Fermented Mangosteen (Garcinia mangostana L.) Supplementation in the Prevention of HPV-Induced Cervical Cancer: From Mechanisms to Clinical Outcomes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Clinical Study Design and Recruitment Criteria
2.3. Food Supplement in Question
2.4. Clinical Diagnosis
2.5. Viral Analyses
2.6. Biological Material Sampling and Processing
2.6.1. Blood Sampling and Processing
2.6.2. Cervical Tissue Sampling and Processing
2.6.3. Cervical Fluid Collecting and Processing
2.7. Assays for Oxidative Markers
2.7.1. Enzymatic Activities
2.7.2. Hydroxyl Radical Scavenging
2.7.3. Nitrotyrosine Determination
2.7.4. Isoprostane Determination
2.8. Assays for Ligands of Apoptosis
2.9. Statistics
3. Results
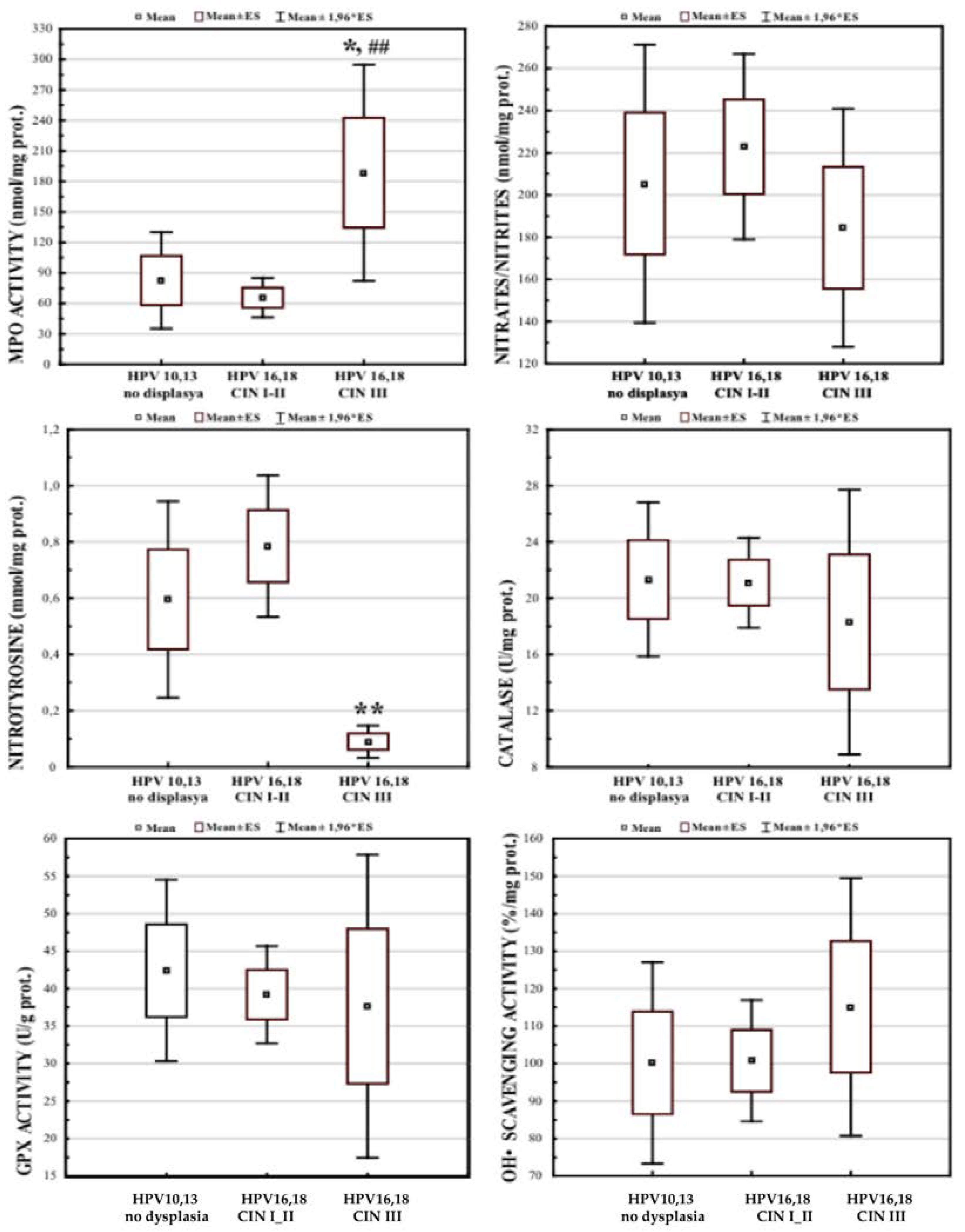
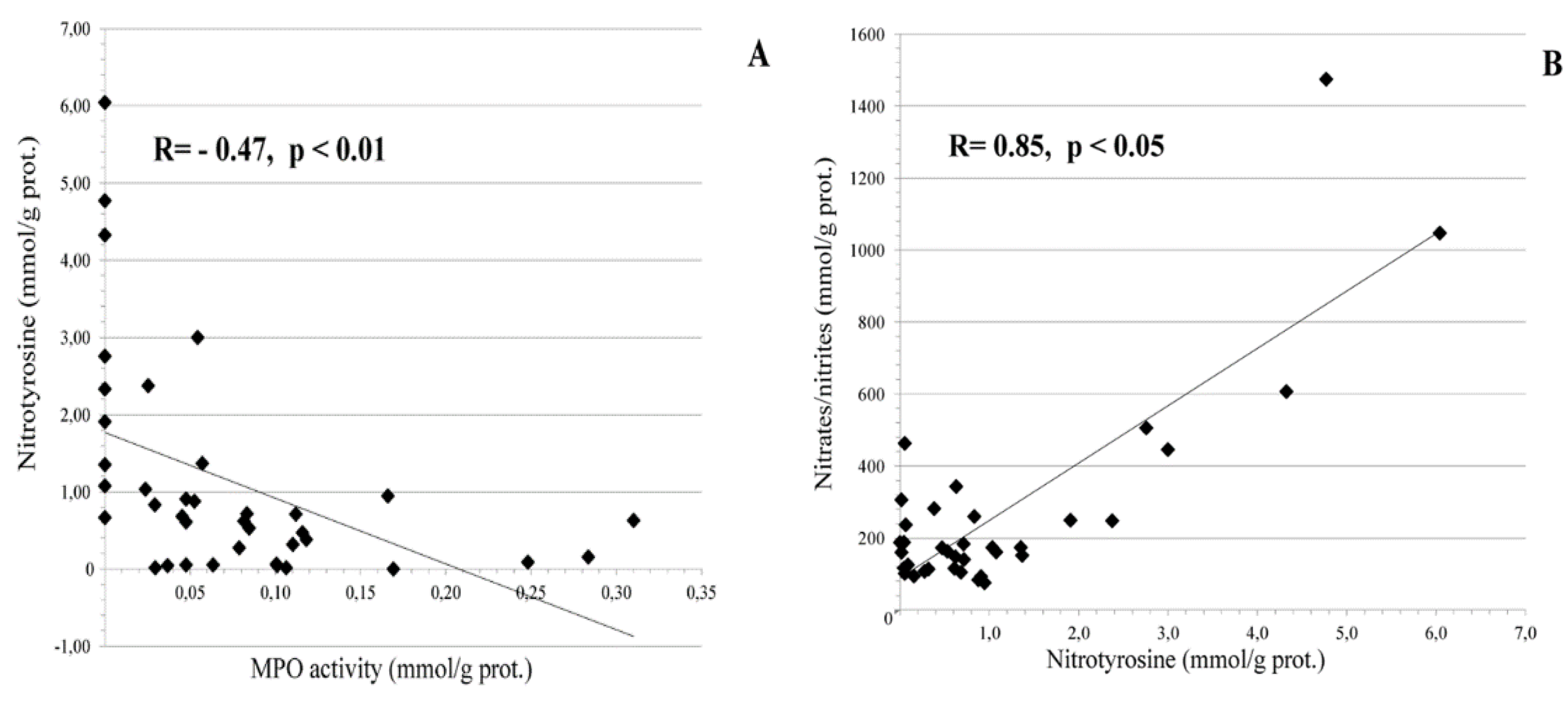
3.1. Local Cervical Oxidative and Nitrosative Markers in HPV-Infected Female Patients at Different Stages of Cervical Carcinogenesis
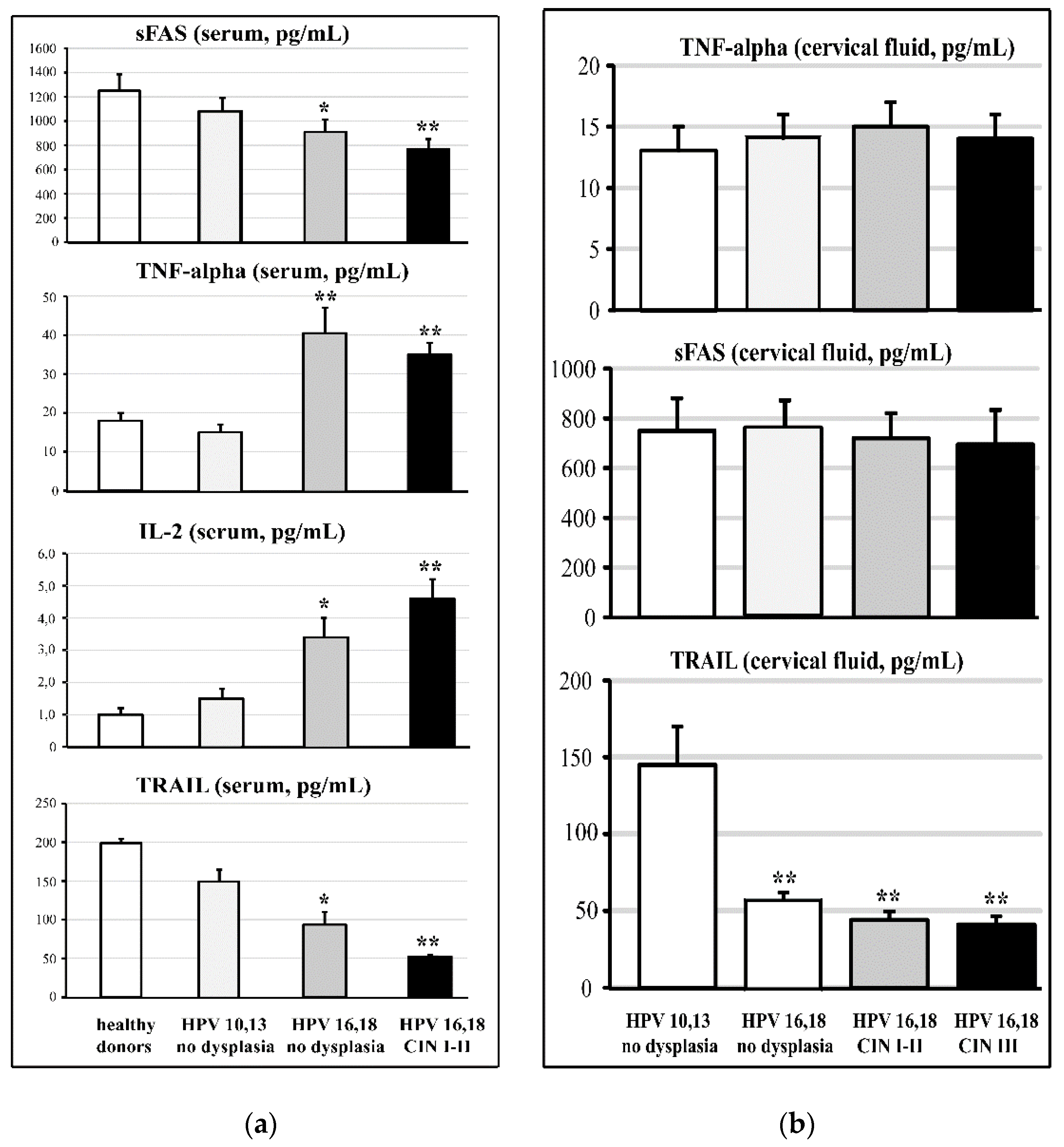
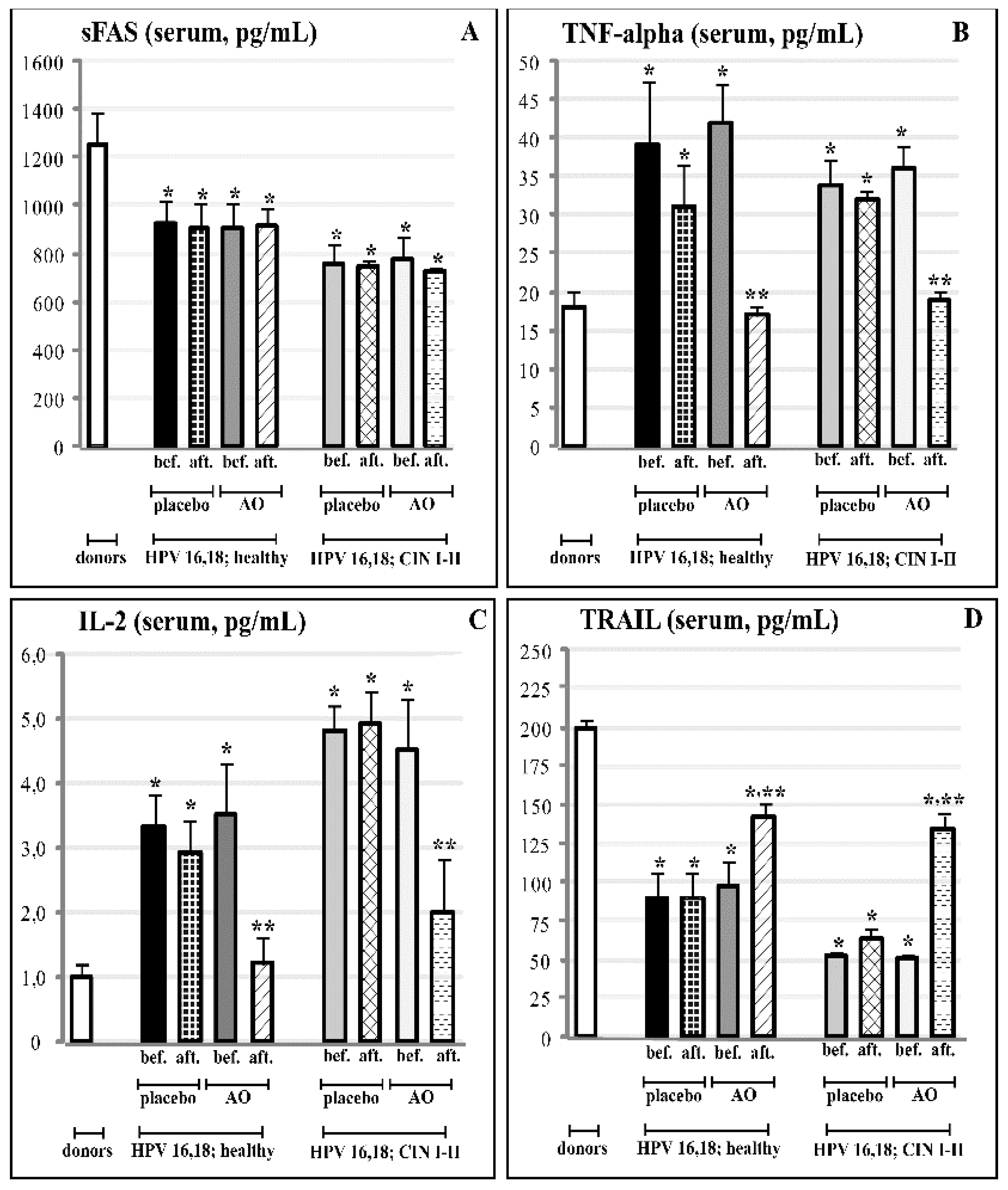
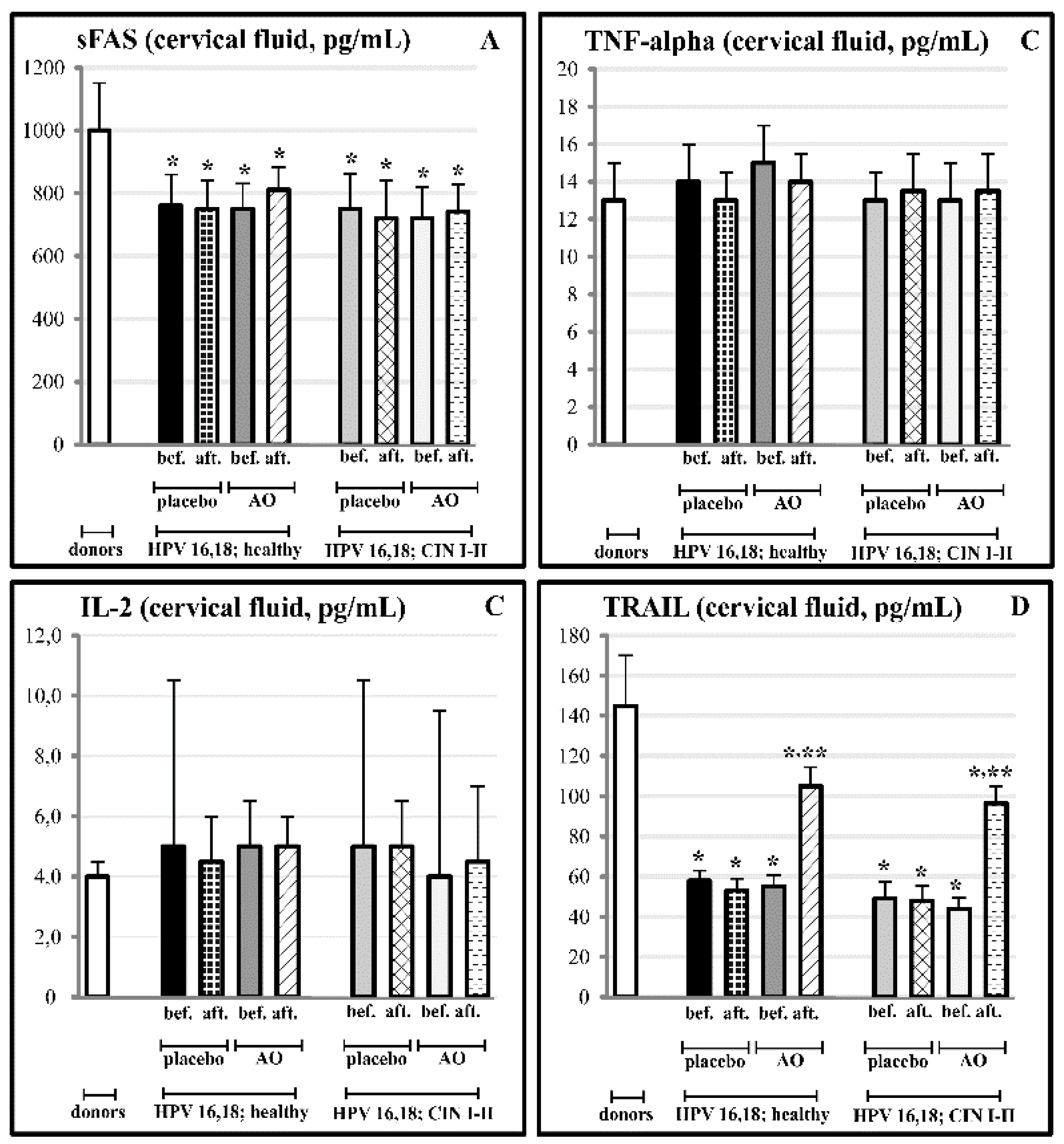
3.2. Cervical and Circulating Ligands of TNF-Alpha Apoptosis in HPV-Infected Female Patients at Different Stages of Cervical Carcinogenesis
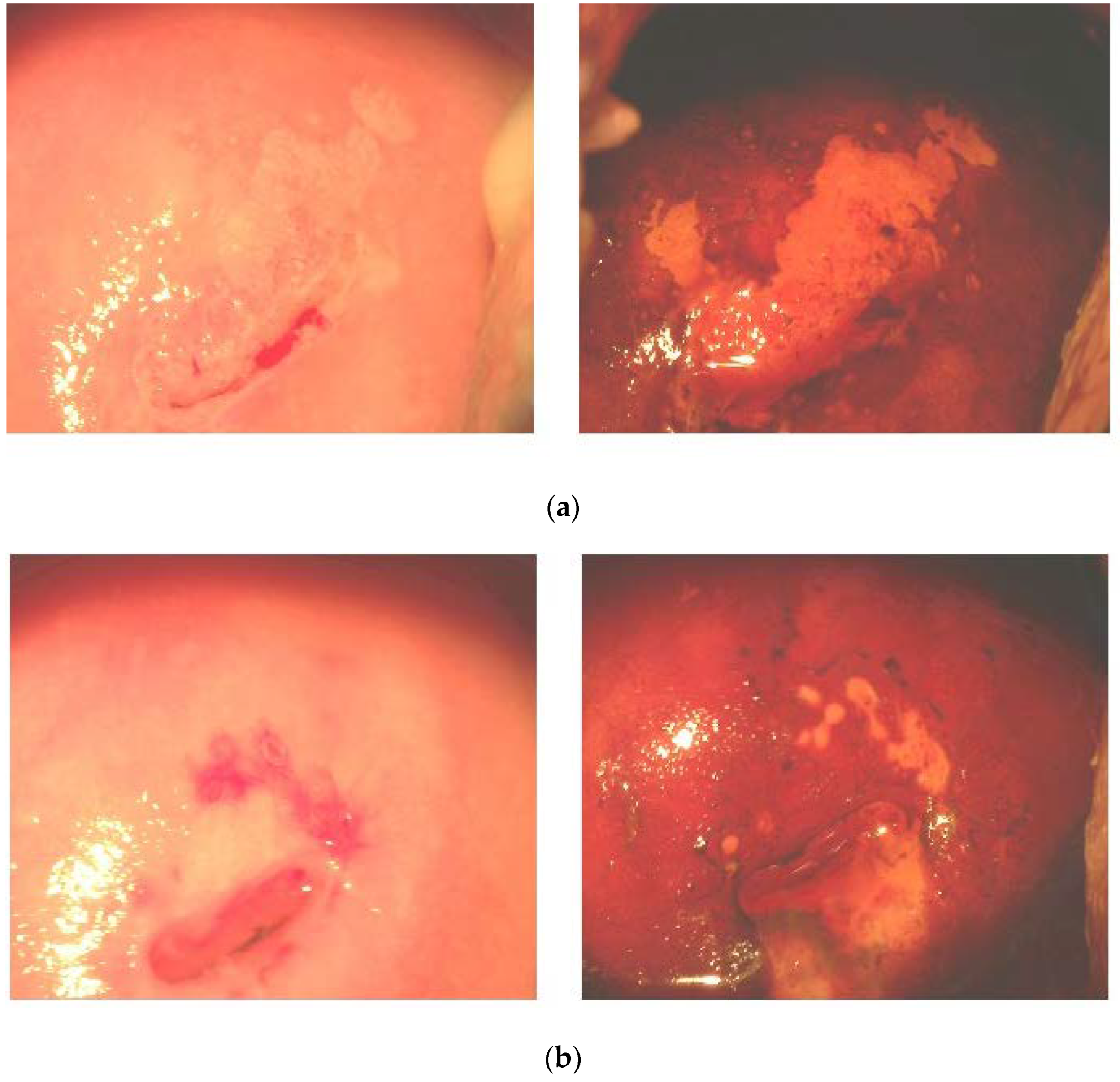
3.3. Effects of FM Supplementation on Macro-Histological Symptoms of Cervical Dysplasia
3.4. Effects of FM Supplementation on Oxidative and Nitrosative Markers in Cervical Tissue
3.5. Effects of Fermented Mangosteen Supplementation on Systemic and Topical (Cervical) Ligands of TNF-Alpha Apoptosis
4. Discussion
- theoretically diminish the load of high-risk HPV [28];
- regulate neutrophil chemotaxis and the degranulation of MPO-containing granules at sites where the virus is harboured;
- increase the bioavailability of NO to fight viruses that would also lead to diminished levels of potentially carcinogenic nitrative agents and nitrotyrosine;
- down-regulate the overexpression of inflammatory cytokines involved in chronic general inflammation;
- deeply affect TRAIL-related events, such as the apoptosis of HPV-infected cells, preventing them from cancerous transformation;
- the presence of numerous active compounds derived from the plant and fermenting microbes in the natural matrix of FM could attenuate their potential individual toxicity, increase bioavailability, and exhibit a synergy between them.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Kawanishi, S.; Hiraku, Y. Oxidative and nitrative DNA damage as biomarker for carcinogenesis with special reference to inflammation. Antioxid. Redox Signal. 2006, 8, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Thanan, R.; Ma, N.; Kawanishi, S. Role of nitrative and oxidative DNA damage in inflammation-related carcinogenesis. J. Biomed. Biotechnol. 2012, 2012, 623019. [Google Scholar] [CrossRef] [PubMed]
- IARC. Chronic infections. In World Cancer Report; Stewart, B.W., Kleihues, P., Eds.; IARC Press: Lyon, France, 2008; pp. 128–135. [Google Scholar]
