Strategies for Efficient Targeting of Tumor Collagen for Cancer Therapy
Abstract
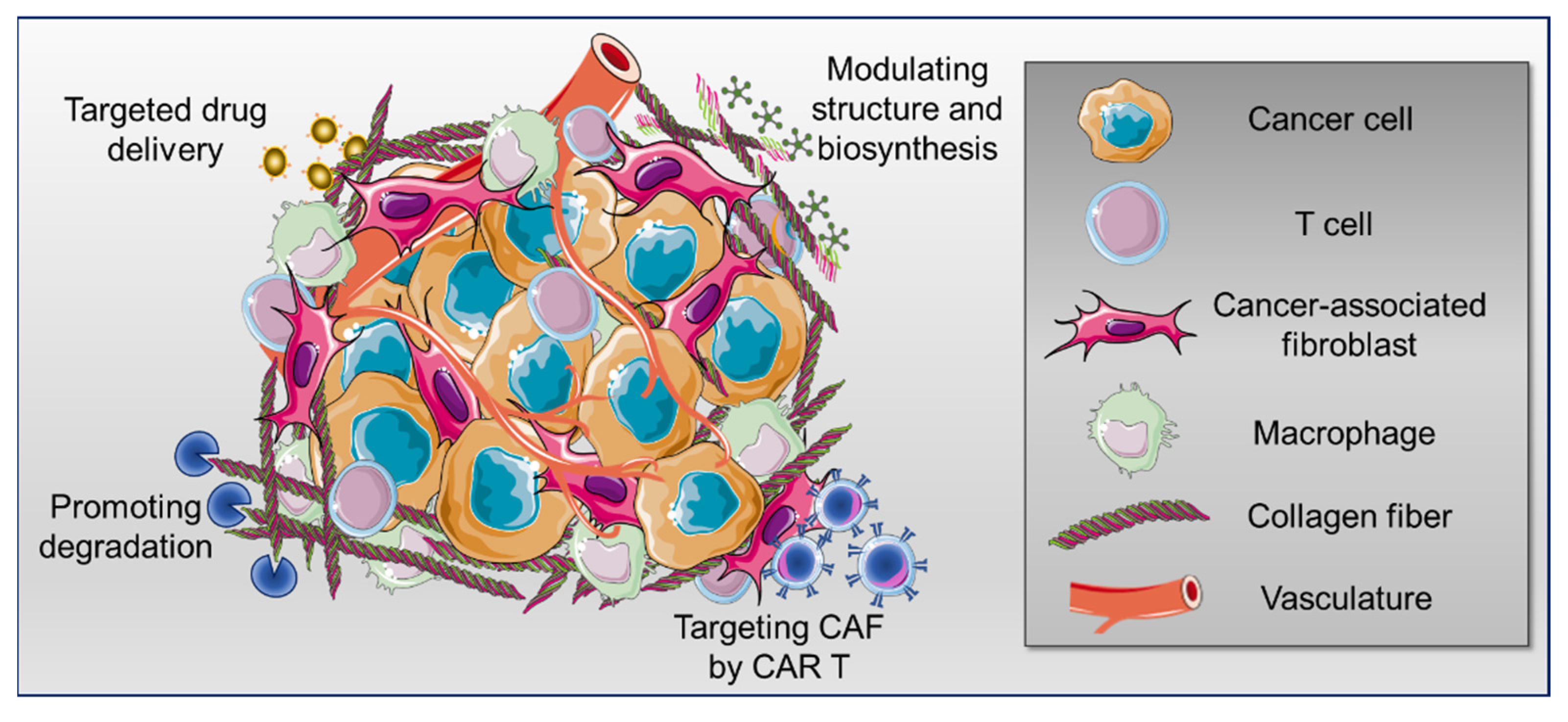
Simple Summary
Abstract
1. Introduction
2. Collagen Targeting for Anticancer Drug Delivery
3. Strategies to Promote Tumor Collagen Degradation
3.1. Collagenase Treatment
3.2. Collagenase Encapsulated Nanoparticles and Hydrogels
3.3. Protein-Free Collagen Degradation
3.4. Collagen-Degrading Bacteria
3.5. Degradation of Tumor Extracellular Matrix Mediated by Armed Oncolytic Virus
4. Strategies to Modulate Collagen Structure and Biosynthesis
4.1. Modulation of Lysyl Oxidase Enzymatic Activity
4.2. Modulation of Collagen Glycation-Related Crosslinking
4.3. Collagen Biosynthesis Inhibition by Antifibrotic Drugs
4.4. Modulation of Proline Incorporation and Hydroxylation
5. Strategies for Targeting Cancer-Associated Fibroblasts as the Major Extracellular Matrix Producers
6. Collagen and Immune Cell Infiltration: A Link to the Efficacy of Immunotherapies
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Kielty, C.M.; Grant, M.E. The collagen family: Structure, assembly, and organization in the extracellular matrix. In Connective tissue and Its Heritable Disorders; Royce, P.M., Steinmann, B., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Bella, J.; Hulmes, D.J.S. Fibrillar collagens. In Fibrous Proteins: Structures and Mechanisms; Springer: Cham, Switzerland, 2017; pp. 457–490. [Google Scholar] [CrossRef]
- Fuller, A.M.; Eisinger-Mathason, T.S.K. Context matters: Response heterogeneity to collagen-targeting approaches in desmoplastic cancers. Cancers 2022, 14, 3132. [Google Scholar] [CrossRef] [PubMed]
- Nissen, N.I.; Karsdal, M.; Willumsen, N. Collagens and cancer associated fibroblasts in the reactive stroma and its relation to Cancer biology. J. Exp. Clin. Cancer Res. 2019, 38, 115. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.V.; Jücker, M. The functional role of extracellular matrix proteins in cancer. Cancers 2022, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.H.; Rodriguez, B.L.; Diao, L.; Chen, L.; Wang, J.; Byers, L.A.; Wei, Y.; Chapman, H.A.; Yamauchi, M.; Behrens, C.; et al. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8+ T cell exhaustion. Nat. Commun. 2020, 11, 4520. [Google Scholar] [CrossRef]
- Klemm, F.; Joyce, J.A. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2014, 25, 198–213. [Google Scholar] [CrossRef]
- Angel, P.M.; Zambrzycki, S.C. Predictive value of collagen in cancer. Adv. Cancer Res. 2022, 154, 15–45. [Google Scholar] [CrossRef]
- Song, K.; Yu, Z.; Zu, X.; Li, G.; Hu, Z.; Xue, Y. Collagen remodeling along cancer progression providing a novel opportunity for cancer diagnosis and treatment. Int. J. Mol. Sci. 2022, 23, 10509. [Google Scholar] [CrossRef]
- Han, W.; Chen, S.; Yuan, W.; Fan, Q.; Tian, J.; Wang, X.; Chen, L.; Zhang, X.; Wei, W.; Liu, R.; et al. Oriented collagen fibers direct tumor cell intravasation. Proc. Natl. Acad. Sci. USA 2016, 113, 11208–11213. [Google Scholar] [CrossRef]
- Ray, A.; Callaway, M.K.; Rodríguez-Merced, N.J.; Crampton, A.L.; Carlson, M.; Emme, K.B.; Ensminger, E.A.; Kinne, A.A.; Schrope, J.H.; Rasmussen, H.R.; et al. Stromal architecture directs early dissemination in pancreatic ductal adenocarcinoma. JCI Insight 2022, 7, e150330. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Nissen, N.I.; Von Arenstorff, C.S.; Karsdal, M.A.; Willumsen, N. Serological assessment of collagen fragments and tumor fibrosis may guide immune checkpoint inhibitor therapy. J. Exp. Clin. Cancer Res. 2021, 40, 326. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, J.; Liu, J.; Wang, Y.; Mu, J.; Zeng, Q.; Deng, S.; Zhou, H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct. Target. Ther. 2021, 6, 218. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L. Stroma as an active player in the development of the tumor microenvironment. Cancer Microenviron. 2014, 8, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 2018, 33, 463–479.e10. [Google Scholar] [CrossRef]
