multicenter, National Cancer Institute Early Detection Research Network Study of [−2]proPSA: Improving Prostate Cancer Detection and Correlating with Cancer Aggressiveness. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1193–1200. [Google Scholar] [CrossRef] [Green Version]
- Mikolajczyk, S.D.; Rittenhouse, H.G. Pro PSA: A more cancer specific form of prostate specific antigen for the early detection of prostate cancer. Keio J. Med. 2003, 52, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Leymarie, N.; Griffin, P.J.; Jonscher, K.; Kolarich, D.; Orlando, R.; McComb, M.; Zaia, J.; Aguilan, J.; Alley, W.R.; Altmann, F.; et al. Interlaboratory Study on Differential Analysis of Protein Glycosylation by Mass Spectrometry: The ABRF Glycoprotein Research Multi-Institutional Study 2012. Mol. Cell. Proteom. 2013, 12, 2935–2951. [Google Scholar] [CrossRef] [Green Version]
- Gilgunn, S.; Conroy, P.J.; Saldova, R.; Rudd, P.M.; O’Kennedy, R.J. Aberrant PSA glycosylation—A sweet predictor of prostate cancer. Nat. Rev. Urol. 2013, 10, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A.; Chait, A.; Hafron, J.M.; Kernen, K.M.; Manickam, K.; Stephenson, A.J.; Wagner, M.; Zhu, H.; Kestranek, A.; Zaslavsky, B.; et al. The Single-parameter, Structure-based IsoPSA Assay Demonstrates Improved Diagnostic Accuracy for Detection of Any Prostate Cancer and High-grade Prostate Cancer Compared to a Concentration-based Assay of Total Prostate-specific Antigen: A Preliminary Report. Eur. Urol. 2017, 72, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Stovsky, M.; Klein, E.A.; Chait, A.; Manickam, K.; Stephenson, A.J.; Wagner, M.; Dineen, M.; Lotan, Y.; Partin, A.; Baniel, J.; et al. Clinical Validation of IsoPSA™, a Single Parameter, Structure Based Assay for Improved Detection of High Grade Prostate Cancer. J. Urol. 2019, 201, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline Phosphatase: An Overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Groot, M.; Kruger, C.B.; Pelger, R.; Groot, C.U.-D. Costs of Prostate Cancer, Metastatic to the Bone, in The Netherlands. Eur. Urol. 2003, 43, 226–232. [Google Scholar] [CrossRef]
- Li, D.; Lv, H.; Hao, X.; Hu, B.; Song, Y. Prognostic value of serum alkaline phosphatase in the survival of prostate cancer: Evidence from a meta-analysis. Cancer Manag. Res. 2018, ume 10, 3125–3139. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, F.G.; Queiroz, M.A.; Ferraro, D.A.; Nunes, R.F.; Dreyer, P.R.; Zaniboni, E.C.; Costa, L.B.; Bastos, D.A.; Marin, J.F.G.; Buchpiguel, C.A. Prostate-specific Membrane Antigen PET: Therapy Response Assessment in Metastatic Prostate Cancer. Radiogr. 2020, 40, 1412–1430. [Google Scholar] [CrossRef]
- Perner, S.; Hofer, M.D.; Kim, R.; Shah, R.B.; Li, H.; Möller, P.; Hautmann, R.E.; Gschwend, J.E.; Kuefer, R.; Rubin, M.A. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum. Pathol. 2007, 38, 696–701. [Google Scholar] [CrossRef]
- Hupe, M.C.; Philippi, C.; Roth, D.; Kümpers, C.; Ribbat-Idel, J.; Becker, F.; Joerg, V.; Duensing, S.; Lubczyk, V.H.; Kirfel, J.; et al. Expression of Prostate-Specific Membrane Antigen (PSMA) on Biopsies Is an Independent Risk Stratifier of Prostate Cancer Patients at Time of Initial Diagnosis. Front. Oncol. 2018, 8, 623. [Google Scholar] [CrossRef]
