Simple Summary
Immunotherapy has revolutionized cancer treatment, as demonstrated by the tremendous success of checkpoint inhibitors in different tumor types. Unfortunately, most patients, particularly patients with non-responsive “cold” tumors, do not benefit from checkpoint inhibitors. Enter “armed” oncolytic viruses, which “cooperate” with checkpoint inhibitors to improve anticancer responses. These are genetically engineered viruses that selectively infect, replicate in, and kill cancer cells but not cells from healthy tissues; in the process, oncolytic viruses express the therapeutic proteins that they are armed with or carry. This effectively turns the infected tumors “hot” and makes them suitable for treatment with checkpoint inhibitors. The most well-studied of all the oncolytic viruses are adenoviruses. These are agents of the common cold, which makes them remarkably safe for clinical use. This review article summarizes the oncolytic adenoviruses in advanced clinical trials and presents strategies to improve their anticancer activity.
Abstract
Oncolytic viruses, colloquially referred to as “living drugs”, amplify themselves and the therapeutic transgenes that they carry to stimulate an immune response both locally and systemically. Remarkable exceptions aside, such as the recent 14-patient trial with the PD-1 inhibitor, dostarlimab, in mismatch repair (MMR) deficient rectal cancer, where the complete response rate was 100%, checkpoint inhibitors are not cure-alls, which suggests the need for a combination partner like oncolytic viruses to prime and augment their activity. This review focuses on adenoviruses, the most clinically investigated of all the oncolytic viruses. It covers specific design features of clinical adenoviral candidates and highlights their potential both alone and in combination with checkpoint inhibitors in clinical trials to turn immunologically “cold” and unresponsive tumors into “hotter” and more responsive ones through a domino effect. Finally, a “mix-and-match” combination of therapies based on the paradigm of the cancer-immunity cycle is proposed to augment the immune responses of oncolytic adenoviruses.
1. Introduction
A major challenge to successful anticancer treatment, especially with immune checkpoint inhibitors (ICBs) specific for CTLA-4, PD-1 and PD-L1 and chimeric antigen T (CAR-T) cell therapy, is the presence of immunologically “cold” or non-T-cell inflamed tumors, which have prompted countermeasures to heat them up vis-à-vis T cell infiltration [1].
One of these countermeasures is oncolytic viruses (OVs), colloquially referred to as “living drugs” [2]. Several oncolytic viruses have received regulatory approval. These include Talimogene laherparepvec (T-VEC), an attenuated herpes simplex virus, type 1 (HSV-1) for melanoma, Delytact (teserpaturev), another HSV-1 virus, in Japan for the treatment of glioma [3], and Rigavir, an unmodified ECHO-7 virus in Latvia, Georgia, and Armenia and H101, an oncolytic adenovirus approved in China [4]. To date, however, the full benefit of combination with checkpoint inhibitors has not been realized in large phase 3 trials, which argues for improvements in the design of OVs. Other OVs have entered clinical trials, including poxviruses, HSV-1, coxsackieviruses, poliovirus, measles virus, Newcastle disease virus (NDV), and reovirus.
The most studied and widely used oncolytic viruses are adenoviruses. These are non-enveloped double-stranded DNA viruses with linear genomes of ~30–38 kb and a fiber-covered icosahedral protein capsid [5,6]. Most genetically engineered oncolytic adenoviruses are derived from Ad serotype 5 (Ad5) and Ad serotype 2 (Ad2), while over 100 different antigenic serotypes and 7 different species (A–G) have been identified, which infect mammals (genus mastadenoviruses) and birds (genus aviadenoviruses) [7]. The adenoviral replication cycle is broadly divided into two temporal phases with early (E1A, E1B, E2A, E2B, E3, and E4) and late (L1–L5) transcription units, as shown in the figure below; the former is responsible for DNA synthesis and the latter for the structural proteins of the Ad virion [8]. The Ad genome is flanked by inverted terminal repeat (ITR) sequences, which initiate replication (Figure 1).
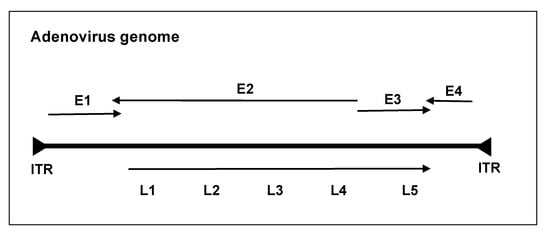
Figure 1.
Simplified schematic of the human adenovirus genome. The adenoviral genome is linear and double-stranded and about 30–38 kb in length. Adenovirus genes are broadly organized into early and late transcription units based on their expression before or after DNA replication. The early transcription units include the early region, E1A, E1B, E2, E3, and E4, and late L1–L5. At each end of the genome are inverted terminal repeats (ITRs), which act as a primer for the host DNA polymerase.
H101, an E1B-55K gene deleted recombinant Ad5 and the successor to ONYX-015, which was the first tumor-specific oncolytic adenovirus (OAV) evaluated in the clinic, received approval from the Chinese FDA for the treatment of nasopharyngeal carcinoma in combination with cisplatin and/or 5-fluorouracil (5-FU) [9,10,11]. Since ONYX-015 and H101, several “generations” of conditionally replicative adenoviruses have followed, which include diverse modifications to E1A and E1B and the capsid, the deletion or partial deletion of E3, and the insertion of therapeutic transgenes.
Cancer is a systemic disease, such that by the time tumors “go live”, that is, reach 1–2 mm in diameter and acquire a vasculature, circulating cancer cells are present [12]. Nevertheless, total local surgical resection is curative in most patients, despite the ab initio systematicity of cancer cells. This suggests that an immune response is involved, possibly from the release of potential tumor antigens, pro-inflammatory cytokines and chemokines, and other danger signals during surgery [13].
Similarly, oncolytic adenoviruses (OAVs) can elicit and redirect both innate and adaptive immune responses to target tumors. This is accomplished through selective infection, replication, and direct elimination of cancer cells, including cancer stem cells, which contribute to therapeutic resistance and recurrence, the release of danger signals and tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs), as well as expression of transgene-encoded immunomodulatory proteins [14].