- Schetter, A.J.; Heegaard, N.Y.; Harris, C.C. Inflammation and cancer: Interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis 2010, 31, 37–49. [Google Scholar] [CrossRef]
- Ohnishi, S.; Ma, N.; Thanan, R.; Pinloar, S.; Hammam, O.; Murata, M.; Kawanishi, S. DNA damage in inflammation-related carcinogenesis and cancer stem cells. Oxid. Med. Cell. Longev. 2013, 2013, 387014. [Google Scholar] [CrossRef] [PubMed]
- Lala, P.K.; Chakraborty, C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001, 2, 149–156. [Google Scholar] [CrossRef]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef]
- De Luca, C.; Kharaeva, Z.; Korkina, L. Is there a role for antioxidants in the prevention of infection-associated carcinogenesis and in the treatment of infection-driven tumors? Curr. Top. Med. Chem. 2015, 15, 120–135. [Google Scholar] [CrossRef]
- Haegens, A.; Van Der Vliet, A.; Butnor, K.J.; Heintz, N.; Taatjes, D.; Hemenway, D.; Vacek, P.; Freeman, B.A.; Hazen, S.L.; Brennan, M.L.; et al. Asbestos-induced lung inflammation and epithelial cell proliferation are altered in myeloperoxidase-null mice. Cancer Res. 2005, 65, 9670–9677. [Google Scholar] [CrossRef]
- Eiserich, J.P.; Hristova, M.; Cross, C.E.; Jones, A.D.; Freeman, B.A.; Halliwell, B.; van der Vliet, A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 1998, 39, 393–397. [Google Scholar] [CrossRef]
- Van Der Vliet, A.; Eiserich, J.P.; Shigenaga, M.K.; Cross, C.E. Reactive nitrogen species and tyrosine nitration in the respiratory tract: Epiphenomena or a pathobiologic mechanism of disease? Am. J. Respir. Crit. Care Med. 1999, 160, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gaut, J.P.; Byun, J.; Tran, H.D.; Lauber, W.M.; Carroll, J.A.; Hotchkiss, R.S.; Belaaouaj, A.; Heinecke, J.W. Myeloperoxidase produces nitrating oxidants in vivo. J. Clin. Investig. 2002, 109, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, J.K.; Privora, H.F.; Wenckebach, G.; Birnboim, H.C. Neutrophils, nitric oxide synthase, and mutations in the mutatect murine tumor model. Am. J. Pathol. 2000, 156, 509–518. [Google Scholar] [CrossRef]
- Nathan, C.; Shiloh, M.U. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 2000, 97, 8841–8848. [Google Scholar] [CrossRef]
- Ishiropoulos, H. Biological tyrosine nitration: A pathophysiological function of nitric oxide and reactive oxygen species. Arch. Biochem. Biophys. 1998, 356, 1–11. [Google Scholar] [CrossRef]
- Klebanoff, S.J.; Nathan, C.F. Nitrite production by stimulated human polymorphonuclear leukocytes supplemented with azide and catalase. Biochem. Biophys. Res. Commun. 1993, 197, 192–196. [Google Scholar] [CrossRef]
- De Marco, F.; Bucaj, E.; Foppoli, C.; Fiorini, A.; Blarzino, C.; Filipi, K.; Giorgi, A.; Schinina, M.E.; Di Domenico, F.; Coccia, R.; et al. Oxidative stress in HPV-driven viral carcinogenesis: Redox proteomics analysis of HPV-16 dysplastic and neoplastic tissues. PLoS ONE 2012, 7, e34366. [Google Scholar] [CrossRef]
- Ryu, H.S.; Chang, K.H.; Chang, S.J.; Kim, M.S.; Joo, H.J.; Oh, K.S. Expression of TRAIL (TNF-related apoptosis-inducing ligand) receptors in cervical cancer. Int. J. Gynecol. Cancer 2000, 10, 417–424. [Google Scholar] [CrossRef]