- Nagaya, N.; Nagata, M.; Lu, Y.; Kanayama, M.; Hou, Q.; Hotta, Z.-U.; China, T.; Kitamura, K.; Matsushita, K.; Isotani, S.; et al. Prostate-specific membrane antigen in circulating tumor cells is a new poor prognostic marker for castration-resistant prostate cancer. PLoS ONE 2020, 15, e0226219. [Google Scholar] [CrossRef] [Green Version]
- Vlachostergios, P.; Zachos, I.; Tzortzis, V. Biomarkers in Prostate-Specific Membrane Antigen Theranostics. Diagnostics 2021, 11, 1108. [Google Scholar] [CrossRef]
- Catalona, W.J.; Partin, A.W.; Sanda, M.G.; Wei, J.T.; Klee, G.G.; Bangma, C.H.; Slawin, K.M.; Marks, L.S.; Loeb, S.; Broyles, D.L.; et al. A Multicenter Study of [-2]Pro-Prostate Specific Antigen Combined With Prostate Specific Antigen and Free Prostate Specific Antigen for Prostate Cancer Detection in the 2.0 to 10.0 ng/ml Prostate Specific Antigen Range. J. Urol. 2011, 185, 1650–1655. [Google Scholar] [CrossRef] [Green Version]
- Filella, X.; Gimenez, N. Evaluation of [-2] proPSA and prostate health index (phi) for the detection of prostate cancer: A systematic review and meta-analysis. Clin. Chem. Lab. Med. 2013, 51, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J. Biomarkers for prostate cancer: Prostate-specific antigen and beyond. Clin. Chem. Lab. Med. 2020, 58, 326–339. [Google Scholar] [CrossRef] [Green Version]
- Jansen, F.H.; van Schaik, R.H.; Kurstjens, J.; Horninger, W.; Klocker, H.; Bektic, J.; Wildhagen, M.F.; Roobol, M.J.; Bangma, C.H.; Bartsch, G. Prostate-Specific Antigen (PSA) Isoform p2PSA in Combination with Total PSA and Free PSA Improves Diagnostic Accuracy in Prostate Cancer Detection. Eur. Urol. 2010, 57, 921–927. [Google Scholar] [CrossRef] [PubMed]
- De La Calle, C.; Patil, D.; Wei, J.T.; Scherr, D.S.; Sokoll, L.; Chan, D.W.; Siddiqui, J.; Mosquera, J.M.; Rubin, M.A.; Sanda, M.G. Multicenter evaluation of the prostate health index to detect aggressive prostate cancer in biopsy naive men. J. Urol. 2015, 194, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Loeb, S.; Sanda, M.G.; Broyles, D.L.; Shin, S.S.; Bangma, C.H.; Wei, J.T.; Partin, A.W.; Klee, G.G.; Slawin, K.M.; Marks, L.S.; et al. The Prostate Health Index Selectively Identifies Clinically Significant Prostate Cancer. J. Urol. 2015, 193, 1163–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olleik, G.; Kassouf, W.; Aprikian, A.; Hu, J.; Vanhuyse, M.; Cury, F.; Peacock, S.; Bonnevier, E.; Palenius, E.; Dragomir, A. Evaluation of New Tests and Interventions for Prostate Cancer Management: A Systematic Review. J. Natl. Compr. Cancer Netw. 2018, 16, 1340–1351. [Google Scholar] [CrossRef] [Green Version]
- Lughezzani, G.; Lazzeri, M.; Buffi, N.M.; Abrate, A.; Mistretta, F.A.; Hurle, R.; Pasini, L.; Castaldo, L.; De Zorzi, S.Z.; Peschechera, R.; et al. Preoperative prostate health index is an independent predictor of early biochemical recurrence after radical prostatectomy: Results from a prospective single-center study. Urol. Oncol. 2015, 33, 337.e7–337.e14. [Google Scholar] [CrossRef]
- Hsieh, P.-F.; Li, W.-J.; Lin, W.-C.; Chang, H.; Chang, C.-H.; Huang, C.-P.; Yang, C.-R.; Chen, W.-C.; Chang, Y.-H.; Wu, H.-C. Combining prostate health index and multiparametric magnetic resonance imaging in the diagnosis of clinically significant prostate cancer in an Asian population. World J. Urol. 2020, 38, 1207–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bussemakers, M.J.; Van Bokhoven, A.; Verhaegh, G.W.; Smit, F.P.; Karthaus, H.F.; Schalken, J.A.; Debruyne, F.M.; Ru, N.; Isaacs, W.B. DD3: A new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999, 59, 5975–5979. [Google Scholar]