- Barrett, R.L.; Puré, E. Cancer-associated fibroblasts and their influence on tumor immunity and immunotherapy. eLife 2020, 9, e57243. [Google Scholar] [CrossRef]
- Peng, L.; Wang, D.; Han, Y.; Huang, T.; He, X.; Wang, J.; Ou, C. Emerging role of cancer-associated fibroblasts-derived exosomes in tumorigenesis. Front. Immunol. 2022, 12, 795372. [Google Scholar] [CrossRef]
- Mhaidly, R.; Mechta-Grigoriou, F. Fibroblast heterogeneity in tumor micro-environment: Role in immunosuppression and new therapies. Semin. Immunol. 2020, 48, 101417. [Google Scholar] [CrossRef]
- Kuczek, D.E.; Larsen, A.M.H.; Thorseth, M.-L.; Carretta, M.; Kalvisa, A.; Siersbæk, M.S.; Simões, A.M.C.; Roslind, A.; Engelholm, L.H.; Noessner, E.; et al. Collagen density regulates the activity of tumor-infiltrating T cells. J. Immunother. Cancer 2019, 7, 68. [Google Scholar] [CrossRef]
- Rømer, A.M.A.; Thorseth, M.-L.; Madsen, D.H. Immune modulatory properties of collagen in cancer. Front. Immunol. 2021, 12, 791453. [Google Scholar] [CrossRef] [PubMed]
- Papanicolaou, M.; Parker, A.L.; Yam, M.; Filipe, E.C.; Wu, S.Z.; Chitty, J.L.; Wyllie, K.; Tran, E.; Mok, E.; Nadalini, A.; et al. Temporal profiling of the breast tumour microenvironment reveals collagen XII as a driver of metastasis. Nat. Commun. 2022, 13, 4587. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Zhang, Z.; Zhu, A.; Xiong, X.; Zhang, J.; Xu, J.; Sy, M.; Li, C. Targeting type I collagen for cancer treatment. Int. J. Cancer 2022, 151, 665–683. [Google Scholar] [CrossRef] [PubMed]
- Abyaneh, H.S.; Regenold, M.; McKee, T.; Allen, C.; Gauthier, M.A. Towards extracellular matrix normalization for improved treatment of solid tumors. Theranostics 2020, 10, 1960–1980. [Google Scholar] [CrossRef] [PubMed]
- I Pareti, F.; Fujimura, Y.; A Dent, J.; Holland, L.Z.; Zimmerman, T.S.; Ruggeri, Z.M. Isolation and characterization of a collagen binding domain in human von Willebrand factor. J. Biol. Chem. 1986, 261, 15310–15315. [Google Scholar] [CrossRef]
- Wahyudi, H.; Reynolds, A.A.; Li, Y.; Owen, S.C.; Yu, S.M. Targeting collagen for diagnostic imaging and therapeutic delivery. J. Control. Release 2016, 240, 323–331. [Google Scholar] [CrossRef]
- Liang, H.; Li, X.; Wang, B.; Chen, B.; Zhao, Y.; Sun, J.; Zhuang, Y.; Shi, J.; Shen, H.; Zhang, Z.; et al. A collagen-binding EGFR antibody fragment targeting tumors with a collagen-rich extracellular matrix. Sci. Rep. 2016, 6, 18205. [Google Scholar] [CrossRef]
- Ishihara, J.; Ishihara, A.; Sasaki, K.; Lee, S.S.-Y.; Williford, J.-M.; Yasui, M.; Abe, H.; Potin, L.; Hosseinchi, P.; Fukunaga, K.; et al. Targeted antibody and cytokine cancer immunotherapies through collagen affinity. Sci. Transl. Med. 2019, 11, eaau3259. [Google Scholar] [CrossRef]
- Mansurov, A.; Ishihara, J.; Hosseinchi, P.; Potin, L.; Marchell, T.M.; Ishihara, A.; Williford, J.-M.; Alpar, A.T.; Raczy, M.M.; Gray, L.T.; et al. Collagen-binding IL-12 enhances tumour inflammation and drives the complete remission of established immunologically cold mouse tumours. Nat. Biomed. Eng. 2020, 4, 531–543. [Google Scholar] [CrossRef]
- Momin, N.; Mehta, N.K.; Bennett, N.R.; Ma, L.; Palmeri, J.R.; Chinn, M.M.; Lutz, E.A.; Kang, B.; Irvine, D.J.; Spranger, S.; et al. Anchoring of intratumorally administered cytokines to collagen safely potentiates systemic cancer immunotherapy. Sci. Transl. Med. 2019, 11, eaaw2614. [Google Scholar] [CrossRef]
- Hu, J.-G.; Pi, J.-K.; Jiang, Y.-L.; Liu, X.-F.; Li-Ling, J.; Xie, H.-Q. Collagen hydrogel functionalized with collagen-targeting IFNA2b shows apoptotic activity in nude mice with xenografted tumors. ACS Biomater. Sci. Eng. 2018, 5, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Jeon, S.I.; Ahn, C.-H.; Shim, M.K.; Kim, K. Emerging albumin-binding anticancer drugs for tumor-targeted drug delivery: Current understandings and clinical translation. Pharmaceutics 2022, 14, 728. [Google Scholar] [CrossRef] [PubMed]
- Rahimizadeh, P.; Yang, S.; Lim, S.I. Albumin: An emerging opportunity in drug delivery. Biotechnol. Bioprocess Eng. 2020, 25, 985–995. [Google Scholar] [CrossRef]
