Checkpoint Inhibitors in Cancer Therapy: Clinical Benefits for Head and Neck Cancers
Abstract
Simple Summary
Abstract
1. Introduction
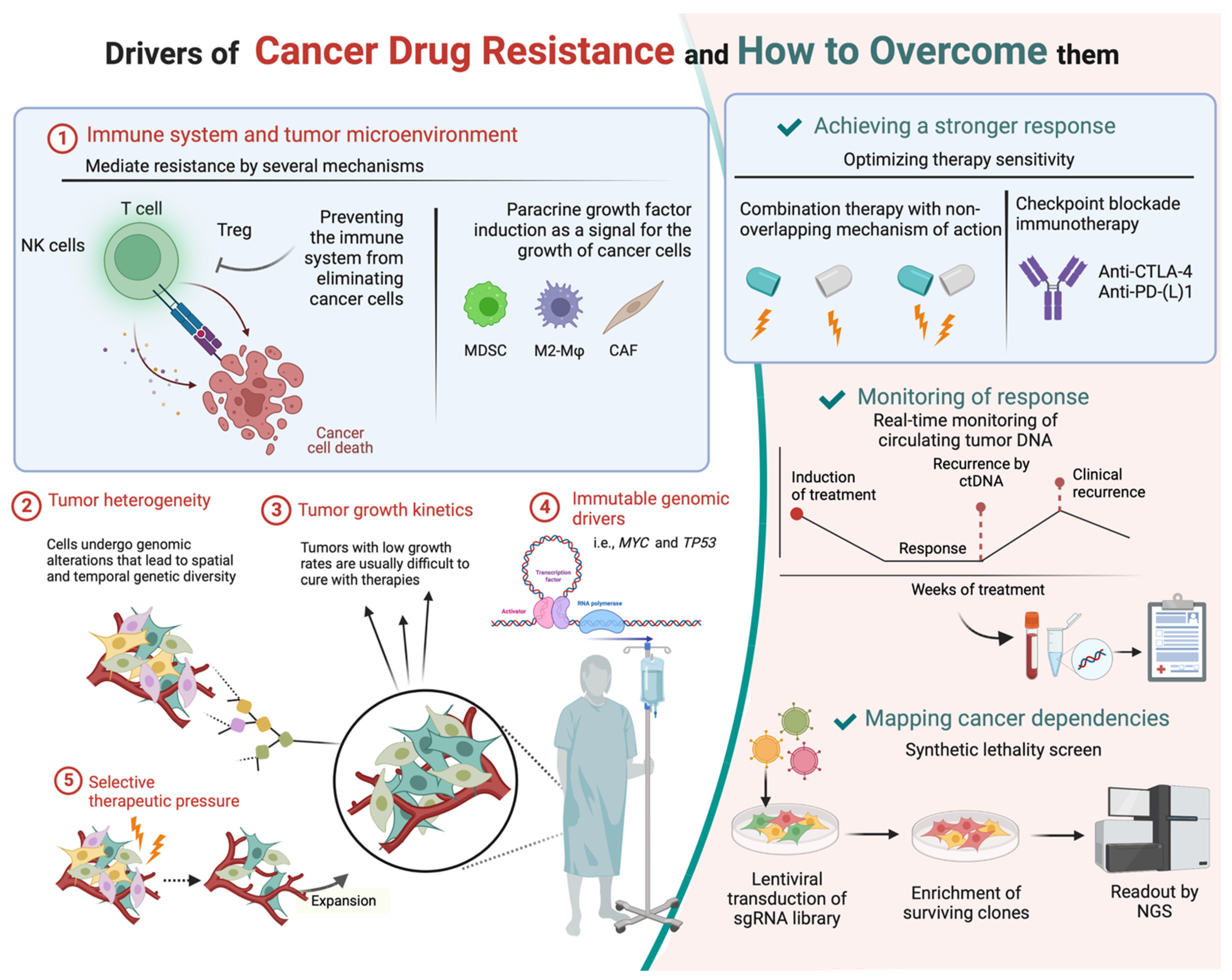
2. Drug Resistance
3. HNSCC Tumor Microenvironment (TME)
4. Development towards ICI Combination Therapy
5. Finding Biomarkers
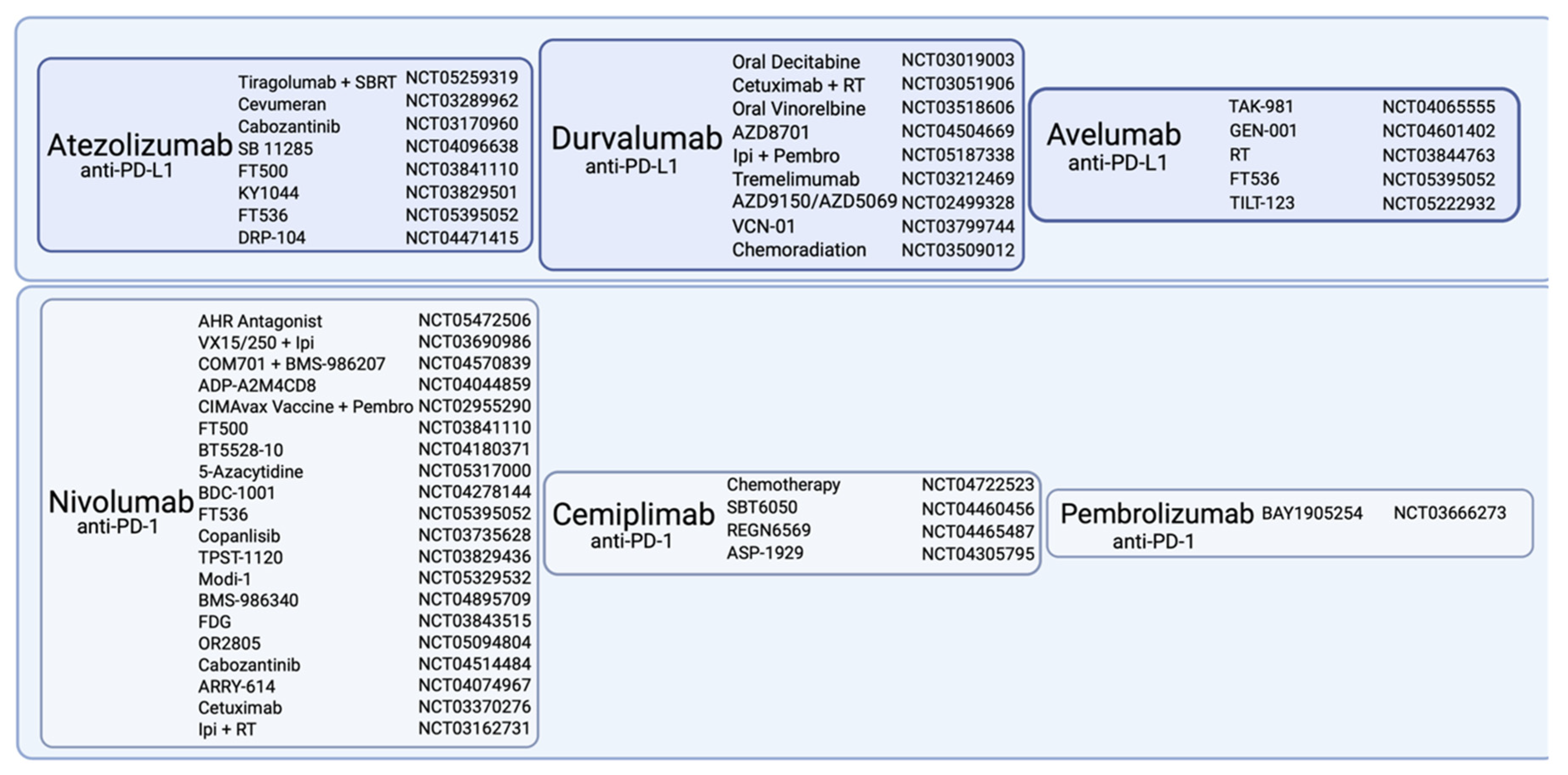
6. Immune Checkpoint Inhibitors
6.1. CTLA-4 Inhibitors
6.2. PD-1 Inhibitors
6.3. PD-L1 Inhibitors
7. Checkpoint Regulators and Combination Therapy
7.1. The Tumor Microenvironment: Cellular Mechanisms which Inhibit T cell Functions
7.2. Strengthening T cell Defense
7.2.1. Costimulatory Agents
7.2.2. T Cell Exhaustion
7.2.3. B7-H3
7.2.4. NKG2A
7.2.5. TLR9
7.2.6. Cellular Therapy
7.3. ICI Combination Therapy against Mechanisms which Inhibit T Cell Functions
8. Combination with Other Immunomodulators
8.1. CXCR2
8.2. STAT3
8.3. EGFR
8.4. VEGF
8.5. PDE5
8.6. SMO
8.7. Aurora Kinase A (AURKA)
8.8. PARP
8.9. EZH2
8.10. PPAR-α
8.11. PTPN2
8.12. TGF-β
8.13. Vaccines Based on Peptide–Protein
8.14. Vaccines Based on Nucleic Acids
9. Common Clinically Applied ICIs of PD-1/PD-L1 Axis and Combination Therapy in Early Clinical Phases
10. Immunotherapy Combined with Chemoradiotherapy
11. Checkpoint Inhibition in Combination with Viral Therapy
12. Side Effects
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Lassen, P.; Eriksen, J.G.; Hamilton-Dutoit, S.; Tramm, T.; Alsner, J.; Overgaard, J. Effect of HPV-Associated P16INK4A Expression on Response to Radiotherapy and Survival in Squamous Cell Carcinoma of the Head and Neck. J. Clin. Oncol. 2009, 27, 1992–1998. [Google Scholar] [CrossRef]
- Sivars, L.; Tani, E.; Näsman, A.; Ramqvist, T.; Munck-Wikland, E.; Dalianis, T. Human Papillomavirus as a Diagnostic and Prognostic Tool in Cancer of Unknown Primary in the Head and Neck Region. Anticancer Res. 2016, 36, 487–493. [Google Scholar]
- Turunen, A.; Rautava, J.; Grénman, R.; Syrjänen, K.; Syrjänen, S. Epstein-Barr Virus (EBV)-Encoded Small RNAs (EBERs) Associated with Poor Prognosis of Head and Neck Carcinomas. Oncotarget 2017, 8, 27328–27338. [Google Scholar] [CrossRef]
- Adelstein, D.; Gillison, M.L.; Pfister, D.G.; Spencer, S.; Adkins, D.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 2.2017. J. Natl. Compr. Canc. Netw. 2017, 15, 761–770. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and Neck Cancer: Changing Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2008, 83, 489–501. [Google Scholar] [CrossRef]
- Xie, X.; O’Neill, W.; Pan, Q. Immunotherapy for Head and Neck Cancer: The Future of Treatment? Expert Opin. Biol. Ther. 2017, 17, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef]
- León, X.; Hitt, R.; Constenla, M.; Rocca, A.; Stupp, R.; Kovács, A.F.; Amellal, N.; Bessa, E.H.; Bourhis, J. A Retrospective Analysis of the Outcome of Patients with Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck Refractory to a Platinum-Based Chemotherapy. Clin. Oncol. (R Coll. Radiol.) 2005, 17, 418–424. [Google Scholar] [CrossRef]