- Filella, X.; Fernández-Galán, E.; Bonifacio, R.F.; Foj, L. Emerging biomarkers in the diagnosis of prostate cancer. Pharm. Pers. Med. 2018, ume 11, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Salami, S.S.; Schmidt, F.; Laxman, B.; Regan, M.M.; Rickman, D.S.; Scherr, D.; Bueti, G.; Siddiqui, J.; Tomlins, S.A.; Wei, J.T.; et al. Combining urinary detection of TMPRSS2:ERG and PCA3 with serum PSA to predict diagnosis of prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 566–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenner, A. PCA3 as a Grade Reclassification Predictor. Nat. Rev. Urol. 2017, 14, 390. [Google Scholar] [CrossRef] [PubMed]
- Schröder, F.H.; Venderbos, L.D.; Bergh, R.C.V.D.; Hessels, D.; van Leenders, G.J.; van Leeuwen, P.J.; Wolters, T.; Barentsz, J.O.; Roobol, M.J. Prostate Cancer Antigen 3: Diagnostic Outcomes in Men Presenting With Urinary Prostate Cancer Antigen 3 Scores ≥100. Urology 2014, 83, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Tomlins, S.A.; Day, J.R.; Lonigro, R.J.; Hovelson, D.H.; Siddiqui, J.; Kunju, L.P.; Dunn, R.L.; Meyer, S.; Hodge, P.; Groskopf, J.; et al. Urine TMPRSS2:ERG Plus PCA3 for Individualized Prostate Cancer Risk Assessment. Eur. Urol. 2016, 70, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Alford, A.V.; Brito, J.M.; Yadav, K.K.; Yadav, S.S.; Tewari, A.K.; Renzulli, J. The Use of Biomarkers in Prostate Cancer Screening and Treatment. Rev. Urol. 2017, 19, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, Z.; Cooperberg, M.R.; Spratt, D.E.; Feng, F.Y. Genomic biomarkers in prostate cancer. Transl. Androl. Urol. 2018, 7, 459–471. [Google Scholar] [CrossRef]
- Vickers, A.J.; Cronin, A.M.; Aus, G.; Pihl, C.-G.; Becker, C.; Pettersson, K.; Scardino, P.T.; Hugosson, J.; Lilja, H. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: Data from the European Randomized Study of Prostate Cancer Screening in Göteborg, Sweden. BMC Med. 2008, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Filella, X.; Foj, L. Emerging biomarkers in the detection and prognosis of prostate cancer. Clin. Chem. Lab. Med. 2015, 53, 963–973. [Google Scholar] [CrossRef]
- Munteanu, V.C.; Munteanu, R.A.; Gulei, D.; Schitcu, V.H.; Petrut, B.; Berindan Neagoe, I.; Achimas Cadariu, P.; Coman, I. PSA Based Biomarkers, Imagistic Techniques and Combined Tests for a Better Diagnostic of Localized Prostate Cancer. Diagnostics 2020, 10, 806. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Ms, A.M.C.; Aus, G.; Pihl, C.-G.; Becker, C.; Pettersson, K.; Scardino, P.T.; Hugosson, J.; Lilja, H. Impact of recent screening on predicting the outcome of prostate cancer biopsy in men with elevated prostate-specific antigen. Cancer 2010, 116, 2612–2620. [Google Scholar] [CrossRef]