One of the main limitations to the success of these OAVs as chemo- and immune-sensitizers is the degree of immunosuppression present in the tumor microenvironment (TME). Multiple mechanisms are responsible for the maintenance of this immunosuppressive phenotype. These mechanisms include upregulation of procancerous factors such as IL-10, IL-18, VEGF, Prostaglandin E, and TGF-β, infiltration of regulatory T cells (Treg cells), and myeloid-derived suppressor cells (MDSCs), increased deposition of extracellular matrix or fibrosis, and overexpression of checkpoint ligands, such as programmed cell death ligand 1 (PDL1) or its cognate receptor, PD-1, cytotoxic T-lymphocyte protein 4 (CTLA4), TIM-3 (HAVcr2), LAG-3 (CD223), TIGIT, B7-H3 (CD276), B7-H4 (VCTN1), downregulation or loss of HLA class I molecules, and A2aR and decreased neoepitope availability [15,16].
The effectiveness of oncolytic adenovirotherapy to bring about cold to hot transformation in the TME critically depends on at least 3 factors: (1) degree of attenuation since the more modifications which are made to the viral genome to increase safety or to ablate native tropism and improve tumor targetability, for example, the more the potency of the virus and its ability to induce a sufficient antitumor response are compromised; (2) choice of immunomodulatory payload since, for example, granulocyte-macrophage colony-stimulating factor (GM-CSF), by far the most widely used transgene in OAVs, may contribute to tumor growth and immunosuppression [17]. In addition, IL-2, IL-12, and Tumor Necrosis Factor Alpha (TNF-a), all well-studied immunostimulatory transgenes, are also associated with immunosuppression (and, interestingly, intralesional mRNA injections of these factors have not performed so well); (3) presence of the coxsackievirus and adenovirus receptor (CAR), which mediates viral attachment and infection [18,19,20].
This review covers specific design features of clinical adenoviral candidates and highlights their potential both alone and in combination with checkpoint inhibitors in clinical trials that were recently completed or are currently active to turn immunologically “cold” and unresponsive tumors into “hotter” and more responsive ones.
3. AdAPT-001 and AdAPT-039 (EpicentRx)
AdAPT-001 is the first conditionally replicating adenovirus to be presented out of familiarity because, full transparency, coauthors Tony Reid and Chris Larson not only conceived and designed AdAPT-001 but also led its clinical development and, more importantly, for this review, because AdAPT-001 presents some distinctive design characteristics, which are instructive to compare and contrast with the other OAVs that follow. Firstly, the AdAPT-001 Ad5 base oncolytic vector is not targeted to tumors through capsid modification, the insertion of exogenous cancer-specific promoters, or hybridization of adenoviral serotypes, strategies that are currently in clinical use to modify the natural tropism of adenoviruses. Rather, AdAPT-001 is detargeted from non-tumor cells through the deletion of a small 50 base pair region located upstream of the E1A initiation site, which contains multiple transcription factor binding sites that are indispensable in non-tumor cells, leading to abortive infection and no or restricted cytolytic activity, but dispensable in tumors, where potent near wild type levels of replication, expression, and cytolytic activity are observed [22,23,24].
The premise behind such a minimal modification is that too many viral additions or deletions may significantly change viral biological features and activity. Due to this “less is more” emphasis, not only is the cytolytic efficiency of AdAPT-001 comparable to that of wild-type virus, but so are the yields during manufacture. In fact, AdAPT-001 is manufacturable to cGMP standards “in house”, which removes reliance on often inefficient and costly contract manufacturing organizations (CMO).
The other modification in AdAPT-001 is the deletion of the E1B19K gene—a Bcl-2 adenoviral homolog that potently inhibits apoptosis [25]—and its replacement with a Transforming Growth Factor-beta (TGF-β) ligand “trap”. This trap is a TGFβ receptor ectodomain-IgG Fc fusion protein, which binds to and neutralizes the immunosuppressive and fibrosis-inducing cytokine, TGF-β [26].
Administered by intratumoral (IT) injection every 2 weeks at a dose of 1 × 1012 vps, AdAPT-001 is currently in Phase I/II study called BETA PRIME (NCT04673942) for patients with treatment-refractory, metastatic cancers both as monotherapy in Part 1, which is almost complete, and in combination with a checkpoint inhibitor in Part 2, which has not yet started. Preliminary data demonstrate that AdAPT-001 is not only well-tolerated but also active in TGFβ-driven tumors.
AdAPT-039 is a folate-targeted nanoparticle formulation of AdAPT-001 to bypass not only pre-existing neutralizing immunity but also the CAR receptor dependency of Ad cell entry. A Phase I trial with AdAPT-039 is scheduled to start shortly.
4. CG0070 (CG Oncology)
CG0070 is a conditionally replicating type 5 adenovirus that selectively replicates in retinoblastoma (Rb) pathway-defective bladder tumor cells. This highly modified virus carries the cancer-selective promoter E2F-1 in place of the wild-type adenovirus E1A promoter and the cytokine granulocyte macrophage colony stimulatory factor (GM-CSF) in place of the deleted E3 region [27]. CG0070 is only used in well confined intravesical bacillus Calmette-Guérin (BCG)-resistant non-muscle invasive bladder cancer (NMIBC), a highly curable tumor type that may nevertheless progress to muscle-invasive disease in the absence of effective treatment [28,29].
In a phase II trial of 66 BCG-unresponsive NMIBC patients that received intravesical CG0070, the 6-month CR was 47% (95% CI 32–62%), 58% in the carcinoma in situ (CIS) group, and 33% in the Ta/T1 group. Treatment was well tolerated. CG0070 is currently under investigation for 110 patients with BCG-unresponsive NMIBC as monotherapy in the phase III registration trial (BOND-003, NCT04452591). It is administered in a weekly induction course x 6 at a dose of 1 × 1012 vps, followed by a second weekly induction course at a dose of 1 × 1012 vps for non-responders, and a maintenance course of weekly x 3 at a dose of 1 × 1012 vps for completer responders. A phase II trial of CG0070 + pembrolizumab (CORE-001, NCT04387461) is also actively recruiting [30,31].
5. Enadenotucirev (EnAd) and NG-350A and NG-641 (Psioxus Therapeutics)
Enadenotucirev (EnAd), formerly ColoAd1, is the product of ‘directed evolution’, having been iteratively pooled and passaged to replicate only in cancer cells and is mostly administered intravenously [32]. EnAd has been investigated in several Phase I clinical trials, which collectively established the safety, tolerability, and pro-immunogenic effects of intravenous and intratumoral administration. These Phase I trials include NCT02028117 (OCTAVE) with EnAd plus paclitaxel in recurrent platinum-resistant ovarian cancer, NCT03916510 in rectal cancer with capecitabine and radiation, and NCT02636036 in solid tumors with the PD-1 inhibitor, nivolumab. However, the only results reported are for OCTAVE, in which enadenotucirev plus paclitaxel demonstrated manageable safety, an encouraging median PFS, and increased tumor immune-cell infiltration [33]. To augment immune responses, two EnAd variants are under investigation in phase I clinical trials: NG-350A (NCT03852511), which expresses a fully agonistic CD40 antibody, and NG-641 (NCT04053283), which quadrivalently expresses the bispecific T-cell engager (BiTE) FAP/CD3, chemokine ligands 9 and 10 (CXCL9 and CXCL10) and interferon alpha (IFNα) [34].