- DeVita, V.T.; Simon, R.M.; Hubbard, S.M.; Young, R.C.; Berard, C.W.; Moxley, J.H.; Frei, E.; Carbone, P.P.; Canellos, G.P. Curability of Advanced Hodgkin’s Disease with Chemotherapy. Long-Term Follow-up of MOPP-Treated Patients at the National Cancer Institute. Ann. Intern. Med. 1980, 92, 587–595. [Google Scholar] [CrossRef]
- Bonadonna, G.; Brusamolino, E.; Valagussa, P.; Rossi, A.; Brugnatelli, L.; Brambilla, C.; De Lena, M.; Tancini, G.; Bajetta, E.; Musumeci, R.; et al. Combination Chemotherapy as an Adjuvant Treatment in Operable Breast Cancer. N. Engl. J. Med. 1976, 294, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Bosl, G.J.; Gluckman, R.; Geller, N.L.; Golbey, R.B.; Whitmore, W.F.; Herr, H.; Sogani, P.; Morse, M.; Martini, N.; Bains, M. VAB-6: An Effective Chemotherapy Regimen for Patients with Germ-Cell Tumors. J. Clin. Oncol. 1986, 4, 1493–1499. [Google Scholar] [CrossRef]
- Citron, M.L.; Berry, D.A.; Cirrincione, C.; Hudis, C.; Winer, E.P.; Gradishar, W.J.; Davidson, N.E.; Martino, S.; Livingston, R.; Ingle, J.N.; et al. Randomized Trial of Dose-Dense versus Conventionally Scheduled and Sequential versus Concurrent Combination Chemotherapy as Postoperative Adjuvant Treatment of Node-Positive Primary Breast Cancer: First Report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J. Clin. Oncol. 2003, 21, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.N.; de Mulder, P.H.; Schornagel, J.H.; Théodore, C.; Fossa, S.D.; van Oosterom, A.T.; Witjes, F.; Spina, M.; van Groeningen, C.J.; de Balincourt, C.; et al. Randomized Phase III Trial of High-Dose-Intensity Methotrexate, Vinblastine, Doxorubicin, and Cisplatin (MVAC) Chemotherapy and Recombinant Human Granulocyte Colony-Stimulating Factor versus Classic MVAC in Advanced Urothelial Tract Tumors: European Organization for Research and Treatment of Cancer Protocol No. 30924. J. Clin. Oncol. 2001, 19, 2638–2646. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer Immunology. Mutational Landscape Determines Sensitivity to PD-1 Blockade in Non-Small Cell Lung Cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; et al. Association of Tumour Mutational Burden with Outcomes in Patients with Advanced Solid Tumours Treated with Pembrolizumab: Prospective Biomarker Analysis of the Multicohort, Open-Label, Phase 2 KEYNOTE-158 Study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Soysal, S.D.; Tzankov, A.; Muenst, S.E. Role of the Tumor Microenvironment in Breast Cancer. Pathobiology 2015, 82, 142–152. [Google Scholar] [CrossRef]
- Wu, T.; Chen, Z.; To, K.K.W.; Fang, X.; Wang, F.; Cheng, B.; Fu, L. Effect of Abemaciclib (LY2835219) on Enhancement of Chemotherapeutic Agents in ABCB1 and ABCG2 Overexpressing Cells in Vitro and in Vivo. Biochem. Pharm. 2017, 124, 29–42. [Google Scholar] [CrossRef]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef] [PubMed]