- Sasaki, K.; Ishihara, J.; Ishihara, A.; Miura, R.; Mansurov, A.; Fukunaga, K.; Hubbell, J.A. Engineered collagen-binding serum albumin as a drug conjugate carrier for cancer therapy. Sci. Adv. 2019, 5, eaaw6081. [Google Scholar] [CrossRef] [PubMed]
- Yasunaga, M.; Manabe, S.; Tarin, D.; Matsumura, Y. Cancer-stroma targeting therapy by cytotoxic immunoconjugate bound to the collagen 4 network in the tumor tissue. Bioconjugate Chem. 2011, 22, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Salarian, M.; Yang, H.; Turaga, R.C.; Tan, S.; Qiao, J.; Xue, S.; Gui, Z.; Peng, G.; Han, H.; Mittal, P.; et al. Precision detection of liver metastasis by collagen-targeted protein MRI contrast agent. Biomaterials 2019, 224, 119478. [Google Scholar] [CrossRef]
- Salarian, M.; Turaga, R.C.; Xue, S.; Nezafati, M.; Hekmatyar, K.; Qiao, J.; Zhang, Y.; Tan, S.; Ibhagui, O.Y.; Hai, Y.; et al. Early detection and staging of chronic liver diseases with a protein MRI contrast agent. Nat. Commun. 2019, 10, 4777. [Google Scholar] [CrossRef]
- Hauge, A.; Rofstad, E.K. Antifibrotic therapy to normalize the tumor microenvironment. J. Transl. Med. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Dolor, A.; Szoka, F.C. Digesting a path forward: The utility of collagenase tumor treatment for improved drug delivery. Mol. Pharm. 2018, 15, 2069–2083. [Google Scholar] [CrossRef]
- García-Olmo, D.; Campos, P.V.; Barambio, J.; Gomez-Heras, S.G.; Vega-Clemente, L.; Olmedillas-Lopez, S.; Guadalajara, H.; Garcia-Arranz, M. Intraperitoneal collagenase as a novel therapeutic approach in an experimental model of colorectal peritoneal carcinomatosis. Sci. Rep. 2021, 11, 503. [Google Scholar] [CrossRef]
- Eikenes, L.; Bruland, S.; Brekken, C.; Davies, C.D.L. Collagenase increases the transcapillary pressure gradient and improves the uptake and distribution of monoclonal antibodies in human osteosarcoma xenografts. Cancer Res. 2004, 64, 4768–4773. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Murty, S.; Gilliland, T.M.; Qiao, P.; Tabtieng, T.; Higbee-Dempsey, E.; Al Zaki, A.; Puré, E.; Tsourkas, A. Nanoparticles functionalized with collagenase exhibit improved tumor accumulation in a murine xenograft model. Part. Part. Syst. Charact. 2014, 31, 1307–1312. [Google Scholar] [CrossRef]
- Zinger, A.; Koren, L.; Adir, O.; Poley, M.; Alyan, M.; Yaari, Z.; Noor, N.; Krinsky, N.; Simon, A.; Gibori, H.; et al. Collagenase nanoparticles enhance the penetration of drugs into pancreatic tumors. ACS Nano 2019, 13, 11008–11021. [Google Scholar] [CrossRef]
- Xu, F.; Huang, X.; Wang, Y.; Zhou, S. A size-changeable collagenase-modified nanoscavenger for increasing penetration and retention of nanomedicine in deep tumor tissue. Adv. Mater. 2020, 32, e1906745. [Google Scholar] [CrossRef]
- Wang, X.; Luo, J.; He, L.; Cheng, X.; Yan, G.; Wang, J.; Tang, R. Hybrid pH-sensitive nanogels surface-functionalized with collagenase for enhanced tumor penetration. J. Colloid Interface Sci. 2018, 525, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Wang, Z.; Chen, B.; Dai, W.; Zhang, H.; He, B.; Wang, X.; Wang, Y.; Zhang, Q. Localized co-delivery of collagenase and trastuzumab by thermosensitive hydrogels for enhanced antitumor efficacy in human breast xenograft. Drug Deliv. 2018, 25, 1495–1503. [Google Scholar] [CrossRef]
- Dong, X.; Liu, H.-J.; Feng, H.-Y.; Yang, S.-C.; Liu, X.-L.; Lai, X.; Lu, Q.; Lovell, J.F.; Chen, H.-Z.; Fang, C. Enhanced drug delivery by nanoscale integration of a nitric oxide donor to induce tumor collagen depletion. Nano Lett. 2019, 19, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Shirai, H.; Tsukada, K. Bacterial proteolytic activity improves drug delivery in tumors in a size, pharmacokinetic, and binding affinity dependent manner—A mechanistic understanding. J. Control. Release 2020, 321, 348–362. [Google Scholar] [CrossRef]
- Lou, X.; Chen, Z.; He, Z.; Sun, M.; Sun, J. Bacteria-mediated synergistic cancer therapy: Small microbiome has a big hope. Nano-Micro Lett. 2021, 13, 37. [Google Scholar] [CrossRef]