- Chih, H.J.; Lee, A.H.; Colville, L.; Binns, C.W.; Xu, D. A review of dietary prevention of human papillomavirus-related infection of the cervix and cervical intraepithelial neoplasia. Nutr. Cancer 2013, 65, 317–328. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef]
- Ono, A.; Koshiyama, M.; Nakagawa, M.; Watanabe, Y.; Ikuta, E.; Seki, K.; Oowaki, M. The Preventive Effect of Dietary Antioxidants on Cervical Cancer Development. Medicina 2020, 56, 604. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, M.; Lee, S.; Jung, W.; Kim, B. Therapeutic potential of natural products in treatment of cervical cancer: A review. Nutrients 2021, 13, 154. [Google Scholar] [CrossRef]
- Koshiyama, M. The effects of the dietary and nutrient intake on gynecological cancers. Healthcare 2019, 7, 88. [Google Scholar] [CrossRef]
- Di Domenico, F.; Foppoli, C.; Coccia, R.; Perluigi, M. Antioxidants in cervical cancer: Chemopreventive and chemotherapeutic effects of polyphenols. Biochim. Biophys. Acta 2012, 1822, 737–747. [Google Scholar] [CrossRef]
- Morosetti, G.; Criscuolo, A.A.; Santi, F.; Perno, C.F.; Piccione, E.; Ciotti, M. Ellagic acid and Annona muricata in the chemoprevention of HPV-related pre-neoplastic lesions of the cervix. Oncol. Lett. 2017, 13, 1880–1884. [Google Scholar] [CrossRef]
- Einbond, L.S.; Zhou, J.; Wu, H.; Mbazor, E.; Song, G.; Balik, M.; DeVoti, J.A.; Redenti, S.; Castellanos, M.R. A novel cancer preventative botanical mixture, TriCurin, inhibits viral transcripts and the growth of W12 cervical cells. harbouring extrachromosomal or integrated HPV16 DNA. Br. J. Cancer 2021, 124, 901–913. [Google Scholar] [CrossRef]
- Polansky, H.; Itzkovitz, E.; Javaherian, A. Human papillomavirus (HPV): Systemic treatment with Gene-Eden-VIR/Novirin safely and effectively clears virus. Drug Des. Dev. Ther. 2017, 11, 575. [Google Scholar] [CrossRef][Green Version]
- Dretcanu, G.; Iuhas, C.I.; Diaconeasa, Z. Involvement of natural polyphenols in the chemoprevention of cervical cancer. Int. J. Mol. Sci. 2021, 22, 8812. [Google Scholar] [CrossRef]
- Rock, C.L.; Michael, C.W.; Reynolds, R.K.; Ruffin, M.T. Prevention of cervix cancer. Crit. Rev. Oncol. Hematol. 2000, 33, 169–185. [Google Scholar] [CrossRef]
- Williams, V.M.; Filippova, M.; Filippov, V.; Payne, K.J.; Duerlsen-Hughes, P. Human papillomavirus type 16 E6* induces oxidative stress and DNA damage. J. Virol. 2014, 88, 6751–6761. [Google Scholar] [CrossRef]
- Cherry, J.J.; Rietz, A.; Malinkevich, A.; Liu, Y.; Xie, M.; Bartolowitz, M.; Jo Davisson, V.; Jo Davisson, V.; Baleja, J.D.; Androphy, E.J. Structure based identification and characterization of flavonoids that disrupt human papillomavirus-16 E6 function. PLoS ONE 2013, 8, e84506. [Google Scholar] [CrossRef] [PubMed]
- Läsche, M.; Urban, H.; Gallwas, J.; Gründker, C. HPV and Other Microbiota; Who’s Good and Who’s Bad: Effects of the Microbial Environment on the Development of Cervical Cancer—A Non-Systematic Review. Cells 2021, 10, 714. [Google Scholar] [CrossRef]