6. ONCOS-102 (Targovax)
ONCOS-102 is an oncolytic adenovirus with a fiber shaft and tail domain of HAdV-5 and a fiber knob domain of HAdV-3. The GM-CSF gene replaces the E3 6.7K/gp19K gene. Accordingly, transduction is mediated by the desmoglein 2 receptor instead of the often downregulated or deficient coxsackie and adenovirus receptor (CAR) to which the fiber from Ad5 binds [35]. The virus carries a deletion of 24 base pairs in the retinoblastoma (Rb) binding domain of the E1A region. Because of this deletion, ONCOS-102 selectively targets only those tumors with Rb protein pathway disruption [36]. A human GM-CSF transgene is inserted in the E1B19K gene region.
ONCOS-102 has completed several clinical trials. Of particular interest is NCT030036, a 20-patient pilot study of ONCOS-102 plus the PD-1 inhibitor, pembrolizumab, in PD-1 inhibitor-refractory melanoma, for which FDA fast track designation was awarded based on a 35% objective response rate (ORR), i.e., complete, or partial responses according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. No DLTs were observed, and the most common adverse events were chills and fever from Ad replication [37].
However, a phase I/II study of ONCOS-102 with durvalumab, an anti-PD-L1 antibody, for the treatment of advanced peritoneal malignancies (NCT02963831) did not meet its efficacy endpoints [38].
7. LoAd-703 (Lokon Pharma)
This is another heavily modified type 5 oncolytic adenovirus with an Ad35 fiber, a 24 base pair deletion in the retinoblastoma (Rb) binding domain of the E1A region, and a partial deletion in E3 to express two transgenes, both of which are under the control of a cytomegalovirus (CMV) promoter: 1) a trimerized (TMZ) form of the membrane-bound CD40 ligand (CD40L), which binds to CD40, a cell surface molecule on antigen-presenting cells, and 2) the ligand for the signaling domain 4-1BB (4-1BBL), which binds to the costimulatory receptor, 4-1BB. CD40/CD40L and 4-1BB/4-1BBL interactions are critical for the development antigen-specific cytotoxic CD8+ T-cell responses [39,40]. Provided that major histocompatibility complex (MHC) class I is present, the co-expression of CD40L and 4-1BBL on LoAd-703-infected tumor cells may synergistically contribute to reverse T cell anergy with checkpoint inhibitors and chemotherapies.
In Phase I/II unresectable or metastatic pancreatic cancer trial (NCT02705196), 18 evaluable patients, the majority of which were previously treated, received intratumoral injections of LoAd-703 plus intravenously delivered nab-paclitaxel and gemcitabine. The safety profile was manageable. The reported overall response rate (ORR) was 44%, the disease control rate (DCR) was 94%, and the median overall survival (OS) was 8.7 months, which compares favorably with an overall historical survival of 6.8 months in patients that receive nab-paclitaxel and gemcitabine [41]. The proportion of T effector memory cells significantly increased while the proportion of T regulatory cells and myeloid-derived suppressor cells significantly decreased. A follow-up clinical trial (NCT02705196), which combines LOAd-703, nab-paclitaxel, and gemcitabine, and the anti-PDL-1 inhibitor atezolizumab is ongoing [42]. Trials are also ongoing in checkpoint inhibitor refractory malignant melanoma with the PD-L1 inhibitor, atezolizumab, (NCT04123470) and in colorectal cancer in combination with atezolizumab (NCT03555149).
8. VCN-01 (Synthetic Biologics, Formerly VCN Biosciences)
VCN-01 is an E1A 24 bp-deleted (for selective replication in Rb deficient tumors), and partially E3 deleted type 5 oncolytic adenovirus that expresses hyaluronidase for degradation of the tumor extracellular matrix (ECM), which is especially prominent in pancreatic adenocarcinoma. To eliminate dependence on CAR binding, the capsid fiber incorporates an arginine glycine aspartate (RGD) integrin-binding motif. In a Phase I trial for patients with pancreatic adenocarcinoma that received intravenously administered VCN-01 nab-paclitaxel plus gemcitabine (NCT02045589), the safety profile was manageable, and viremia was observed as well as increased levels of immune biomarkers [43].
9. OBP-301 (Telomelysin) (Oncolys BioPharma)
OBP-301 is an ‘unarmed’ type 5 adenovirus in which the human telomerase reverse transcriptase (hTERT) promoter has been inserted upstream of the E1 genes to drive tumor-specific expression.
In a Phase I dose-escalation study of endoscopic intratumoral injection of 1010, 1011, and 1012 vp of OBP-301 (Telomelysin) with 60 Gy radiotherapy over 6 weeks in 13 esophageal cancer (NCT03213054) patients deemed unfit for standard treatments, the objective response rate was 91.7%, and the complete response rate was 83.3% in stage I and 60.0% in stage II/III concomitant with massive infiltration of CD8+ cells and increased PD-L1 expression [44]. This suggests clinical synergy with a checkpoint inhibitor.
A Phase II trial in combination with pembrolizumab in esophagogastric adenocarcinoma (NCT03921021) is ongoing.
10. DNX-2401 (Tasadenoturev) (DNAtrix—A Spin-Off of University of Texas MD Anderson Cancer Center)
DNX-2401 is an E1A 24 bp-deleted type 5 oncolytic adenovirus that selectively replicates in Rb-deficient tumors, and that incorporates an arginine glycine aspartate (RGD) integrin-binding motif, which mediates viral attachment and entry instead of CAR.
In a phase I trial (NCT00805376), 37 patients with recurrent malignant glioma received a single intratumoral injection of DNX-2401 over eight dose levels (group A; n = 25) or underwent intratumoral injection through a permanently implanted catheter, followed 14 days later by en bloc resection to acquire post-treatment specimens (group B; n = 12). In group A, 20% of patients survived > 3 years from treatment, and a ≥ 95% tumor reduction was observed in 3 patients, resulting in > 3 years of progression-free survival. Analyses of post-treatment surgical specimens documented direct virus-induced oncolysis and infiltration of CD8+ cells [45].