- Ebelt, N.; Zamloot, V.; Zuniga, E.; Passi, K.; Sobocinski, L.; Young, C.; Blazar, B.; Manuel, E. Collagenase-expressing Salmonella targets major collagens in pancreatic cancer leading to reductions in immunosuppressive subsets and tumor growth. Cancers 2021, 13, 3565. [Google Scholar] [CrossRef] [PubMed]
- Achard, C.; Surendran, A.; Wedge, M.-E.; Ungerechts, G.; Bell, J.; Ilkow, C.S. Lighting a fire in the tumor microenvironment using oncolytic immunotherapy. EBioMedicine 2018, 31, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Everts, A.; Bergeman, M.; McFadden, G.; Kemp, V. Simultaneous tumor and stroma targeting by oncolytic viruses. Biomedicines 2020, 8, 474. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Lee, Y.-S.; Kim, H.; Huang, J.-H.; Yoon, A.-R.; Yun, C.-O. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. JNCI: J. Natl. Cancer Inst. 2006, 98, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Choi, I.-K.; Lee, H.-S.; Yan, H.H.; Son, M.K.; Ahn, H.M.; Hong, J.; Yun, C.-O.; Hong, S.-S. Oncolytic adenovirus expressing relaxin (YDC002) enhances therapeutic efficacy of gemcitabine against pancreatic cancer. Cancer Lett. 2017, 396, 155–166. [Google Scholar] [CrossRef]
- Choi, I.-K.; Lee, Y.-S.; Yoo, J.Y.; Yoon, A.-R.; Kim, H.; Kim, D.-S.; Seidler, D.G.; Kim, J.-H.; Yun, C.-O. Effect of decorin on overcoming the extracellular matrix barrier for oncolytic virotherapy. Gene Ther. 2009, 17, 190–201. [Google Scholar] [CrossRef]
- Li, Y.; Hong, J.; Oh, J.-E.; Yoon, A.-R.; Yun, C.-O. Potent antitumor effect of tumor microenvironment-targeted oncolytic adenovirus against desmoplastic pancreatic cancer. Int. J. Cancer 2017, 142, 392–413. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, C.; Tian, W.; Qin, J.; Chen, J.; Zhang, Q.; Fang, L.; Zheng, J. Efficacy of an oncolytic adenovirus driven by a chimeric promoter and armed with decorin against renal cell carcinoma. Hum. Gene Ther. 2020, 31, 651–663. [Google Scholar] [CrossRef]
- Oh, E.; Choi, I.-K.; Hong, J.; Yun, C.-O. Oncolytic adenovirus coexpressing interleukin-12 and decorin overcomes Treg-mediated immunosuppression inducing potent antitumor effects in a weakly immunogenic tumor model. Oncotarget 2016, 8, 4730–4746. [Google Scholar] [CrossRef]
- Cheng, J.; Sauthoff, H.; Huang, Y.; I Kutler, D.; Bajwa, S.; Rom, W.; Hay, J.G. Human matrix metalloproteinase-8 gene delivery increases the oncolytic activity of a replicating adenovirus. Mol. Ther. 2007, 15, 1982–1990. [Google Scholar] [CrossRef]
- Wan, P.K.-T.; Ryan, A.J.; Seymour, L.W. Beyond cancer cells: Targeting the tumor microenvironment with gene therapy and armed oncolytic virus. Mol. Ther. 2021, 29, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fang, L.; Wang, X.; Yuan, S.; Li, W.; Tian, W.; Chen, J.; Zhang, Q.; Zhang, Y.; Zhang, Q.; et al. Oncolytic adenovirus-mediated expression of decorin facilitates CAIX-targeting CAR-T therapy against renal cell carcinoma. Mol. Ther. Oncolytics 2021, 24, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.D.; Duffy, M.R.; Lei-Rossmann, J.; Muntzer, A.; Scott, E.M.; Hagel, J.; Campo, L.; Bryant, R.J.; Verrill, C.; Lambert, A.; et al. An oncolytic virus expressing a T-cell engager simultaneously targets cancer and immunosuppressive stromal cells. Cancer Res. 2018, 78, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- De Sostoa, J.; Fajardo, C.A.; Moreno, R.; Ramos, M.D.; Farrera-Sal, M.; Alemany, R. Targeting the tumor stroma with an oncolytic adenovirus secreting a fibroblast activation protein-targeted bispecific T-cell engager. J. Immunother. Cancer 2019, 7, 19. [Google Scholar] [CrossRef]
- Heidbuechel, J.P.W.; Engeland, C.E. Oncolytic viruses encoding bispecific T cell engagers: A blueprint for emerging immunovirotherapies. J. Hematol. Oncol. 2021, 14, 63. [Google Scholar] [CrossRef]
- Egeblad, M.; Rasch, M.G.; Weaver, V.M. Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol. 2010, 22, 697–706. [Google Scholar] [CrossRef]
- Wang, T.-H.; Hsia, S.-M.; Shieh, T.-M. Lysyl oxidase and the tumor microenvironment. Int. J. Mol. Sci. 2016, 18, 62. [Google Scholar] [CrossRef]