- Preci, D.P.; Almeida, A.; Weiler, A.L.; Mukai Franciosi, M.L.; Cardoso, A.M. Oxidative damage and antioxidants in cervical cancer. Int. J. Gynecol. Cancer 2021, 31, 265–271. [Google Scholar] [CrossRef]
- Calaf, G.M.; Urzua, U.; Termini, L.; Aguaio, F. Oxidative stress in female cancers. Oncotarget 2018, 9, 23824–23842. [Google Scholar] [CrossRef]
- Foppoli, C.; De Marco, F.; Cini, C.; Perluigi, M. Redox control of viral carcinogenesis: The human papillomavirus paradigm. Biochim. Biophys. Acta 2015, 1850, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Gregorio, A.; Manzo-Merino, J.; Lizano, M. Cellular redox, cancer and human papillomavirus. Virus Res. 2018, 246, 35–45. [Google Scholar] [CrossRef]
- Lin, H.Y.; Fu, Q.; Kao, Y.H.; Tseng, T.S.; Reiss, K.; Cameron, J.E.; Ronis, M.J.; Su, J.; Nair, N.; Chang, H.-M.; et al. Antioxidants Associated with Oncogenic Human Papillomavirus Infection in Women. J. Infect. Dis. 2021, 224, 1520–1528. [Google Scholar] [CrossRef]
- Georgescu, S.R.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Sarbu, M.I.; Matei, C.; Nicolae, I.; Tocut, S.M.; Popa, M.I.; Tampa, M. New Insights in the Pathogenesis of HPV Infection and the Associated Carcinogenic Processes: The Role of Chronic Inflammation and Oxidative Stress. J. Immunol. Res. 2018, 2018, 5315816. [Google Scholar] [CrossRef]
- Khan, F.; Pandey, P.; Upadhyay, T.K.; Jafri, A.; Jha, N.K.; Mishra, R.; Singh, V. Anti-cancerous effect of rutin against HPV-C33A cervical cancer cells via G0/G1 cycle arrest and apoptotic induction. Endocr. Metab. Immune Drug Targets 2020, 20, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Soto, A.F.; Salas-Vidal, E.; Milan-Pacheco, C.; Sanchez-Carranza, J.N.; Peralta-Zaragoza, O.; Gonzalez-Maya, L. Quercetin induces G2 phase arrest and apoptosis with the activation of p53 in an E6 expression-independent manner in HPV-positive human cervical cancer-derived cells. Mol. Med. Rep. 2019, 19, 2097–2106. [Google Scholar] [CrossRef]
- Gutierrez-Orozco, F.; Failla, M.L. Biological activities and bioavailability of mangosteen xanthons: A critical review of the current evidence. Nutrients 2013, 5, 3163–3183. [Google Scholar] [CrossRef]
- Liu, Z.; Antalek, M.; Nguyen, L.; Li, X.; Tian, X.; Le, A.; Zi, X. The effect of gartanin, a naturally-occurring xanthone in mangosteen juice, on the mTOR pathway, autophagy, apoptosis and the growth of human urinary bladder cancer cell lines. Nutr. Cancer 2013, 65, 68–77. [Google Scholar] [CrossRef]
- Shan, T.; Ma, Q.; Gui, K.; Liu, J.; Li, W.; Wang, F.; Wu, E. Xanthones from mangosteen extracts as natural chemopreventive agents: Potential anticancer drugs. Curr. Mol. Med. 2011, 11, 666–677. [Google Scholar] [CrossRef]
- Chin, Y.-W.; Kinghorn, A.D. Structural characterization, biological effects, and synthetic studies on xanthones from mangosteen (Garcinia mangostana), a popular botanical dietary supplement. Mini Rev. Org. Chem. 2008, 5, 355–364. [Google Scholar] [CrossRef]