In another Phase I trial (NCT03178032), 12 newly diagnosed pediatric patients (3–12 years old) with diffuse intrinsic pontine glioma (DIPG), an untreatable and universally fatal brain tumor, received a single infusion of DNX-2401 through a catheter placed in the cerebellar peduncle at doses of 1 × 1010 (first four patients) or 5 × 1010 (next eight patients) viral particles (vp) followed by subsequent radiotherapy. Activity was demonstrated with a partial response in three patients and stable disease in eight patients. Median progression-free survival was 10.7 months. Median overall survival was 17.8 months. However, four treatment-related Grade 3 neurological adverse events occurred [46]. Based on these results, DNX-2401 has been granted FDA Fast Track and Orphan designation and EMA PRIME and Orphan designation.
In a Phase II study (NCT02798406) where 49 patients with recurrent glioblastoma (GBM) received 200 mg pembrolizumab every three weeks + a single intratumoral injection of DNX-2401, the median overall survival was 12.5 months, which compares favorably with the standard of care agents, lomustine and temozolomide, where the median overall survival is approximately7.2 months. The adverse event profile was manageable. A Phase 3 trial in recurrent GBM is reportedly planned [47].
11. Ad5-yCD/mutTKSR39rep-hIL12 (Henry Ford Health System)
Like ONYX-015 and H101, this is an E1B-55k-deleted Ad5 virus with an insertion of yeast cytosine deaminase (yCD), mutant herpes simplex thymidine kinaseSR39 (TKSR39), and human interleukin (IL)-12, which is under investigation in pancreatic and prostate cancer. yCD and TKSR39 convert the prodrugs 5-fluorocytosine (5-FC) and valganciclovir to their toxic forms in infected cells. In a Phase I trial, 12 patients with metastatic pancreatic cancer (T2N0M1-T4N1M1) received intratumoral injections of Ad5-yCD/mutTKSR39rep-hIL12 at escalating doses (1 × 1011, 3 × 1011, or 1 × 1012 viral particles) in combination with 5-fluorocytosine (5-FC) therapy for 7 days followed by chemotherapy (FOLFIRINOX or gemcitabine/albumin-bound paclitaxel (nab-paclitaxel)). The safety profile was acceptable; no MTD was reached, which is encouraging because systemic administration of IL-12 is associated with severe dose-limiting toxicities [48]. The median overall survival (OS) of the 6 patients that received Ad5-yCD/mutTKSR39rep-hIL12 at a dose of 1 × 1012 viral particles was 18.1 months, which exceeds the OS that is historically associated with FOLFIRINOX and gemcitabine + nab-paclitaxel of 11.1 and 6.8 months, respectively, although it is difficult to draw conclusions about efficacy based on such a small sample size [49].
12. CELYVIR (Hospital Infantil Universitario Niño Jesús Madrid, Spain)
CELYVIR is an intravenously administered formulation of autologous mesenchymal stem cells (MSCs) that carry ICOVIR-5, a heavily modified Ad5 dependent on an aberrant RB pathway, with a 24 base pair deletion, an RGD insertion, and an E2F-1 promoter insertion that failed to demonstrate activity in a Phase I melanoma trial [50].
In a Phase I pediatric trial (1–18 years) with advanced relapsed/refractory solid tumors, 15 patients, 9 of whom were evaluable, received Celyvir manufactured with MSCs collected from a bone marrow aspirate and then given IV weekly for 6 weeks at doses from 2 × 106 cells/Kg and 2 × 104 viral particles (vp) per cell. The safety profile was tolerable. Stable disease was reported in two patients with neuroblastoma, but no radiologic responses were seen [51].
A table, which summarizes these adenoviral clinical candidates is shown below (Table 1).

Table 1.
Examples of key design features and status of oncolytic adenoviral clinical candidates *.
13. Discussion and Conclusions
The use of immunotherapy to treat cancer is a highly en vogue topic with literally thousands of published articles on it and multiple clinical trials underway. Amid all the (understandably) enthusiastic claims about the “game-changing” properties of checkpoint inhibitors (CPIs) and the promise of total tumor eradication, which most recently occurred with the PD-1 inhibitor, dostarlimab, in 14 locally advanced mismatch repair (MMR) deficiency rectal cancer patients [52], success stories are limited to a small subset of the treatment population in an even smaller subset of proimmunogenic and genetically unstable tumors such as NSCLC, melanoma, and MMR deficient rectal cancer [53]. Rarer still are those patients with long-term durable remissions since acquired resistance after initial response to CPIs is the rule, not the exception [54]. Moreover, despite a series of positive trials with anti-PD-1/L1 checkpoint inhibitors, several other immunotherapies such as the anti-TIGIT agent, tiragolumab, the engineered interleukin-2, nemvaleukin alfa, and indoleamine 2,3-dioxygenase 1 inhibitors (IDO1) have failed [55]. Also, the combination of checkpoint inhibitors and targeted therapies, while promising, is still early stage and associated with complex and unexpected toxicities [56]. This is the thorniest challenge in oncology—how, and in combination with what exactly (exactly being the operative word), to overcome resistance to checkpoint inhibitors, and immunotherapy, in general, so that all patients durably benefit, regardless of tumor type and pre-existing immunogenicity of tumor type.
To date, no universal solution has presented itself with combinations that include other checkpoint inhibitors, cytokines, cytokine inhibitors, epigenetic inhibitors, adoptive cell transfer, antiangiogenics, bispecific T cell engagers, chemotherapies, radiotherapy, targeted therapies, antitumor vaccines, oncolytic viruses, etc. In this regard, the seminal cancer-immunity cycle (CIC) model proposed by Mellman and Chen may serve as a useful guidepost [57]. This CIC comprises seven stepwise events, which are: release of cancer antigens from damaged or dying tumor cells (step 1); antigen presentation by dendritic cells (step 2); priming phase (T cell activation) (step 3); trafficking or migration of cytotoxic T lymphocytes (CTLs) to the tumor (step 4); infiltration of cytotoxic T lymphocytes into tumor tissue (step 5); recognition of cancer antigens presented by the HLA class I molecules of tumor cells (step 6); effector phase (destruction of tumor cells) (step 7). An eighth step is proposed in this review: reversal of immunosuppression, given how deleterious the effects of immunosuppression are on tumor-specific immune responses.
As shown in the figure below (Figure 2), sine qua non conditions for response to checkpoint inhibitors are the activation and enrichment of CTLs at the tumor sites, according to steps 1–5, indicative of a “hot” TME, as well as reversal or removal of immunosuppression (step 8).