- Setargew, Y.F.; Wyllie, K.; Grant, R.D.; Chitty, J.L.; Cox, T.R. Targeting lysyl oxidase family meditated matrix cross-linking as an anti-stromal therapy in solid tumours. Cancers 2021, 13, 491. [Google Scholar] [CrossRef]
- Saatci, O.; Kaymak, A.; Raza, U.; Ersan, P.G.; Akbulut, O.; Banister, C.E.; Sikirzhytski, V.; Tokat, U.M.; Aykut, G.; Ansari, S.A.; et al. Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat. Commun. 2020, 11, 2416. [Google Scholar] [CrossRef]
- Rosell-García, T.; Paradela, A.; Bravo, G.; Dupont, L.; Bekhouche, M.; Colige, A.; Rodriguez-Pascual, F. Differential cleavage of lysyl oxidase by the metalloproteinases BMP1 and ADAMTS2/14 regulates collagen binding through a tyrosine sulfate domain. J. Biol. Chem. 2019, 294, 11087–11100. [Google Scholar] [CrossRef]
- Nicolas-Boluda, A.; Vaquero, J.; Vimeux, L.; Guilbert, T.; Barrin, S.; Kantari-Mimoun, C.; Ponzo, M.; Renault, G.; Deptula, P.; Pogoda, K.; et al. Tumor stiffening reversion through collagen crosslinking inhibition improves T cell migration and anti-PD-1 treatment. eLife 2021, 10, e58688. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Saraiva, N.; Rijo, P.; Fernandes, A. LOXL2 inhibitors and breast cancer progression. Antioxidants 2021, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Baldari, S.; Di Rocco, G.; Toietta, G. Current biomedical use of copper chelation therapy. Int. J. Mol. Sci. 2020, 21, 1069. [Google Scholar] [CrossRef]
- Liu, Y.L.; Bager, C.L.; Willumsen, N.; Ramchandani, D.; Kornhauser, N.; Ling, L.; Cobham, M.; Andreopoulou, E.; Cigler, T.; Moore, A.; et al. Tetrathiomolybdate (TM)-associated copper depletion influences collagen remodeling and immune response in the pre-metastatic niche of breast cancer. npj Breast Cancer 2021, 7, 108. [Google Scholar] [CrossRef]
- Yamagishi, S.-I.; Matsui, T.; Fukami, K. Role of receptor for advanced glycation end products (RAGE) and its ligands in cancer risk. Rejuvenation Res. 2015, 18, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Schneider, I.; Lindner, C.; Gonzalez, I.; Morales, M.A. The RAGE/multiligand axis: A new actor in tumor biology. Biosci. Rep. 2022, 42, BSR20220395. [Google Scholar] [CrossRef] [PubMed]
- Krisanits, B.A.; Woods, P.; Nogueira, L.M.; Woolfork, D.D.; Lloyd, C.E.; Baldwin, A.; Frye, C.C.; Peterson, K.D.; Cosh, S.D.; Guo, Q.-J.; et al. Non-enzymatic glycoxidation linked with nutrition enhances the tumorigenic capacity of prostate cancer epithelia through AGE mediated activation of RAGE in cancer associated fibroblasts. Transl. Oncol. 2022, 17, 101350. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, M.; Jang, S. Molecular characteristics of RAGE and advances in small-molecule inhibitors. Int. J. Mol. Sci. 2021, 22, 6904. [Google Scholar] [CrossRef]
- Bansode, S.; Bashtanova, U.; Li, R.; Clark, J.; Müller, K.H.; Puszkarska, A.; Goldberga, I.; Chetwood, H.H.; Reid, D.G.; Colwell, L.J.; et al. Glycation changes molecular organization and charge distribution in type I collagen fibrils. Sci. Rep. 2020, 10, 3397. [Google Scholar] [CrossRef]
- Palanissami, G.; Paul, S.F.D. RAGE and its ligands: Molecular interplay between glycation, inflammation, and hallmarks of cancer—A review. Horm. Cancer 2018, 9, 295–325. [Google Scholar] [CrossRef]
- McGaha, T.L.; Bona, C.; Phelps, R.G.; Spiera, H. Halofuginone, an inhibitor of type-I collagen synthesis and skin sclerosis, blocks transforming-growth-factor-β-mediated Smad3 activation in fibroblasts. J. Investig. Dermatol. 2002, 118, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Elahi-Gedwillo, K.Y.; Carlson, M.; Zettervall, J.; Provenzano, P.P. Antifibrotic therapy disrupts stromal barriers and modulates the immune landscape in pancreatic ductal adenocarcinoma. Cancer Res. 2019, 79, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.; Le, A.; Weaver, V.M.; Werb, Z. Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget 2016, 7, 82889–82901. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Funaki, S.; Fukui, E.; Kimura, K.; Kanou, T.; Ose, N.; Minami, M.; Shintani, Y. Effects of pirfenidone targeting the tumor microenvironment and tumor-stroma interaction as a novel treatment for non-small cell lung cancer. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Mediavilla-Varela, M.; Boateng, K.; Noyes, D.; Antonia, S.J. The anti-fibrotic agent pirfenidone synergizes with cisplatin in killing tumor cells and cancer-associated fibroblasts. BMC Cancer 2016, 16, 176. [Google Scholar] [CrossRef]