- Karim, A.A.; Azlan, A. Fruit pod extracts as a source of nutraceuticals and pharmaceuticals. Molecules 2012, 17, 11931–11946. [Google Scholar] [CrossRef]
- Sun, X.; Fu, P.; Xie, L.; Chai, S.; Xu, Q.; Zeng, L.; Wang, X.; Jiang, N.; Sang, M. Resveratrol inhibits the progression of cervical cancer by suppressing the transcription and expression of HPV E6 and E7 genes. Int. J. Mol. Med. 2021, 47, 335–345. [Google Scholar] [CrossRef]
- Shin, S.-A.; Moon, S.Y.; Kim, W.-Y.; Paek, S.-M.; Park, H.H.; Lee, C.S. Structure-based classification and anti-cancer effects of plant metabolites. Int. J. Mol. Sci. 2018, 19, 2651. [Google Scholar] [CrossRef]
- Korkina, L.G.; Pastore, S.; De Luca, C.; Kostyuk, V.A. Metabolism of plant polyphenols in the skin: Beneficial versus deleterious effects. Curr. Drug Metab. 2008, 9, 710–729. [Google Scholar] [CrossRef]
- Korkina, L.G.; De Luca, C.; Kostyuk, V.A.; Pastore, S. Plant polyphenols and tumors: From mechanisms to therapies, prevention, and protection against toxicity of anti-cancer treatments. Curr. Med. Chem. 2009, 16, 3943–3965. [Google Scholar] [CrossRef]
- Chilton, S.N.; Burton, J.P.; Reid, G. Inclusion of fermented foods in food guides around the world. Nutrients 2015, 7, 390–404. [Google Scholar] [CrossRef]
- Vuong, T.; Mallet, J.-F.; Ouzounova, M.; Rahbar, S.; Hernandez-Vargas, H.; Herceg, Z.; Matar, C. Role of a polyphenol-enriched preparation of chemoprevention of mammalian carcinoma through cancer stem cells and inflammatory pathways modulation. J. Trans. Med. 2016, 14, 13. [Google Scholar] [CrossRef]
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Rani, P.R. Fermented fruits and vegetables of Asia: A potential source of probiotics. Biotechnol. Res. Int. 2014, 2014, 250424. [Google Scholar] [CrossRef] [PubMed]
- Pessione, E.; Cirrincione, S. Bioactive molecules released in food by lactic acid bacteria: Encrypted peptides and biogenic amines. Front. Microbiol. 2016, 7, 876. [Google Scholar] [CrossRef]
- Cuzick, J.; Beverley, E.; Ho, L.; Terry, G.; Sapper, H.; Mielzynska, I.; Lorincz, A.; Chan, W.K.; Krausz, T.; Soutter, B. HPV testing in primary screening of older women. Br. J. Cancer 1999, 8, 554–558. [Google Scholar] [CrossRef]
- Franco, E.L.; Duarte-Franco, E.; Ferenczy, A. Cervical cancer: Epidemiology, prevention, and role of human papillomavirus infection. J. Can. Med. Assoc. 2001, 164, 1017–1025. [Google Scholar]
- Franco, E.L. Chapter 13: Primary screening of cervical cancer with human papilloma virus tests. J. Natl. Cancer Inst. Monogr. 2003, 31, 89–96. [Google Scholar] [CrossRef][Green Version]
- Kowluru, R.A.; Kanwar, M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr. Metab. 2007, 4, 8. [Google Scholar] [CrossRef]
- Saidel-Sulkowska, E.M.; Lipinski, B.; Windom, H.; Audhya, T.; McGinnis, W. Oxidative stress in autism: Elevated cerebellar 3-nitrotyrosine levels. Am. J. Biochem. Biotechnol. 2008, 4, 73–84. [Google Scholar]
- Walsh, S.W.; Vaughan, J.E.; Wang, Y.; Roberts, L.J., II. Placental isoprostane is significantly increased in preeclampsia. FASEB J. 2000, 14, 1289–1296. [Google Scholar] [CrossRef]