- Parekh, D.J.; Punnen, S.; Sjoberg, D.D.; Asroff, S.W.; Bailen, J.L.; Cochran, J.S.; Concepcion, R.; David, R.D.; Deck, K.B.; Dumbadze, I.; et al. A multi-institutional prospective trial in the USA confirms that the 4Kscore accu-rately identifies men with high-grade prostate cancer. Eur Urol. 2015, 68, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Kohaar, I.; Petrovics, G.; Srivastava, S. A Rich Array of Prostate Cancer Molecular Biomarkers: Opportunities and Challenges. Int. J. Mol. Sci. 2019, 20, 1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, G.I.; Regis, F.; Castelli, T.; Favilla, V.; Privitera, S.; Giardina, R.; Cimino, S.; Morgia, G. A systematic review and meta-analysis of the diagnostic accuracy of prostate health index and 4-kallikrein panel score in predicting overall and high-grade prostate cancer. Clin. Genitourin. Cancer 2017, 15, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Rector, T.S.; Taylor, B.C.; Wilt, T.J. Systematic review of prognostic tests. In Methods Guide for Medical Test Reviews; Chang, S.M., Matchar, D.B., Smetana, G.W., Umscheid, C.A., Eds.; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2012; Chapter 12. [Google Scholar]
- Ström, P.; Nordström, T.; Aly, M.; Egevad, L.; Grönberg, H.; Eklund, M. The Stockholm-3 Model for Prostate Cancer Detection: Algorithm Update, Biomarker Contribution, and Reflex Test Potential. Eur. Urol. 2018, 74, 204–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grönberg, H.; Eklund, M.; Picker, W.; Aly, M.; Jäderling, F.; Adolfsson, J.; Landquist, M.; Haug, E.S.; Ström, P.; Carlsson, S.; et al. Prostate Cancer Diagnostics Using a Combination of the Stockholm3 Blood Test and Multiparametric Magnetic Resonance Imaging. Eur. Urol. 2018, 74, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.; Coleman, I.; Yuan, W.; Sprenger, C.; Dolling, D.; Rodrigues, D.N.; Russo, J.W.; Figueiredo, I.; Bertan, C.; Seed, G.; et al. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J. Clin. Investig. 2018, 129, 192–208. [Google Scholar] [CrossRef] [Green Version]
- Friedlander, T.W.; Pritchard, C.C.; Beltran, H. Personalizing Therapy for Metastatic Prostate Cancer: The Role of Solid and Liquid Tumor Biopsies. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 358–369. [Google Scholar] [CrossRef]
- Markowski, M.C.; Silberstein, J.L.; Eshleman, J.R.; Eisenberger, M.A.; Luo, J.; Antonarakis, E.S. Clinical Utility of CLIA-Grade AR-V7 Testing in Patients With Metastatic Castration-Resistant Prostate Cancer. JCO Precis. Oncol. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Shore, N.D.; Kella, N.; Moran, B.; Boczko, J.; Bianco, F.J.; Crawford, E.D.; Davis, T.; Roundy, K.M.; Rushton, K.; Grier, C.; et al. Impact of the Cell Cycle Progression Test on Physician and Patient Treatment Selection for Localized Prostate Cancer. J. Urol. 2016, 195, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Swanson, G.P.; Fisher, G.; Brothman, A.R.; Berney, D.M.; Reid, J.E.; Mesher, D.; Speights, V.O.; Stankiewicz, E.; Foster, C.S.; et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: A retrospective study. Lancet Oncol. 2011, 12, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Cuzick, J.; Berney, D.M.; Fisher, G.; Mesher, D.; Møller, H.; Reid, J.E.; Perry, M.; Park, J.; Younus, A.; Gutin, A.; et al. Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br. J. Cancer 2012, 106, 1095–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohler, J.L.; Armstrong, A.J.; Bahnson, R.R.; D’Amico, A.V.; Davis, B.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; Horwitz, E.M.; et al. Prostate Cancer, Version 1.2016. J. Natl. Compr. Cancer Netw. 2016, 14, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Dall’Era, M.A.; Maddala, T.; Polychronopoulos, L.; Gallagher, J.R.; Febbo, P.G.; Denes, B.S. Utility of the Oncotype DX ® Prostate Cancer Assay in Clinical Practice for Treatment Selection in Men Newly Diagnosed with Prostate Cancer: A Retrospective Chart Review Analysis. Urol. Pract. 2015, 2, 343–348. [Google Scholar] [CrossRef]