- Diop-Frimpong, B.; Chauhan, V.P.; Krane, S.; Boucher, Y.; Jain, R.K. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl. Acad. Sci. USA 2011, 108, 2909–2914. [Google Scholar] [CrossRef]
- Xia, T.; He, Q.; Shi, K.; Wang, Y.; Yu, Q.; Zhang, L.; Zhang, Q.; Gao, H.; Ma, L.; Liu, J. Losartan loaded liposomes improve the antitumor efficacy of liposomal paclitaxel modified with pH sensitive peptides by inhibition of collagen in breast cancer. Pharm. Dev. Technol. 2016, 23, 13–21. [Google Scholar] [CrossRef]
- Cortes, E.; Lachowski, D.; Rice, A.; Thorpe, S.; Robinson, B.; Yeldag, G.; Lee, D.; Ghemtio, L.; Rombouts, K.; del Rio Hernández, A.E. Tamoxifen mechanically deactivates hepatic stellate cells via the G protein-coupled estrogen receptor. Oncogene 2018, 38, 2910–2922. [Google Scholar] [CrossRef]
- Özdemir, B.C.; Pentcheva-Hoang, T.; Carstens, J.L.; Zheng, X.; Wu, C.-C.; Simpson, T.R.; Laklai, H.; Sugimoto, H.; Kahlert, C.; Novitskiy, S.V.; et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014, 25, 719–734. [Google Scholar] [CrossRef]
- Uitto, J.; Prockop, D.J. Incorporation of proline analogues into collagen polypeptides Effects on the production of extracellular procollagen and on the stability of the triple-helical structure of the molecule. Biochim. et Biophys. Acta (BBA) Protein Struct. 1974, 336, 234–251. [Google Scholar] [CrossRef]
- Abayasiriwardana, K.S.; Wood, M.K.; Prêle, C.M.; Birnie, K.A.; Robinson, B.W.; Laurent, G.J.; McAnulty, R.J.; Mutsaers, S.E. Inhibition of collagen production delays malignant mesothelioma tumor growth in a murine model. Biochem. Biophys. Res. Commun. 2019, 510, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Vasta, J.D.; Raines, R.T. Collagen prolyl 4-hydroxylase as a therapeutic target. J. Med. Chem. 2018, 61, 10403–10411. [Google Scholar] [CrossRef] [PubMed]
- Gilkes, D.M.; Chaturvedi, P.; Bajpai, S.; Wong, C.C.; Wei, H.; Pitcairn, S.; Hubbi, M.E.; Wirtz, D.; Semenza, G.L. Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer Res. 2013, 73, 3285–3296. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Behring, M.; Kim, H.-G.; Bajpai, P.; Chakravarthi, B.V.; Gupta, N.; Elkholy, A.; Al Diffalha, S.; Varambally, S.; Manne, U. Targeting P4HA1 with a small molecule inhibitor in a colorectal cancer PDX model. Transl. Oncol. 2020, 13, 100754. [Google Scholar] [CrossRef]
- Shi, R.; Gao, S.; Zhang, J.; Xu, J.; Graham, L.M.; Yang, X.; Li, C. Collagen prolyl 4-hydroxylases modify tumor progression. Acta Biochim. et Biophys. Sin. 2021, 53, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhou, L.; Li, D.; Andl, T.; Zhang, Y. Cancer-associated fibroblasts build and secure the tumor microenvironment. Front. Cell Dev. Biol. 2019, 7, 60. [Google Scholar] [CrossRef]
- Mieulet, V.; Garnier, C.; Kieffer, Y.; Guilbert, T.; Nemati, F.; Marangoni, E.; Renault, G.; Chamming’s, F.; Vincent-Salomon, A.; Mechta-Grigoriou, F. Stiffness increases with myofibroblast content and collagen density in mesenchymal high grade serous ovarian cancer. Sci. Rep. 2021, 11, 4219. [Google Scholar] [CrossRef]
- Harryvan, T.J.; Verdegaal, E.M.E.; Hardwick, J.C.H.; Hawinkels, L.J.A.C.; van der Burg, S.H. Targeting of the cancer-associated fibroblast—T-cell axis in solid malignancies. J. Clin. Med. 2019, 8, 1989. [Google Scholar] [CrossRef]
- Aghajanian, H.; Kimura, T.; Rurik, J.G.; Hancock, A.S.; Leibowitz, M.S.; Li, L.; Scholler, J.; Monslow, J.; Lo, A.; Han, W.; et al. Targeting cardiac fibrosis with engineered T cells. Nature 2019, 573, 430–433. [Google Scholar] [CrossRef]
- Bughda, R.; Dimou, P.; D’Souza, R.R.; Klampatsa, A. Fibroblast activation protein (FAP)-targeted CAR-T cells: Launching an attack on tumor stroma. ImmunoTargets Ther. 2021, 10, 313–323. [Google Scholar] [CrossRef]