- Takahashi, T.; Tanaka, M.; Inazawa, J.; Abe, T.; Suda, T.; Nagata, S. Human Fas ligand: Gene structure, chromosomal location and species specificity. Int. Immunol. 1994, 6, 1567–1574. [Google Scholar] [CrossRef]
- Itoh, N.; Yonehara, S.; Ishii, A.; Yonehara, M.; Mizushima, S.-I.; Hase, A.; Seto, Y.; Nagata, S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 1991, 66, 233–234. [Google Scholar] [CrossRef]
- Fernandes, J.V.; De Medeiros Fernandes, T.A.; De Azevedo, J.C.; Cobucci, R.N.; De Carvalho, M.G.; Andrade, V.S.; De Araujo, J.M.G. Link between chronic inflammation and human papillomavirus-induced carcinogenesis (Review). Oncol. Lett. 2015, 9, 1015–1026. [Google Scholar] [CrossRef]
- Akagi, K.; Li, J.; Broutian, T.R.; Padilla-Nash, H.; Xiao, W.; Jiang, B.; Rocco, J.W.; Tecnos, T.N.; Kumar, B.; Wangsa, D.; et al. Genome-wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability. Genome Res. 2014, 24, 185–199. [Google Scholar] [CrossRef]
- Jiang, Q.; Hurst, J.K. Relative chlorinating, nitrating, and oxidizing capabilities of neutrophils determined with phagocytosable probes. J. Biol. Chem. 1997, 272, 32767–32772. [Google Scholar] [CrossRef]
- Chapman, A.L.; Morrissey, B.M.; Vasu, V.T.; Juarez, M.M.; Houghton, J.S.; Li, C.S.; Cross, C.E.; Eiserich, J.P. Myeloperoxidase-dependent oxidative metabolism of nitric oxide in the cystic fibrosis airway. J. Cyst. Fibros. 2010, 9, 84–92. [Google Scholar] [CrossRef]
- Beckman, J.S. Oxidative damage and tyrosine nitration from peroxynitrite. Chem. Res. Toxicol. 1996, 9, 836–844. [Google Scholar] [CrossRef]
- Van Der Vliet, A.; Eiserich, J.P.; Kaur, H.; Cross, C.E.; Halliwell, B. Nitrotyrosine as biomarker for reactive nitrogen species. Methods Enzymol. 1996, 269, 175–184. [Google Scholar]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2005. [Google Scholar]
- Van Der Vliet, A.; Hristova, M.; Cross, C.E.; Eiserich, J.P.; Goldcorn, T. Peroxynitrite induces covalent dimerization of epidermal growth factor receptors in A431 epidermoid carcinoma cells. J. Biol. Chem. 1998, 273, 31860–31866. [Google Scholar] [CrossRef]
- Potapovich, A.I.; Lulli, D.; Fidanza, P.; Kostyuk, V.A.; De Luca, C.; Pastore, S.; Korkina, L. Plant polyphenols differentially modulate inflammatory responses of human keratinocytes by interfering with activation of transcription factors NFκB and AhR and EGFR-ERK pathway. Toxicol. Appl. Pharmacol. 2011, 255, 138–149. [Google Scholar] [CrossRef]
- Pastore, S.; Lulli, D.; Maurelli, R.; Dellambra, E.; De Luca, C.; Korkina, L.G. Resveratrol induces long-lasting IL-8 expression and peculiar EGFR activation/distribution in human keratinocytes: Mechanisms and implications for skin administration. PLoS ONE 2013, 8, e59632. [Google Scholar] [CrossRef]
- Korkina, L.G.; Pastore, S.; Dellambra, E.; De Luca, C. New molecular and cellular targets for chemoprevention and treatment of skin tumors by plant polyphenols: A critical review. Curr. Med. Chem. 2013, 20, 852–868. [Google Scholar]