- Giancotti, F.G. Deregulation of Cell Signaling in Cancer. FEBS Lett. 2014, 588, 2558–2570. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2020, 56, 15. [Google Scholar] [CrossRef]
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.-W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The Head and Neck Cancer Immune Landscape and Its Immunotherapeutic Implications. JCI Insight 2016, 1, e89829. [Google Scholar] [CrossRef]
- Miyara, M.; Sakaguchi, S. Natural Regulatory T Cells: Mechanisms of Suppression. Trends Mol. Med. 2007, 13, 108–116. [Google Scholar] [CrossRef]
- Freudlsperger, C.; Bian, Y.; Contag Wise, S.; Burnett, J.; Coupar, J.; Yang, X.; Chen, Z.; Van Waes, C. TGF-β and NF-ΚB Signal Pathway Cross-Talk Is Mediated through TAK1 and SMAD7 in a Subset of Head and Neck Cancers. Oncogene 2013, 32, 1549–1559. [Google Scholar] [CrossRef]
- Yao, S.; Zhu, Y.; Chen, L. Advances in Targeting Cell Surface Signalling Molecules for Immune Modulation. Nat. Rev. Drug Discov. 2013, 12, 130–146. [Google Scholar] [CrossRef]
- Ferris, R.L. Immunology and Immunotherapy of Head and Neck Cancer. J. Clin. Oncol. 2015, 33, 3293–3304. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef]
- Cristina, V.; Herrera-Gómez, R.G.; Szturz, P.; Espeli, V.; Siano, M. Immunotherapies and Future Combination Strategies for Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 5399. [Google Scholar] [CrossRef]
- Wessely, A.; Waltera, A.; Reichert, T.E.; Stöckl, S.; Grässel, S.; Bauer, R.J. Induction of ALP and MMP9 Activity Facilitates Invasive Behavior in Heterogeneous Human BMSC and HNSCC 3D Spheroids. FASEB J. 2019, 33, 11884–11893. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, A.; Narine, K.; De Neve, W.; Mareel, M.; Bracke, M.; De Wever, O. Resident and Bone Marrow-Derived Mesenchymal Stem Cells in Head and Neck Squamous Cell Carcinoma. Oral Oncol. 2010, 46, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Koontongkaew, S. The Tumor Microenvironment Contribution to Development, Growth, Invasion and Metastasis of Head and Neck Squamous Cell Carcinomas. J. Cancer 2013, 4, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Liotta, F.; Querci, V.; Mannelli, G.; Santarlasci, V.; Maggi, L.; Capone, M.; Rossi, M.C.; Mazzoni, A.; Cosmi, L.; Romagnani, S.; et al. Mesenchymal Stem Cells Are Enriched in Head Neck Squamous Cell Carcinoma, Correlates with Tumour Size and Inhibit T-Cell Proliferation. Br. J. Cancer 2015, 112, 745–754. [Google Scholar] [CrossRef]
- Liu, C.; Billet, S.; Choudhury, D.; Cheng, R.; Haldar, S.; Fernandez, A.; Biondi, S.; Liu, Z.; Zhou, H.; Bhowmick, N.A. Bone Marrow Mesenchymal Stem Cells Interact with Head and Neck Squamous Cell Carcinoma Cells to Promote Cancer Progression and Drug Resistance. Neoplasia 2021, 23, 118–128. [Google Scholar] [CrossRef]