- Kakarla, S.; Chow, K.K.; Mata, M.; Shaffer, D.R.; Song, X.-T.; Wu, M.-F.; Liu, H.; Wang, L.L.; Rowley, D.R.; Pfizenmaier, K.; et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol. Ther. 2013, 21, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Schuberth, P.C.; Hagedorn, C.; Jensen, S.M.; Gulati, P.; Broek, M.V.D.; Mischo, A.; Soltermann, A.; Jüngel, A.; Belaunzaran, O.M.; Stahel, R.; et al. Treatment of malignant pleural mesothelioma by fibroblast activation protein-specific re-directed T cells. J. Transl. Med. 2013, 11, 187. [Google Scholar] [CrossRef]
- Wang, L.-C.S.; Lo, A.; Scholler, J.; Sun, J.; Majumdar, R.S.; Kapoor, V.; Antzis, M.; Cotner, C.E.; Johnson, L.A.; Durham, A.C.; et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res. 2013, 2, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.; Wang, L.-C.S.; Scholler, J.; Monslow, J.; Avery, D.; Newick, K.; O’Brien, S.; Evans, R.A.; Bajor, D.J.; Clendenin, C.; et al. Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res. 2015, 75, 2800–2810. [Google Scholar] [CrossRef] [PubMed]
- Gulati, P.; Rühl, J.; Kannan, A.; Pircher, M.; Schuberth, P.; Nytko, K.J.; Pruschy, M.N.; Sulser, S.; Haefner, M.D.; Jensen, S.M.; et al. Aberrant Lck signal via CD28 costimulation augments antigen-specific functionality and tumor control by redirected T cells with PD-1 blockade in humanized mice. Clin. Cancer Res. 2018, 24, 3981–3993. [Google Scholar] [CrossRef]
- Curioni, A.; Britschgi, C.; Hiltbrunner, S.; Bankel, L.; Gulati, P.; Weder, W.; Opitz, I.; Lauk, O.; Caviezel, C.; Knuth, A.; et al. A phase I clinical trial of malignant pleural mesothelioma treated with locally delivered autologous anti-FAP-targeted CAR T-cells. Ann. Oncol. 2019, 30, v501. [Google Scholar] [CrossRef]
- Hiltbrunner, S.; Britschgi, C.; Schuberth, P.; Bankel, L.; Nguyen-Kim, T.; Gulati, P.; Weder, W.; Opitz, I.; Lauk, O.; Caviezel, C.; et al. Local delivery of CAR T cells targeting fibroblast activation protein is safe in patients with pleural mesothelioma: First report of FAPME, a phase I clinical trial. Ann. Oncol. 2020, 32, 120–121. [Google Scholar] [CrossRef]
- Tran, E.; Chinnasamy, D.; Yu, Z.; Morgan, R.A.; Lee, C.-C.; Restifo, N.P.; Rosenberg, S.A. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J. Exp. Med. 2013, 210, 1125–1135. [Google Scholar] [CrossRef]
- Thorseth, M.-L.; Carretta, M.; Jensen, C.; Mølgaard, K.; Jürgensen, H.J.; Engelholm, L.H.; Behrendt, N.; Willumsen, N.; Madsen, D.H. Uncovering mediators of collagen degradation in the tumor microenvironment. Matrix Biol. Plus 2022, 13, 100101. [Google Scholar] [CrossRef]
- Jungwirth, U.; van Weverwijk, A.; Evans, R.J.; Jenkins, L.; Vicente, D.; Alexander, J.; Gao, Q.; Haider, S.; Iravani, M.; Isacke, C.M. Impairment of a distinct cancer-associated fibroblast population limits tumour growth and metastasis. Nat. Commun. 2021, 12, 3516. [Google Scholar] [CrossRef]
- Jenkins, L.; Jungwirth, U.; Avgustinova, A.; Iravani, M.; Mills, A.P.; Haider, S.; Harper, J.; Isacke, C.M. Cancer-associated fibroblasts suppress CD8+ T cell infiltration and confer resistance to immune checkpoint blockade. Cancer Res. 2022, 82, 2904–2917. [Google Scholar] [CrossRef] [PubMed]
- Belhabib, I.; Zaghdoudi, S.; Lac, C.; Bousquet, C.; Jean, C. Extracellular matrices and cancer-associated fibroblasts: Targets for cancer diagnosis and therapy? Cancers 2021, 13, 3466. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Han, C.; Wang, S.; Fang, P.; Ma, Z.; Xu, L.; Yin, R. Cancer-associated fibroblasts: An emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 2019, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Salmon, H.; Franciszkiewicz, K.; Damotte, D.; Dieu-Nosjean, M.-C.; Validire, P.; Trautmann, A.; Mami-Chouaib, F.; Donnadieu, E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Investig. 2012, 122, 899–910. [Google Scholar] [CrossRef]
- Bougherara, H.; Mansuet-Lupo, A.; Alifano, M.; Ngo, C.; Damotte, D.; Le Frère-Belda, M.-A.; Donnadieu, E.; Peranzoni, E. Real-time imaging of resident T cells in human lung and ovarian carcinomas reveals how different tumor microenvironments control T lymphocyte migration. Front. Immunol. 2015, 6, 500. [Google Scholar] [CrossRef]