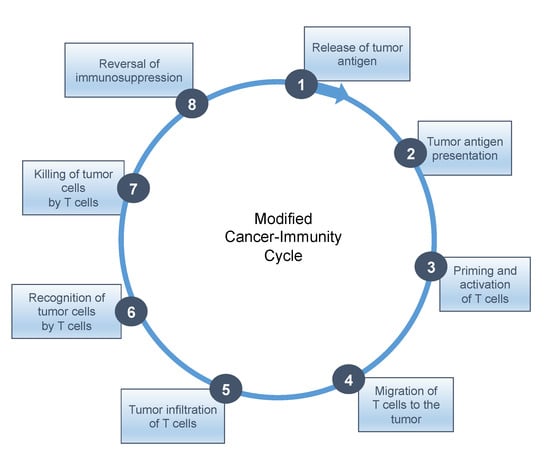
Figure 2.
Modified cancer immunity cycle with the addition of immunosuppression.
Of all the treatment modalities that directly lyse tumor cells, such as chemotherapy, radiotherapy, targeted therapy, and oncolytic viruses, potentially leading to the release of tumor antigens from dying tumor cells for presentation to and activation of T cells (steps 1, 2, 3), only oncolytic viruses are self-amplifying. The importance of self-amplification is that it eliminates the need to infect every tumor cell at the treatment time since thousands and thousands of progeny infectious viruses emerge during cell lysis in a self-sustaining loop. This releases an abundance of pathogenic viral DNA and proteins as potential immune adjuvants to draw in responding immune cells (steps 4 and 5) as well as highly immunogenic tumor-specific antigens (TSAs) or neoantigens that can elicit T cell responses. Moreover, genome replication also amplifies therapeutic transgene protein production in situ [23].
Nevertheless, despite these immunomodulatory properties that suggest near-perfect complementarity with checkpoint inhibitors, oncolytic viruses have failed to completely deliver on their promise as immune sensitizers par excellence, which has engendered a degree of skepticism and disillusionment [58]. To the extent that oncolytic viruses (OVs) have disappointed/fallen short of expectations regarding therapeutic efficacy, it must be acknowledged that third and fourth generation OVs, armed with different therapeutic transgenes, represent a substantial improvement over first-generation versions, which lack them.
That said, OVs are a tractable platform, which lends itself to rational design and the advancement of strategies to address the following key challenges: (i) intense immunosuppression in and around the tumors, which “turns off” infiltrating T cells, (ii) “overengineering” of the oncolytic viruses for better safety and tumor selectivity, which potentially comes at the expense of potency (iii) the insertion into viruses of paradoxically dichotomous transgenes like GM-CSF, IL-12, IL-2, and TNF-a, which are ostensibly strong immune adjuvants but which also have been linked to immunosuppression, and (iv) downregulation or absence of the specific cell surface viral receptor on tumor cells, mediating viral entry, which in the case of adenovirus type V is CAR [59,60,61,62]. Added to these limitations is the rapid neutralization of seroprevalent viruses like adenoviruses by pre-existing antibodies and memory T cells when delivered systemically, although a reversible association with blood cells may protect adenoviruses, in particular, from opsonizing immunity [63]; also, minimal viral clearance has been demonstrated with intra-arterial administration [64].
A potential workaround to several of these challenges, since it is difficult, if not impossible, to design one virus which “does it all” in the absence of significant attenuation and loss of potency, is the sequential administration of different therapies that individually target specific steps of the cancer immunity cycle.
As an example, illustrated below in Figure 3, to maximize oncolysis and release of tumor antigen with priming and activation of T cells for steps 1 and 2, a more minimally modified oncolytic adenovirus such as AdAPT-001, whose replication kinetics out of all the clinical candidates that have been presented in this review are probably the most like wild type Ad5, might be administered initially. An additional potential benefit of AdAPT-001/AdAPT-039 is its neutralization of the immunosuppressive cytokine, TGF-β, which contributes to rampant T cell dysfunction in the TME (step 8).
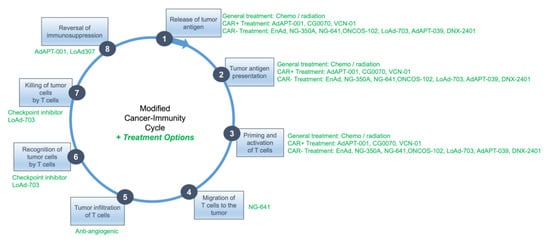
Figure 3.
Therapeutic options based on cancer-immunity cycle.
Strong downregulation or absence of the coxsackie and adenovirus receptor (CAR) might instead prompt the use of non-CAR dependent viruses such as AdAPT-039, ONCOS-102, Enadenotucirev (EnAd), NG-350A, NG-641 or LoAd-703. As NG-641 expresses the chemokines CXCL9 and CXCL 10, which mediate immune cell trafficking [65], and LoAd-307 encodes the costimulatory ligands CD40L and 4-1BBL, the use of these viruses may also potentiate steps 4 (NG-641) and 6 and 7 (LoAd-307).
The administration of these OAVs would be followed approximately one week later, after peak viral load has been achieved, with the administration of an antiangiogenic therapy to restore normal vessel function for better immune effector cell infiltration in step 5. Since the so-called “vascular normalization window” is transient, lasting between 3–5 days with most antiangiogenic agents, checkpoint inhibitors might be administered during this time to enhance T cell recognition and cytotoxicity for steps 6 and 7.
Different permutations of this approximately 3-week cycle are possible. For example, separate oncolytic adenoviruses may work well together in a prime-boost regimen or combination with radiotherapy, chemotherapy, or targeted therapy. Furthermore, since the vascular normalization window may vary from patient to patient, the use of imaging technologies such as MRI (DCE-MRI and BOLD-MRI), dynamic contrast-enhanced ultrasonography (DCE-US), computed tomography, and positron emission tomography (PET) and serum markers such as soluble VEGFR (sFlt1) may determine it more precisely on an individual basis [66].
In conclusion, since 2011, when the first checkpoint inhibitor, ipilimumab (Yervoy), was approved, the oncology profession has been in the midst of a cold war with cancer since most tumors outside of melanoma and NSCLC are immunologically cold or checkpoint inhibitor unresponsive. The fundamental challenge in oncology is how, and using what combinatorial strategy, to transition from a cold war to a hot one so that the 70–80% of patients with tumors, which are currently checkpoint inhibitor unresponsive, benefit from them. In this context, oncolytic adenoviruses, which were introduced in 1996 and which target more than one of the steps of the cancer-immunity cycle, may prime the immunological landscape for more robust, long-lasting checkpoint inhibitor-led responses, especially in combination with canonical cytotoxics, targeted therapies, antiangiogenics, radiation therapy, and possibly other oncolytic viruses.