- Cullen, J.; Rosner, I.L.; Brand, T.C.; Zhang, N.; Tsiatis, A.C.; Moncur, J.; Ali, A.; Chen, Y.; Knezevic, D.; Maddala, T.; et al. A biopsy-based 17-gene genomic prostate score predicts recurrence after radical prostatectomy and adverse surgical pathology in a racially diverse population of men with clinically low- and intermediate-risk prostate cancer. Eur. Urol. 2015, 68, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Brajtbord, J.S.; Leapman, M.S.; Cooperberg, M.R. The CAPRA score at 10 years: Contemporary perspectives and analysis of sup-porting studies. Eur. Urol. 2017, 71, 705–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, E.A.; Cooperberg, M.R.; Magi-Galluzzi, C.; Simko, J.P.; Falzarano, S.M.; Maddala, T.; Chan, J.; Li, J.; Cowan, J.E.; Tsiatis, A.C.; et al. A 17-gene Assay to Predict Prostate Cancer Aggressiveness in the Context of Gleason Grade Heterogeneity, Tumor Multifocality, and Biopsy Undersampling. Eur. Urol. 2014, 66, 550–560. [Google Scholar] [CrossRef] [Green Version]
- Cullen, J.; Kuo, H.-C.; Shan, J.; Lu, R.; Aboushwareb, T.; Eeden, S.K.V.D. The 17-Gene Genomic Prostate Score Test as a Predictor of Outcomes in Men with Unfavorable Intermediate Risk Prostate Cancer. Urol. 2020, 143, 103–111. [Google Scholar] [CrossRef]
- Zhao, S.G.; Chang, S.L.; Spratt, D.E.; Erho, N.; Yu, M.; Ashab, H.A.D.; Alshalalfa, M.; Speers, C.; Tomlins, S.A.; Davicioni, E.; et al. Development and validation of a 24-gene predictor of response to postoperative radiotherapy in prostate cancer: A matched, retrospective analysis. Lancet Oncol. 2016, 17, 1612–1620. [Google Scholar] [CrossRef]
- Erho, N.; Crisan, A.; Vergara, I.A.; Mitra, A.P.; Ghadessi, M.; Buerki, C.; Bergstralh, E.J.; Kollmeyer, T.; Fink, S.; Haddad, Z.; et al. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS ONE 2013, 8, e66855. [Google Scholar]
- Dalela, D.; Santiago-Jiménez, M.; Yousefi, K.; Karnes, R.J.; Ross, A.E.; Den, R.B.; Freedland, S.J.; Schaeffer, E.M.; Dicker, A.P.; Menon, M.; et al. Genomic classifier augments the role of pathological features in identifying optimal candidates for adjuvant radiation therapy in patients with prostate cancer: Development and internal validation of a multivariable prognostic model. J. Clin. Oncol. 2017, 35, 1982–1990. [Google Scholar] [CrossRef]
- Cucchiara, V.; Cooperberg, M.R.; Dall’Era, M.; Lin, D.W.; Montorsi, F.; Schalken, J.A.; Evans, C.P. Genomic Markers in Prostate Cancer Decision Making. Eur. Urol. 2018, 73, 572–582. [Google Scholar] [CrossRef]
- Moul, J.W. Prostate specific antigen only progression of prostate cancer. J. Urol. 2000, 163, 1632–1642. [Google Scholar] [CrossRef]
- Roach, M., III; Hanks, G.; Thames, H., Jr.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef]
- Jairath, N.K.; Pra, A.D.; Vince, R.; Dess, R.T.; Jackson, W.C.; Tosoian, J.J.; McBride, S.M.; Zhao, S.G.; Berlin, A.; Mahal, B.A.; et al. A Systematic Review of the Evidence for the Decipher Genomic Classifier in Prostate Cancer. Eur. Urol. 2021, 79, 374–383. [Google Scholar] [CrossRef]
- NCCN. Clinical Practice Guidelines in Oncology—Prostate Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 13 November 2021).