- Peranzoni, E.; Rivas-Caicedo, A.; Bougherara, H.; Salmon, H.; Donnadieu, E. Positive and negative influence of the matrix architecture on antitumor immune surveillance. Cell. Mol. Life Sci. 2013, 70, 4431–4448. [Google Scholar] [CrossRef]
- Ray, A.; Provenzano, P.P. Aligned forces: Origins and mechanisms of cancer dissemination guided by extracellular matrix architecture. Curr. Opin. Cell Biol. 2021, 72, 63–71. [Google Scholar] [CrossRef]
- Sun, X.; Wu, B.; Chiang, H.-C.; Deng, H.; Zhang, X.; Xiong, W.; Liu, J.; Rozeboom, A.M.; Harris, B.T.; Blommaert, E.; et al. Tumour DDR1 promotes collagen fibre alignment to instigate immune exclusion. Nature 2021, 599, 673–678. [Google Scholar] [CrossRef]
- Liu, W.; Gao, M.; Li, L.; Chen, Y.; Fan, H.; Cai, Q.; Shi, Y.; Pan, C.; Liu, J.; Cheng, L.S.; et al. Homeoprotein SIX1 compromises antitumor immunity through TGF-β-mediated regulation of collagens. Cell. Mol. Immunol. 2021, 18, 2660–2672. [Google Scholar] [CrossRef]
- Gao, H.; Tian, Q.; Zhou, Y.; Zhu, L.; Lu, Y.; Ma, Y.; Feng, J.; Jiang, Y.; Wang, B. 3D collagen fiber concentration regulates Treg cell infiltration in triple negative breast cancer. Front. Immunol. 2022, 13, 904418. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, S.; Tavormina, J.; Tampe, D.; Zeisberg, M.; Wang, H.; Mahadevan, K.K.; Wu, C.-J.; Sugimoto, H.; Chang, C.-C.; et al. Oncogenic collagen I homotrimers from cancer cells bind to α3β1 integrin and impact tumor microbiome and immunity to promote pancreatic cancer. Cancer Cell 2022, 40, 818–834. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, J.; Herjan, T.; Hong, L.; Liao, Y.; Liu, C.; Vasu, K.; Wang, H.; Thompson, A.; Fox, P.L.; et al. IL-17–induced HIF1α drives resistance to anti–PD-L1 via fibroblast-mediated immune exclusion. J. Exp. Med. 2022, 219, e20210693. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Massagué, J. TGF-beta inhibition and immunotherapy: Checkmate. Immunity 2018, 48, 626–628. [Google Scholar] [CrossRef]
- Horn, L.A.; Chariou, P.L.; Gameiro, S.R.; Qin, H.; Iida, M.; Fousek, K.; Meyer, T.J.; Cam, M.; Flies, D.; Langermann, S.; et al. Remodeling the tumor microenvironment via blockade of LAIR-1 and TGF-β signaling enables PD-L1–mediated tumor eradication. J. Clin. Investig. 2022, 132, e155148. [Google Scholar] [CrossRef]
- Jiang, H.; Hegde, S.; Knolhoff, B.L.; Zhu, Y.; Herndon, J.M.; Meyer, M.A.; Nywening, T.M.; Hawkins, W.G.; Shapiro, I.M.; Weaver, D.T.; et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 2016, 22, 851–860. [Google Scholar] [CrossRef]
- Chen, Y.; Kim, J.; Yang, S.; Wang, H.; Wu, C.-J.; Sugimoto, H.; LeBleu, V.S.; Kalluri, R. Type I collagen deletion in αSMA+ myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell 2021, 39, 548–565. [Google Scholar] [CrossRef]
- McAndrews, K.M.; Chen, Y.; Darpolor, J.K.; Zheng, X.; Yang, S.; Carstens, J.L.; Li, B.; Wang, H.; Miyake, T.; Correa de Sampaio, P.; et al. Identification of functional heterogeneity of carcinoma-associated fibroblasts with distinct IL6-mediated therapy resistance in pancreatic cancer. Cancer Discov. 2022, 12, 1580–1597. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldari, S.; Di Modugno, F.; Nisticò, P.; Toietta, G. Strategies for Efficient Targeting of Tumor Collagen for Cancer Therapy. Cancers 2022, 14, 4706. https://doi.org/10.3390/cancers14194706
Baldari S, Di Modugno F, Nisticò P, Toietta G. Strategies for Efficient Targeting of Tumor Collagen for Cancer Therapy. Cancers. 2022; 14(19):4706. https://doi.org/10.3390/cancers14194706
Chicago/Turabian StyleBaldari, Silvia, Francesca Di Modugno, Paola Nisticò, and Gabriele Toietta. 2022. "Strategies for Efficient Targeting of Tumor Collagen for Cancer Therapy" Cancers 14, no. 19: 4706. https://doi.org/10.3390/cancers14194706
APA StyleBaldari, S., Di Modugno, F., Nisticò, P., & Toietta, G. (2022). Strategies for Efficient Targeting of Tumor Collagen for Cancer Therapy. Cancers, 14(19), 4706. https://doi.org/10.3390/cancers14194706