Author Contributions
B.O. and T.R. conceived and originally wrote the manuscript. B.G., A.P.C., C.R., and S.C. significantly contributed to the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
B.O., S.C. and T.R. are employees of EpicentRx.
References
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Marchini, A.; Ilkow, C.S.; Melcher, A. Oncolytic Virus Immunotherapy. Cancers 2021, 13, 3672. [Google Scholar] [CrossRef] [PubMed]
- Bernstock, J.D.; Hoffman, S.E.; Chen, J.A.; Gupta, S.; Kappel, A.D.; Smith, T.R.; Chiocca, E.A. The current landscape of oncolytic herpes simplex viruses as novel therapies for brain malignancies. Viruses 2021, 13, 1158. [Google Scholar] [CrossRef] [PubMed]
- Donina, S.; Strele, I.; Proboka, G.; Auziņš, J.; Alberts, P.; Jonsson, B.; Venskus, D.; Muceniece, A. Adapted ECHO-7 virus Rigvir immunotherapy (oncolytic virotherapy) prolongs survival in melanoma patients after surgical excision of the tumour in a retrospective study. Melanoma Res. 2015, 25, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.; Silk, A.W.; Kane, M.P.; Kaufman, H.L. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J. ImmunoTherapy Cancer 2016, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.; Oronsky, B.; Scicinski, J.; Fanger, G.R.; Stirn, M.; Oronsky, A.; Reid, T.R. Going viral: A review of replication-selective oncolytic adenoviruses. Oncotarget 2015, 6, 19976–19989. [Google Scholar] [CrossRef]
- Ghebremedhin, B. Human adenovirus: Viral pathogen with increasing importance. Eur. J. Microbiol. Immunol. 2014, 4, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Charman, M.; Herrmann, C.; Weitzman, M.D. Viral and cellular interactions during adenovirus DNA replication. FEBS Lett. 2019, 593, 3531–3550. [Google Scholar] [CrossRef]
- Lu, W.; Zheng, S.; Li, X.F.; Huang, J.J.; Zheng, X.; Li, Z. Intra-tumor injection of H101, a recombinant adenovirus, in combination with chemotherapy in patients with advanced cancers: A pilot phase II clinical trial. World J. Gastroenterol. 2004, 10, 3634–3638. [Google Scholar] [CrossRef]
- Bischoff, J.R.; Kirn, D.H.; Williams, A.; Heise, C.; Horn, S.; Muna, M.; Ng, L.; Nye, J.A.; Sampson-Johannes, A.; Fattaey, A.; et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 1996, 274, 373–376. [Google Scholar] [CrossRef]
- Koscielny, S.; Tubiana, M. Parallel progression of tumour and metastases. Nat. Rev. Cancer 2010, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Larson, C.; Reid, T.R. Case Series: Abscopal Benefit of Surgery in 3 Immunotherapy-Treated Patients with Unresectable Cancer. J. Investig. Med. High Impact Case Rep. 2018, 6, 2324709618786319. [Google Scholar] [CrossRef] [PubMed]
- Mantwill, K.; Naumann, U.; Seznec, J.; Girbinger, V.; Lage, H.; Surowiak, P.; Beier, D.; Mittelbronn, M.; Schlegel, J.; Holm, P.S. YB-1 dependent oncolytic adenovirus efficiently inhibits tumor growth of glioma cancer stem like cells. J. Transl. Med. 2013, 11, 216. [Google Scholar] [CrossRef]
- Larson, C.; Oronsky, B.; Oronsky., A.; Knox, S.J.; Sher, D.; Reid, T.R. TGF-beta: A master immune regulator. Expert Opin. Ther. Targets 2020, 24, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Pearl, T.M.; Markert, J.M.; Cassady, K.A.; Ghonime, M.G. Oncolytic Virus-Based Cytokine Expression to Improve Immune Activity in Brain and Solid Tumors. Mol. Ther. Oncolytics 2019, 13, 14–21. [Google Scholar] [CrossRef]
- Tahtinen, S.; Kaikkonen, S.; Merisalo-Soikkeli, M.; Gronberg-Vaha-Koskela, S.; Kanerva, A.; Parviainen, S.; Vähä-Koskela, M.; Hemminki, A. Favorable alteration of tumor microenvironment by immunomodulatory cytokines for efficient T-cell therapy in solid tumors. PLoS ONE 2015, 10, e0131242. [Google Scholar] [CrossRef]
- Pol, J.G.; Caudana, P.; Paillet, J.; Piaggio, E.; Kroemer, G. Effects of interleukin-2 in immunostimulation and immunosuppression. J. Exp. Med. 2020, 217, e20191247. [Google Scholar] [CrossRef] [PubMed]
- Pistoia, V.; Raffaghello, L. Unveiling the role of TNF-α in mesenchymal stromal cell-mediated immunosuppression. Eur. J. Immunol. 2014, 44, 352–356. [Google Scholar] [CrossRef]
- McDonald, D.; Stockwin, L.; Matzow, T.; Zajdel, B.M.; Blair, G.E. Coxsackie and adenovirus receptor (CAR)-dependent and major histocompatibility complex (MHC) class I-independent uptake of recombinant adenoviruses into human tumour cells. Gene Ther. 1999, 6, 1512–1519. [Google Scholar] [CrossRef]
- Ungerechts, G.; Bossow, S.; Leuchs, B.; Holm, P.S.; Rommelaere, J.; Coffey, M.; Coffin, R.; Bell, J.; Nettelbeck, D.M. Moving oncolytic viruses into the clinic: Clinical-grade production, purification, and characterization of diverse oncolytic viruses. Methods Clin. Dev. 2016, 3, 16018. [Google Scholar] [CrossRef]
- Hedjran, F.; Shantanu, K.; Tony, R. Deletion analysis of Ad5 E1a transcriptional control region: Impact on tumor-selective expression of E1a and E1b. Cancer Gene Ther. 2011, 18, 717–723. [Google Scholar] [CrossRef]
- Larson, C.; Oronsky, B.; Varner, G.; Caroen, S.; Burbano, E.; Insel, E.; Hedjran, F.; Reid, T.R. A practical guide to the handling and administration of personalized transcriptionally attenuated oncolytic adenoviruses (PTAVs). Oncoimmunology 2018, 7, e1478648. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, M.; Reid, T.R.; Larson, C.; Oronsky, B.; Morris, J.C. Extended treatment with MY-NEOVAX, personalized neoantigen-enhanced oncolytic viruses, for two end-stage cancer patients. Oxf. Med. Case Rep. 2019, 2019, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.C.; Hallden, G.; Wang, Y.; Brooks, G.; Francis, J.; Lemoine, N.; Kirn, D. An E1B-19 kDa gene deletion mutant adenovirus demonstrates tumor necrosis factor-enhanced cancer selectivity and enhanced oncolytic potency. Mol. Ther. 2004, 9, 786–803. [Google Scholar] [CrossRef]