- Peltanova, B.; Raudenska, M.; Masarik, M. Effect of Tumor Microenvironment on Pathogenesis of the Head and Neck Squamous Cell Carcinoma: A Systematic Review. Mol. Cancer 2019, 18, 63. [Google Scholar] [CrossRef]
- Rowan, B.G.; Lacayo, E.A.; Sheng, M.; Anbalagan, M.; Gimble, J.M.; Jones, R.K.; Joseph, W.J.; Friedlander, P.L.; Chiu, E.S. Human Adipose Tissue-Derived Stromal/Stem Cells Promote Migration and Early Metastasis of Head and Neck Cancer Xenografts. Aesthet. Surg. J. 2016, 36, 93–104. [Google Scholar] [CrossRef]
- Scherzed, A.; Hackenberg, S.; Froelich, K.; Kessler, M.; Koehler, C.; Hagen, R.; Radeloff, A.; Friehs, G.; Kleinsasser, N. BMSC Enhance the Survival of Paclitaxel Treated Squamous Cell Carcinoma Cells in Vitro. Cancer Biol. Ther. 2011, 11, 349–357. [Google Scholar] [CrossRef]
- Zhang, W.; Matrisian, L.M.; Holmbeck, K.; Vick, C.C.; Rosenthal, E.L. Fibroblast-Derived MT1-MMP Promotes Tumor Progression in Vitro and in Vivo. BMC Cancer 2006, 6, 52. [Google Scholar] [CrossRef][Green Version]
- Corrales, L.; Ajona, D.; Rafail, S.; Lasarte, J.J.; Riezu-Boj, J.I.; Lambris, J.D.; Rouzaut, A.; Pajares, M.J.; Montuenga, L.M.; Pio, R. Anaphylatoxin C5a Creates a Favorable Microenvironment for Lung Cancer Progression. J. Immunol. 2012, 189, 4674–4683. [Google Scholar] [CrossRef]
- Fang, J.; Li, X.; Ma, D.; Liu, X.; Chen, Y.; Wang, Y.; Lui, V.W.Y.; Xia, J.; Cheng, B.; Wang, Z. Prognostic Significance of Tumor Infiltrating Immune Cells in Oral Squamous Cell Carcinoma. BMC Cancer 2017, 17, 375. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Bellile, E.; Thomas, D.; McHugh, J.; Rozek, L.; Virani, S.; Peterson, L.; Carey, T.E.; Walline, H.; Moyer, J.; et al. Tumor Infiltrating Lymphocytes and Survival in Patients with Head and Neck Squamous Cell Carcinoma. Head Neck 2016, 38, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Pio, R.; Ajona, D.; Lambris, J.D. Complement Inhibition: A Promising Concept for Cancer Treatment. Semin Immunol. 2013, 25, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Quezada, S.A.; Peggs, K.S.; Simpson, T.R.; Allison, J.P. Shifting the Equilibrium in Cancer Immunoediting: From Tumor Tolerance to Eradication. Immunol. Rev. 2011, 241, 104–118. [Google Scholar] [CrossRef]
- De Costa, A.-M.A.; Schuyler, C.A.; Walker, D.D.; Young, M.R.I. Characterization of the Evolution of Immune Phenotype during the Development and Progression of Squamous Cell Carcinoma of the Head and Neck. Cancer Immunol. Immunother. 2012, 61, 927–939. [Google Scholar] [CrossRef]
- Morisada, M.; Clavijo, P.E.; Moore, E.; Sun, L.; Chamberlin, M.; Van Waes, C.; Hodge, J.W.; Mitchell, J.B.; Friedman, J.; Allen, C.T. PD-1 Blockade Reverses Adaptive Immune Resistance Induced by High-Dose Hypofractionated but Not Low-Dose Daily Fractionated Radiation. Oncoimmunology 2018, 7, e1395996. [Google Scholar] [CrossRef]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Targeting the PD-1/B7-H1(PD-L1) Pathway to Activate Anti-Tumor Immunity. Curr. Opin. Immunol. 2012, 24, 207–212. [Google Scholar] [CrossRef]