- Leyten, G.H.J.M.; Hessels, D.; Smit, F.P.; Jannink, S.A.; De Jong, H.; Melchers, W.; Cornel, E.B.; De Reijke, T.M.; Vergunst, H.; Kil, P.; et al. Identification of a Candidate Gene Panel for the Early Diagnosis of Prostate Cancer. Clin. Cancer Res. 2015, 21, 3061–3070. [Google Scholar] [CrossRef] [Green Version]
- Van Neste, L.; Hendriks, R.J.; Dijkstra, S.; Trooskens, G.; Cornel, E.B.; Jannink, S.A.; de Jong, H.; Hessels, D.; Smit, F.P.; Melchers, W.J.; et al. Detection of high-grade prostate cancer using a urinary molecular biomarker-based risk score. Eur. Urol. 2016, 70, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Lendínez-Cano, G.; Ojeda-Claro, A.V.; Gómez-Gómez, E.; Jimenez, P.M.; Martin, J.F.; Dominguez, J.F.; Amores, J.; Cozar, J.M.; Bachiller, J.; Juárez, A.; et al. Prospective study of diagnostic accuracy in the detection of high-grade prostate cancer in biopsy-naïve patients with clinical suspicion of prostate cancer who underwent the Select MDx test. Prostate 2021, 81, 857–865. [Google Scholar] [CrossRef] [PubMed]
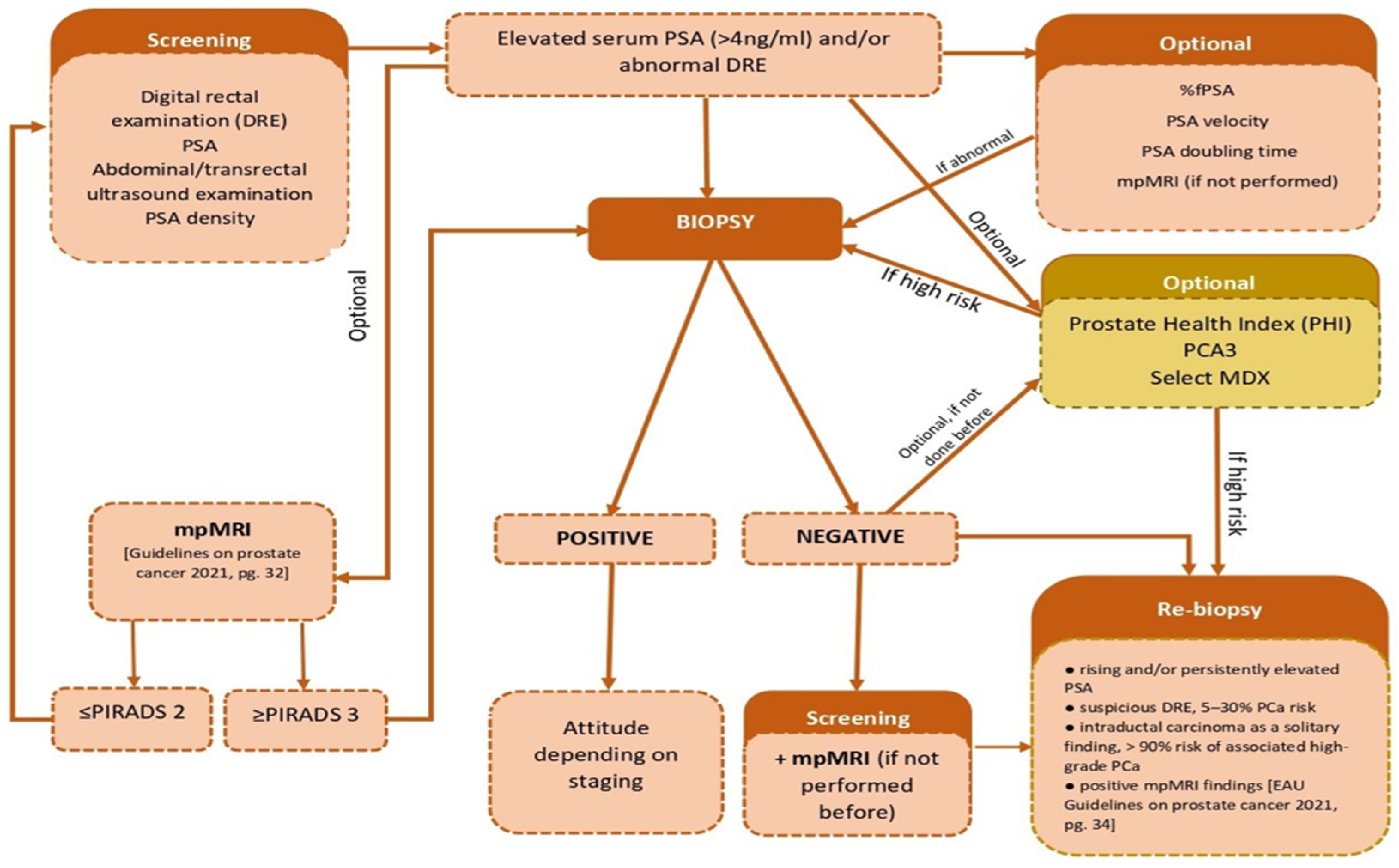
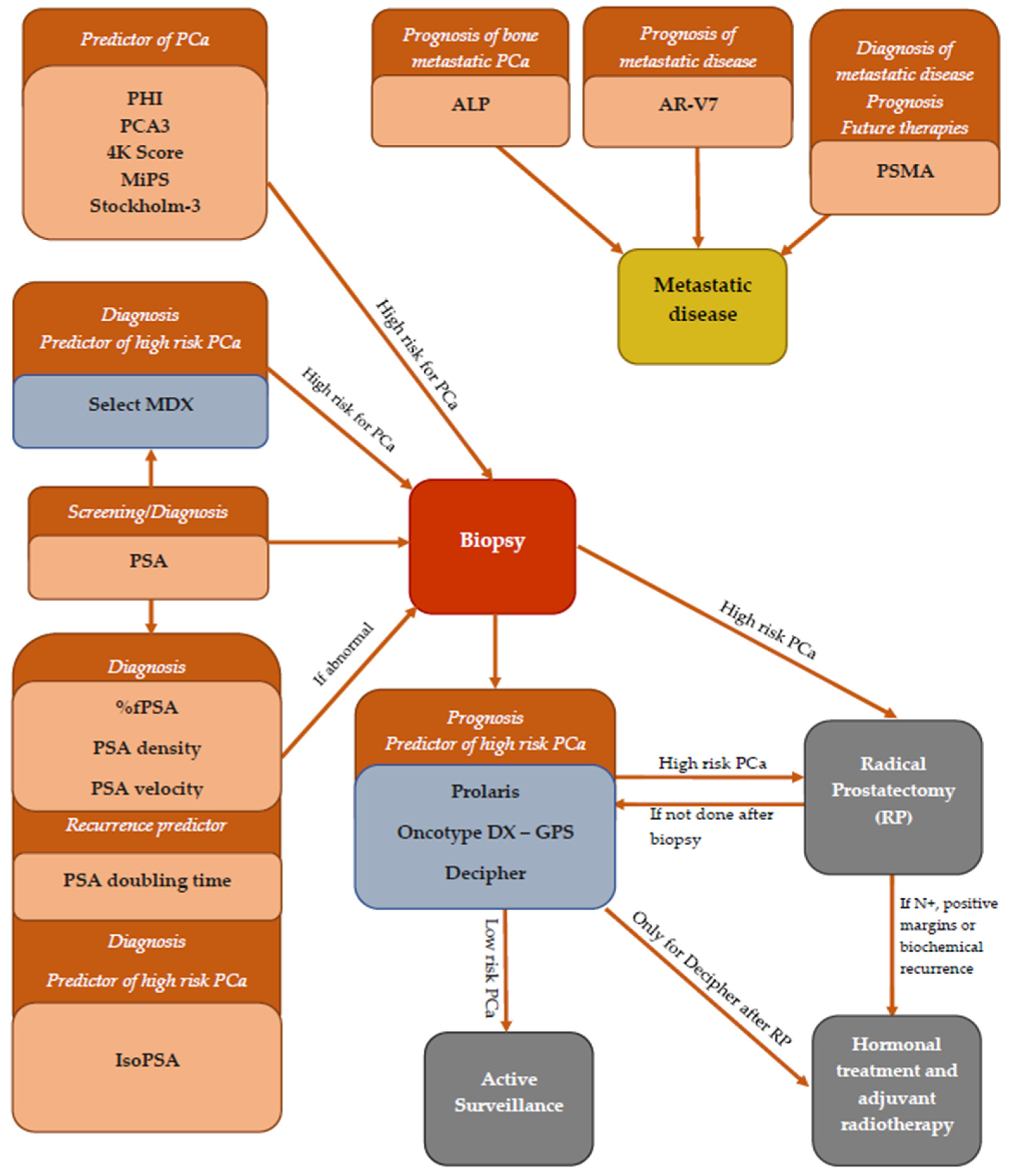
| Biomarker | Role | Sample |
|---|---|---|