- Larson, C.; Oronsky, B.; Abrouk, N.E.; Oronsky, A.; Reid, T.R. Toxicology and biodistribution of AdAPT-001, a replication-competent type 5 adenovirus with a trap for the immunosuppressive cytokine, TGF-beta. Am. J. Cancer Res. 2021, 11, 5184–5189. [Google Scholar]
- Burke, J.M.; Lamm, D.L.; Meng, M.V.; Nemunaitis, J.J.; Stephenson, J.J.; Arseneau, J.C.; Aimi, J.; Lerner, S.; Yeung, A.W.; Kazarian, T.; et al. A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J. Urol. 2012, 188, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hallden, G.; Hill, R.; Anand, A.; Liu, T.C.; Francis, J.; Brooks, G.; Lemoine, N.; Kirn, D. E3 gene manipulations affect oncolytic adenovirus activity in immunocompetent tumor models. Nat. Biotechnol. 2003, 21, 1328–1335. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. Non-muscle-invasive Bladder Cancer (TaT1 and CIS) EAU Guidelines. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef]
- Li, R.; Steinberg, G.D.; Uchio, E.M.; Lamm, D.L.; Shah, P.; Kamat, A.M.; Bivalacqua, T.; Packiam, V.T.; Chisamore, M.J.; McAdory, J.; et al. CORE1: Phase 2, single arm study of CG0070 combined with pembrolizumab in patients with non-muscle invasive bladder cancer (NMIBC) unresponsive to Bacillus Calmette-Guerin (BCG). In Proceedings of the AACR Annual Meeting 2022, New Orleans, LA, USA, 8–13 April 2022. Abstract CT036. [Google Scholar]
- Packiam, V.T.; Lamm, D.L.; Barocas, D.A.; Trainer, A.; Fand, B.; Davis, R.L., III; Clark, W.; Kroeger, M.; Dumbadze, I.; Chamie, K.; et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol. Oncol. 2018, 36, 440–447. [Google Scholar] [CrossRef]
- Kuhn, I.; Harden, P.; Bauzon, M.; Chartier, C.; Nye, J.; Thorne, S.; Reid, T.; Ni, S.; Lieber, A.; Fisher, K.; et al. Directed evolution generates a novel oncolytic virus for the treatment of colon cancer. PLoS ONE 2008, 3, e2409. [Google Scholar] [CrossRef]
- Moreno, V.; Barretina-Ginesta, M.P.; García-Donas, J.; Jayson, G.C.; Roxburgh, P.; Vázquez, R.M.; Michael, A.; Antón-Torres, A.; Brown, R.; Krige, D.; et al. Safety and efficacy of the tumor-selective adenovirus enadenotucirev with or without paclitaxel in platinum-resistant ovarian cancer: A phase 1 clinical trial. J. Immunother. Cancer 2021, 9, e003645. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, O.; Dos Santos, J.M.; Hemminki, A. Oncolytic viruses for cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Kuryk, L.; Møller, A.W.; Jaderberg, M. Combination of immunogenic oncolytic adenovirus ONCOS-102 with anti-PD-1 pembrolizumab exhibits synergistic antitumor effect in humanized A2058 melanoma huNOG mouse model. Oncoimmunology 2018, 8, e1532763. [Google Scholar] [CrossRef] [PubMed]
- Kuryk, L.; Rodella, G.; Staniszewska, M.; Pancer, K.W.; Wieczorek, M.; Salmaso, S.; Caliceti, P.; Garofalo, M. Novel Insights into Mesothelioma Therapy: Emerging Avenues and Future Prospects. Front. Oncol. 2022, 12, 916839. [Google Scholar] [CrossRef] [PubMed]
- Ranki, T.; Pesonen, S.; Hemminki, A.; Partanen, K.; Kairemo, K.; Alanko, T.; Lundin, J.; Linder, N.; Turkki, R.; Ristimäki, A.; et al. Phase I study with ONCOS-102 for the treatment of solid tumors–an evaluation of clinical response and exploratory analyses of immune markers. J. Immunother. Cancer 2016, 4, 17. [Google Scholar] [CrossRef]
- Zamarin, D.; Odunsi, K.; Zsiros, E.; Slomovitz, B.M.; Pimentel, A.; Duska, L.R.; Reilley, M.; Nemunaitis, J.J.; Hamouda, D.M.; Patel, H.; et al. Study to evaluate intraperitoneal (IP) ONCOS-102 with systemic durvalumab in patients with peritoneal disease who have epithelial ovarian (OC) or metastatic colorectal cancer (CRC): Phase 2 results. J. Clin. Oncol. 2022, 40 (Suppl. S16), 2600. [Google Scholar] [CrossRef]
- Cella, M.; Scheidegger, D.; Palmer-Lehmann, K.; Lane, P.; Lanzavecchia, A.; Alber, G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 1996, 184, 747–752. [Google Scholar] [CrossRef]
- Maher, J.; Davies, E.T. Targeting cytotoxic T lymphocytes for cancer immunotherapy. Br. J. Cancer 2004, 91, 817–821. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Musher, B.L.; Smaglo, B.G.; Abidi, W.; Othman, M.; Patel, K.; Jawaid, S.; Jing, J.; Brisco, A.; Wenthe, J.; Eriksson, E.; et al. A phase I/II study of LOAd703, a TMZ-CD40L/4-1BBL-armed oncolytic adenovirus, combined with nab-paclitaxel and gemcitabine in advanced pancreatic cancer. J. Clin. Oncol. 2022, 40 (Suppl. S16), 4138. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Bazan-Peregrino, M.; Gil-Martín, M.; Álvarez, R.; Macarulla, T.; Riesco-Martinez, M.C.; Verdaguer, H.; Guillén-Ponce, C.; Farrera-Sal, M.; Moreno, R.; et al. Phase I, multicenter, open-label study of intravenous VCN-01 oncolytic adenovirus with or without nab-paclitaxel plus gemcitabine in patients with advanced solid tumors. J. Immunother. Cancer 2022, 10, e003255. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, Y.; Tazawa, H.; Tanabe, S.; Kanaya, N.; Noma, K.; Koujima, T.; Kashima, H.; Kato, T.; Kuroda, S.; Kikuchi, S.; et al. Phase I dose-escalation study of endoscopic intratumoral injection of OBP-301 (Telomelysin) with radiotherapy in oesophageal cancer patients unfit for standard treatments. Eur. J. Cancer 2021, 153, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Larraya, J.G.; Garcia-Moure, M.; Labiano, S.; Patiño-García, A.; Dobbs, J.; Gonzalez-Huarriz, M.; Zalacain, M.; Marrodan, L.; Martinez-Velez, N.; Puigdelloses, M.; et al. Oncolytic DNX-2401 Virus for Pediatric Diffuse Intrinsic Pontine Glioma. N. Engl. J. Med. 2022, 386, 2471–2481. [Google Scholar] [CrossRef]