- Farkona, S.; Diamandis, E.P.; Blasutig, I.M. Cancer Immunotherapy: The Beginning of the End of Cancer? BMC Med. 2016, 14, 73. [Google Scholar] [CrossRef]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Pauken, K.E. The Diverse Functions of the PD1 Inhibitory Pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 Inhibitors as a Form of Cancer Immunotherapy: A Comprehensive Review of Registration Trials and Future Considerations. J. Immunother. Cancer 2018, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab Alone or with Chemotherapy versus Cetuximab with Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-048): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Argiris, A.; Gillison, M.; Ferris, R.L.; Harrington, K.; Sanchez, T.K.; Baudelet, C.; Geese, W.J.; Shaw, J.; Haddad, R. A Randomized, Open-Label, Phase 3 Study of Nivolumab in Combination with Ipilimumab vs Extreme Regimen (Cetuximab + Cisplatin/Carboplatin + Fluorouracil) as First-Line Therapy in Patients with Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck-CheckMate 651. Ann. Oncol. 2016, 27, vi350. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Weiss, J.; Baxi, S.S.; Ahn, M.-J.; Fayette, J.; Gillison, M.L.; Machiels, J.-P.H.; Takahashi, S.; Melillo, G.; Franks, A.; et al. A Phase 3, Randomized, Open-Label Study of First-Line Durvalumab (MEDI4736) ± Tremelimumab versus Standard of Care (SoC.; EXTREME Regimen) in Recurrent/Metastatic (R/M) SCCHN: KESTREL. J. Clin. Oncol. 2016, 34, TPS6101. [Google Scholar] [CrossRef]
- Licitra, L.F.; Haddad, R.I.; Even, C.; Tahara, M.; Dvorkin, M.; Ciuleanu, T.-E.; Clement, P.M.; Mesia, R.; Kutukova, S.I.; Zholudeva, L.; et al. EAGLE: A Phase 3, Randomized, Open-Label Study of Durvalumab (D) with or without Tremelimumab (T) in Patients (Pts) with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma (R/M HNSCC). J. Clin. Oncol. 2019, 37, 6012. [Google Scholar] [CrossRef]
- Ferris, R.L.; Haddad, R.; Even, C.; Tahara, M.; Dvorkin, M.; Ciuleanu, T.E.; Clement, P.M.; Mesia, R.; Kutukova, S.; Zholudeva, L.; et al. Durvalumab with or without Tremelimumab in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: EAGLE, a Randomized, Open-Label Phase III Study. Ann. Oncol. 2020, 31, 942–950. [Google Scholar] [CrossRef]
- Tao, Y.; Aupérin, A.; Sun, X.; Sire, C.; Martin, L.; Coutte, A.; Lafond, C.; Miroir, J.; Liem, X.; Rolland, F.; et al. Avelumab-Cetuximab-Radiotherapy versus Standards of Care in Locally Advanced Squamous-Cell Carcinoma of the Head and Neck: The Safety Phase of a Randomised Phase III Trial GORTEC 2017-01 (REACH). Eur. J. Cancer 2020, 141, 21–29. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ferris, R.L.; Beck, J.T.; Harrington, K.; Haddad, R.I.; Bourhis, J.; Tahara, M.; Geraldes, M.; Nuyten, D.S.A.; Goldberg, Z.; et al. JAVELIN Head and Neck 100: A Phase 3 Trial of Avelumab in Combination with Chemoradiotherapy (CRT) vs CRT for 1st-Line Treatment of Locally Advanced Squamous Cell Carcinoma of the Head and Neck (LA SCCHN). J. Clin. Oncol. 2017, 35, TPS6093. [Google Scholar] [CrossRef]