| Prostate Specific Antigen (PSA) | Screening Diagnosis | Blood |
| Free/Total PSA ratio (%fPSA) | Diagnosis | Blood |
| PSA density | Diagnosis | Blood |
| PSA velocity | Prognosis | Blood |
| PSA doubling time | Recurrence predictor | Blood |
| Iso PSA | Diagnosis Predictor of high risk PCa | Blood |
| Alkaline Phosphatase (ALP) | Prognosis of bone metastatic PCa | Blood |
| Prostate specific membrane antigen (PSMA) | Diagnosis of metastatic disease Prognosis Future therapies | Tissue Blood (circulating tumor cells) |
| Prostate Cancer Antigen 3 (PCA3) | Diagnosis | Urine |
| Androgen receptor variant 7 (AR V-7) | Prognosis of metastatic disease | Tissue Blood (circulating tumor cells) |
| Test | Analytes Detected | Fluid |
|---|---|---|
| Prostate Health Index (PHI) | PSA, fPSA, [−2]ProPSA | Serum |
| Prostate cancer antigen (PCA3) | PCA3 | Urine collected after prostate massage |
| Four-kallikrein panel (4K Score) | PSA, fPSA, iPSA, khK2 | Serum or plasma |
| MiPS | PCA3, TMPRSS2-ERG | Urine |
| Stockholm-3 (STHLM3) | PSA, fPSA, hK2, MIC 1, MSMB, genetic markers | Serum |
| Test | Role | Sample |
|---|---|---|
| Prolaris | Prognosis Predictor of high risk disease | Tissue |