- Zadeh, G.; Daras, M.; Cloughesy, T.F.; Colman, H.; Kumthekar, P.U.; Chen, C.C.; Aiken, R.; Groves, M.D.; Ong, S.; Ramakrishna, R.; et al. Phase 2 Multicenter Study of the Oncolytic Adenovirus DNX-2401 (Tasadenoturev) in Combination with Pembrolizumab for Recurrent Glioblastoma; Captive Study (Keynote-192). Neuro-Oncol. 2022, 22, ii237. [Google Scholar] [CrossRef]
- Lee, J.C.; Shin, D.W.; Park, H.; Kim, J.; Youn, Y.; Kim, J.H.; Kim, J.; Hwang, J.H. Tolerability and safety of EUS-injected adenovirus-mediated double-suicide gene therapy with chemotherapy in locally advanced pancreatic cancer: A phase 1 trial. Orig. Artic. Clin. Endosc. 2020, 92, 1044–1052.e1. [Google Scholar] [CrossRef]
- Barton, K.N.; Siddiqui, F.; Pompa, R.; Freytag, S.O.; Khan, G.; Dobrosotskaya, I.; Ajlouni, M.; Zhang, Y.; Cheng, J.; Movsas, B.; et al. Phase I trial of oncolytic adenovirus-mediated cytotoxic and interleukin-12 gene therapy for the treatment of metastatic pancreatic cancer. Mol. Ther. Oncolytics. 2020, 20, 94–104. [Google Scholar] [CrossRef]
- García, M.; Moreno, R.; Gil-Martin, M.; Cascallò, M.; de Olza, M.O.; Cuadra, C.; Piulats, J.M.; Navarro, V.; Domenech, M.; Alemany, R.; et al. A Phase 1 Trial of Oncolytic Adenovirus ICOVIR-5 Administered Intravenously to Cutaneous and Uveal Melanoma Patients. Hum. Gene Ther. 2019, 30, 352–364. [Google Scholar] [CrossRef]
- Ramirez, M.; Ruano, D.; Moreno, L.; Lassaletta, A.; Sirvent, F.J.B.; Andión, M.; Hernández, C.; González-Murillo, A.; Melen, G.; Alemany, R.; et al. First-in-child trial of celyvir (autologous mesenchymal stem cells carrying the oncolytic virus ICOVIR-5) in patients with relapsed and refractory pediatric solid tumors. J. Clin. Oncol. 2018, 36 (Suppl. S15), 10543. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; Dika, I.H.E.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Li, X.; Shao, C.; Shi, Y.; Han, W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J. Hematol. Oncol. 2018, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.J.; Hellmann, M.D. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell. 2020, 37, 443–455. [Google Scholar] [CrossRef]
- Corke, L.; Sacher, A. New Strategies and Combinations to Improve Outcomes in Immunotherapy in Metastatic Non-Small-Cell Lung Cancer. Curr. Oncol. 2021, 29, 38–55. [Google Scholar] [CrossRef]
- Prieto, P.A.; Reuben, A.; Cooper, Z.A.; Wargo, J.A. Targeted Therapies Combined with Immune Checkpoint Therapy. Cancer J. 2016, 22, 138–146. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sze, D.Y.; Reid, T.R.; Rose, S.C. Oncolytic virotherapy. J. Vasc. Interv. Radiol. 2013, 24, 1115–1122. [Google Scholar] [CrossRef]
- Parmiani, G.; Castelli, C.; Pilla, L.; Santinami, M.; Colombo, M.P.; Rivoltini, L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann. Oncol. 2007, 18, 226–232. [Google Scholar] [CrossRef]
- Montfort, A.; Colacios, C.; Levade, T.; Andrieu-Abadie, N.; Meyer, N.; Ségui, B. The TNF Paradox in Cancer Progression and Immunotherapy. Front. Immunol. 2019, 10, 1818. [Google Scholar] [CrossRef]
- Hensen, L.C.M.; Hoeben, R.C.; Bots, S.T.F. Adenovirus Receptor Expression in Cancer and Its Multifaceted Role in Oncolytic Adenovirus Therapy. Int. J. Mol. Sci. 2020, 21, 6828. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Oxenius, A. Interleukin 2: From immunostimulation to immunoregulation and back again. EMBO Rep. 2007, 8, 1142–1148. [Google Scholar] [CrossRef]
- Zafar, S.; Quixabeira, D.; Kudling, T.V.; Cervera-Carrascon, V.; Santos, J.M.; Grönberg-Vähä-Koskela, S.; Zhao, F.; Aronen, P.; Heiniö, C.; Havunen, R.; et al. Ad5/3 is able to avoid neutralization by binding to erythrocytes and lymphocytes. Cancer Gene Ther. 2021, 28, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Reid, T.; Galanis, E.; Abbruzzese, J.; Sze, D.; Wein, L.M.; Andrews, J.; Randlev, B.; Heise, C.; Uprichard, M.; Hatfield, M.; et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): Phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002, 62, 6070–6079. [Google Scholar] [PubMed]
- Märkl, F.; Huynh, D.; Endres, S.; Kobold, S. Utilizing chemokines in cancer immunotherapy. Trends Cancer. 2022, 8, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xiao, H.; Liu, X.; Wang, Z.; Zhang, Q.; Wei, N.; Guo, X. Vascular Normalization: A New Window Opened for Cancer Therapies. Front. Oncol. 2021, 11, 719836. [Google Scholar] [CrossRef]
- Opyrchal, M.; Aderca, I.; Galanis, E. Phase I clinical trial of locoregional administration of the oncolytic adenovirus ONYX-015 in combination with mitomycin-C, doxorubicin, and cisplatin chemotherapy in patients with advanced sarcomas. In Methods in Molecular Biology; Springer: New York, NY, USA, 2009; Volume 542, pp. 705–717. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).