- Machiels, J.-P.; Tao, Y.; Burtness, B.; Tahara, M.; Licitra, L.; Rischin, D.; Waldron, J.; Simon, C.; Gregoire, V.; Harrington, K.; et al. Pembrolizumab given Concomitantly with Chemoradiation and as Maintenance Therapy for Locally Advanced Head and Neck Squamous Cell Carcinoma: KEYNOTE-412. Future Oncol. 2020, 16, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Mehra, R.; Seiwert, T.Y.; Gupta, S.; Weiss, J.; Gluck, I.; Eder, J.P.; Burtness, B.; Tahara, M.; Keam, B.; Kang, H.; et al. Efficacy and Safety of Pembrolizumab in Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma: Pooled Analyses after Long-Term Follow-up in KEYNOTE-012. Br. J. Cancer 2018, 119, 153–159. [Google Scholar] [CrossRef]
- Hanna, G.J.; Lizotte, P.; Cavanaugh, M.; Kuo, F.C.; Shivdasani, P.; Frieden, A.; Chau, N.G.; Schoenfeld, J.D.; Lorch, J.H.; Uppaluri, R.; et al. Frameshift Events Predict Anti–PD-1/L1 Response in Head and Neck Cancer. JCI Insight 2018, 3, e98811. [Google Scholar] [CrossRef]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor Mutational Burden as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef]
- Haddad, R.I.; Seiwert, T.Y.; Chow, L.Q.M.; Gupta, S.; Weiss, J.; Gluck, I.; Eder, J.P.; Burtness, B.; Tahara, M.; Keam, B.; et al. Genomic Determinants of Response to Pembrolizumab in Head and Neck Squamous Cell Carcinoma (HNSCC). J. Clin. Oncol. 2017, 35, 6009. [Google Scholar] [CrossRef]
- Carreno, B.M.; Collins, M. BTLA: A New Inhibitory Receptor with a B7-like Ligand. Trends Immunol. 2003, 24, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.L. PD-1 Signaling in Primary T Cells. Immunol. Rev. 2009, 229, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 Expression by Tumour-Associated Macrophages Inhibits Phagocytosis and Tumour Immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef]
- Zandberg, D.P.; Strome, S.E. The Role of the PD-L1:PD-1 Pathway in Squamous Cell Carcinoma of the Head and Neck. Oral. Oncol. 2014, 50, 627–632. [Google Scholar] [CrossRef]
- Sondak, V.K.; Smalley, K.S.M.; Kudchadkar, R.; Grippon, S.; Kirkpatrick, P. Ipilimumab. Nat. Rev. Drug Discov. 2011, 10, 411–412. [Google Scholar] [CrossRef]