| Oncotype DX—GPS | Prognosis Predictor of high risk disease | Tissue |
| Decipher | Prognosis Predictor of high risk disease Indicator for treatment | Tissue |
| Select MDX | Diagnosis Predictor of high risk disease | Urine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantin, T.; Savu, D.A.; Bucur, Ș.; Predoiu, G.; Constantin, M.M.; Jinga, V. The Role and Significance of Bioumoral Markers in Prostate Cancer. Cancers 2021, 13, 5932. https://doi.org/10.3390/cancers13235932
Constantin T, Savu DA, Bucur Ș, Predoiu G, Constantin MM, Jinga V. The Role and Significance of Bioumoral Markers in Prostate Cancer. Cancers. 2021; 13(23):5932. https://doi.org/10.3390/cancers13235932
Chicago/Turabian StyleConstantin, Traian, Diana Alexandra Savu, Ștefana Bucur, Gabriel Predoiu, Maria Magdalena Constantin, and Viorel Jinga. 2021. "The Role and Significance of Bioumoral Markers in Prostate Cancer" Cancers 13, no. 23: 5932. https://doi.org/10.3390/cancers13235932
APA StyleConstantin, T., Savu, D. A., Bucur, Ș., Predoiu, G., Constantin, M. M., & Jinga, V. (2021). The Role and Significance of Bioumoral Markers in Prostate Cancer. Cancers, 13(23), 5932. https://doi.org/10.3390/cancers13235932