- Bellocq, A.; Antoine, M.; Flahault, A.; Philippe, C.; Crestani, B.; Bernaudin, J.F.; Mayaud, C.; Milleron, B.; Baud, L.; Cadranel, J. Neutrophil alveolitis in bronchioloalveolar carcinoma: Induction by tumor-derived interleukin-8 and relation to clinical outcome. Am. J. Pathol. 1998, 152, 83–92. [Google Scholar]
- Brew, R.; Southern, S.A.; Flanagan, B.F.; McDicken, I.W.; Christmas, S.E. Detection of interleukin-8 mRNA and protein in human colorectal carcinoma cells. Eur. J. Cancer 1996, 32, 2142–2147. [Google Scholar] [CrossRef]
- Negus, R.P.; Stamp, G.W.; Hadley, J.; Balkwill, F.R. Quanitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am. J. Pathol. 1997, 150, 1723–1734. [Google Scholar]
- Carrero, Y.; Callejas, D.; Alaña, F.; Silva, C.; Mindiola, R.; Mosquera, J. Increased vascular endothelial growth factor expression, CD3-positive cell infiltration, and oxidative stress in premalignant lesions of the cervix. Cancer 2009, 115, 3680–3688. [Google Scholar] [CrossRef]
- Shacter, E.; Beecham, E.J.; Covey, J.M.; Kohn, K.W.; Potter, M. Activated neutrophils induce prolonged DNA damage in neighbouring cells. Carcinogenesis 1988, 9, 2297–2304. [Google Scholar] [CrossRef]
- Tamir, S.; Tannenbaum, S.R. The role of nitric oxide (NO) in the carcinogenic process. Biochim. Biophys. Acta Rev. Cancer 1996, 1288, F31–F36. [Google Scholar] [CrossRef]
- Felley-Bosco, E. Role of nitric oxide in genotoxicity: Implication for carcinogenesis. Cancer Metastasis Rev. 1998, 17, 25–37. [Google Scholar] [CrossRef]
- Halim, T.A.; Farooqi, A.A.; Zaman, F. Nip the HPV encoded evil in the cancer bud: HPV reshapes signaling landscapes. Cancer Cell Int. 2013, 13, 61. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Wayne, E.; Rana, K.; Schaffer, C.B.; King, M.R. TRAIL-coated leukocytes that kill cancer cells in the circulation. Proc. Natl. Acad. Sci. USA 2014, 111, 930–935. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.M. Sensitization for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol. Cancer Res. 2004, 64, 337–346. [Google Scholar] [CrossRef]
- Ren, B.; Kwah, M.X.Y.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xian, X.; Ho, P.C.L.; Wang, L.; Ong, P.S. Resveratrol for cancer therapy: Challenges and future perspectiveas. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef]
- Pastore, S.; Lulli, D.; Pascarella, A.; Maurelli, R.; Dellambra, E.; Potapovich, A.; Kostyuk, V.; De Luca, C.; Korkina, L. Resveratrol enhances solar UV induced responses in normal human epidermal keratinocytes. Photochem. Photobiol. 2012, 88, 1522–1530. [Google Scholar] [CrossRef]
- Wang, D.; Upadhyaya, B.; Liu, Y.; Knudsen, D.; Dey, M. Phenetyl isothiocyanate upregulates death receptors 4 and 5 and inhibits proliferation in human cancer stem-like cells. BMC Cancer 2014, 14, 591. [Google Scholar] [CrossRef]
- Lin, X.; Farooqui, A.A. Cucurbitacin mediated regulation of deregulated oncogenic signaling cascades and non-coding RNAs in different cancers: Spotlight on JAK/STAT, Wnt/β-catenin, mTOR, TRAIL-mediated pathways. Semin. Cancer Biol. 2021, 73, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Butt, G.; El-Zanaby, S.A.; Attar, R.; Sabitaliyevich, U.Y.; Jovic, J.J.; Tang, K.F.; Naureen, H.; Xu, B. Luteolin mediated targeting of protein network and microRNAs in different cancers: Focus on JAK-STAT, NOTCH, mTOR and TRAIL-mediated signaling pathways. Pharmacol. Res. 2020, 160, 105188. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Pinheiro, M.; Granja, A.; Farabegoli, F.; Reis, S.; Attar, R.; Sabitalievich, U.Y.; Xu, B.; Ahmad, A. EGCG Mediated Targeting of Deregulated Signaling Pathways and Non-Coding RNAs in Different Cancers: Focus on JAK/STAT, Wnt/β-Catenin, TGF/SMAD, NOTCH, SHH/GLI, and TRAIL Mediated Signaling Pathways. Cancers 2020, 12, 951. [Google Scholar] [CrossRef] [PubMed]

| Group | Number of Subjects | Age Range |
|---|---|---|
| HPV10, HPV13, healthy | 42 | 21–44 year |
| HPV16, HPV18, CIN I + CIN II | 75 | 24–45 year |
| HPV16, HPV18, CIN III | 45 | 28–53 year |
| Healthy female controls | 15 | 25–47 year |
| Group | Number of Subjects | Age Range |
|---|---|---|
| HPV16, HPV18, no dysplasia Group 1—placebo Group 2—fermented mangosteen (FM) | 152 70 82 | 25–45 year |
| HPV16, HPV18, CIN I + CIN II Group 3—placebo Group 4—fermented mangosteen (FM) | 98 48 50 | 25–52 year |
| Healthy controls without viral infections | 30 | 27–42 year |
| Marker | Before Treatment | After Treatment |
|---|---|---|
| Myeloperoxidase, nmol/mg | 76 ± 12 | 5.9 ± 1.3 ** |
| Nitrates/Nitrites, nmol/mg | 207 ± 18 | 147 ± 30 * |
| Catalase, U/mg | 20 ± 1.8 | 15 ± 3 |
| Isoprostanes, ng/mg | 1.1 ± 0.2 | 0.9 ± 0.2 |
| Nitrotyrosine, nmol/mg | 1.1 ± 0.2 | 0.5 ± 0.3 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharaeva, Z.; Trakhtman, P.; Trakhtman, I.; De Luca, C.; Mayer, W.; Chung, J.; Ibragimova, G.; Korkina, L. Fermented Mangosteen (Garcinia mangostana L.) Supplementation in the Prevention of HPV-Induced Cervical Cancer: From Mechanisms to Clinical Outcomes. Cancers 2022, 14, 4707. https://doi.org/10.3390/cancers14194707
Kharaeva Z, Trakhtman P, Trakhtman I, De Luca C, Mayer W, Chung J, Ibragimova G, Korkina L. Fermented Mangosteen (Garcinia mangostana L.) Supplementation in the Prevention of HPV-Induced Cervical Cancer: From Mechanisms to Clinical Outcomes. Cancers. 2022; 14(19):4707. https://doi.org/10.3390/cancers14194707
Chicago/Turabian StyleKharaeva, Zaira, Pavel Trakhtman, Ilya Trakhtman, Chiara De Luca, Wolfgang Mayer, Jessie Chung, Galina Ibragimova, and Liudmila Korkina. 2022. "Fermented Mangosteen (Garcinia mangostana L.) Supplementation in the Prevention of HPV-Induced Cervical Cancer: From Mechanisms to Clinical Outcomes" Cancers 14, no. 19: 4707. https://doi.org/10.3390/cancers14194707
APA StyleKharaeva, Z., Trakhtman, P., Trakhtman, I., De Luca, C., Mayer, W., Chung, J., Ibragimova, G., & Korkina, L. (2022). Fermented Mangosteen (Garcinia mangostana L.) Supplementation in the Prevention of HPV-Induced Cervical Cancer: From Mechanisms to Clinical Outcomes. Cancers, 14(19), 4707. https://doi.org/10.3390/cancers14194707