- Lutzky, J.; Wolchok, J.; Hamid, O.; Lebbe, C.; Pehamberger, H.; Linette, G.; de Pril, V.; Ibrahim, R.; Hoos, A.; O’Day, S. Association between Immune-Related Adverse Events (IrAEs) and Disease Control or Overall Survival in Patients (Pts) with Advanced Melanoma Treated with 10 Mg/Kg Ipilimumab in Three Phase II Clinical Trials. J. Clin. Oncol. 2009, 27, 9034. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Weber, J.S.; Maio, M.; Neyns, B.; Harmankaya, K.; Chin, K.; Cykowski, L.; de Pril, V.; Humphrey, R.; Lebbé, C. Four-Year Survival Rates for Patients with Metastatic Melanoma Who Received Ipilimumab in Phase II Clinical Trials. Ann. Oncol. 2013, 24, 2174–2180. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Mankor, J.M.; Disselhorst, M.J.; Poncin, M.; Baas, P.; Aerts, J.G.J.V.; Vroman, H. Efficacy of Nivolumab and Ipilimumab in Patients with Malignant Pleural Mesothelioma Is Related to a Subtype of Effector Memory Cytotoxic T Cells: Translational Evidence from Two Clinical Trials. EBioMedicine 2020, 62, 103040. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.-J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef]
- Argiris, A.; Harrington, K.; Tahara, M.; Ferris, R.L.; Gillison, M.; Fayette, J.; Daste, A.; Koralewski, P.; Mesia Nin, R.; Saba, N.F.; et al. LBA36 Nivolumab (N) + Ipilimumab (I) vs EXTREME as First-Line (1L) Treatment (Tx) for Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck (R/M SCCHN): Final Results of CheckMate 651. Ann. Oncol. 2021, 32, S1310–S1311. [Google Scholar] [CrossRef]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, Durable Tumor Remission, and Long-Term Safety in Patients with Advanced Melanoma Receiving Nivolumab. J. Clin. Oncol. 2014, 32, 1020–1030. [Google Scholar] [CrossRef]
- Haddad, R.; Concha-Benavente, F.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Kasper, S.; Vokes, E.E.; Worden, F.; et al. Nivolumab Treatment beyond RECIST-defined Progression in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck in CheckMate 141: A Subgroup Analysis of a Randomized Phase 3 Clinical Trial. Cancer 2019, 125, 3208–3218. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Motzer, R.J.; Hammers, H.J.; Redman, B.G.; Kuzel, T.M.; Tykodi, S.S.; Plimack, E.R.; Jiang, J.; Waxman, I.M.; Rini, B.I. Safety and Efficacy of Nivolumab in Patients With Metastatic Renal Cell Carcinoma Treated Beyond Progression: A Subgroup Analysis of a Randomized Clinical Trial. JAMA Oncol. 2016, 2, 1179–1186. [Google Scholar] [CrossRef]
- Long, G.V.; Weber, J.S.; Larkin, J.; Atkinson, V.; Grob, J.-J.; Schadendorf, D.; Dummer, R.; Robert, C.; Márquez-Rodas, I.; McNeil, C.; et al. Nivolumab for Patients With Advanced Melanoma Treated Beyond Progression: Analysis of 2 Phase 3 Clinical Trials. JAMA Oncol. 2017, 3, 1511–1519. [Google Scholar] [CrossRef]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune Checkpoint Inhibitors: Recent Progress and Potential Biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Chow, L.Q.M.; Haddad, R.; Gupta, S.; Mahipal, A.; Mehra, R.; Tahara, M.; Berger, R.; Eder, J.P.; Burtness, B.; Lee, S.-H.; et al. Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort. J. Clin. Oncol. 2016, 34, 3838–3845. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Nabell, L.; Wong, D.J.L.; Day, T.A.; Daniels, G.A.; Milhem, M.M.; Deva, S.; Jameson, M.B.; Guntinas-Lichius, O.; Almubarak, M.; et al. Phase 1b/2, Open Label, Multicenter Study of Intratumoral SD-101 in Combination with Pembrolizumab in Anti-PD-1 Treatment Naïve Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma (HNSCC). J. Clin. Oncol. 2019, 37, 6039. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive Correlates of Response to the Anti-PD-L1 Antibody MPDL3280A in Cancer Patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Pai-Scherf, L.; Blumenthal, G.M.; Li, H.; Subramaniam, S.; Mishra-Kalyani, P.S.; He, K.; Zhao, H.; Yu, J.; Paciga, M.; Goldberg, K.B.; et al. FDA Approval Summary: Pembrolizumab for Treatment of Metastatic Non-Small Cell Lung Cancer: First-Line Therapy and Beyond. Oncologist 2017, 22, 1392–1399. [Google Scholar] [CrossRef]
- Aragon-Ching, J.B. Pembrolizumab Use in Bladder Cancer: A Tale of Two Trials. Nat. Rev. Urol. 2021, 18, 577–578. [Google Scholar] [CrossRef]
- Maly, J.; Alinari, L. Pembrolizumab in Classical Hodgkin’s Lymphoma. Eur. J. Haematol. 2016, 97, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Kato, K. Pembrolizumab for the Treatment of Esophageal Cancer. Expert Opin. Biol. Ther. 2020, 20, 1143–1150. [Google Scholar] [CrossRef]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Babiker, H.; Brana, I.; Mahadevan, D.; Owonikoko, T.; Calvo, E.; Rischin, D.; Moreno, V.; Papadopoulos, K.P.; Crittenden, M.; Formenti, S.; et al. Phase I Trial of Cemiplimab, Radiotherapy, Cyclophosphamide, and Granulocyte Macrophage Colony-Stimulating Factor in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Oncol. 2021, 26, e1508–e1513. [Google Scholar] [CrossRef] [PubMed]
- Philips, G.K.; Atkins, M. Therapeutic Uses of Anti-PD-1 and Anti-PD-L1 Antibodies. Int. Immunol. 2015, 27, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Colevas, A.D.; Bahleda, R.; Braiteh, F.; Balmanoukian, A.; Brana, I.; Chau, N.G.; Sarkar, I.; Molinero, L.; Grossman, W.; Kabbinavar, F.; et al. Safety and Clinical Activity of Atezolizumab in Head and Neck Cancer: Results from a Phase I Trial. Ann. Oncol. 2018, 29, 2247–2253. [Google Scholar] [CrossRef]
- Hecht, M.; Gostian, A.O.; Eckstein, M.; Rutzner, S.; von der Grün, J.; Illmer, T.; Hautmann, M.G.; Klautke, G.; Laban, S.; Brunner, T.; et al. Safety and Efficacy of Single Cycle Induction Treatment with Cisplatin/Docetaxel/ Durvalumab/Tremelimumab in Locally Advanced HNSCC: First Results of CheckRad-CD8. J. Immunother. Cancer 2020, 8, e001378. [Google Scholar] [CrossRef]
- Guigay, J.; Lee, K.-W.; Patel, M.R.; Daste, A.; Wong, D.J.; Goel, S.; Gordon, M.S.; Gutierrez, M.; Balmanoukian, A.; Le Tourneau, C.; et al. Avelumab for Platinum-Ineligible/Refractory Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck: Phase Ib Results from the JAVELIN Solid Tumor Trial. J. Immunother. Cancer 2021, 9, e002998. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ferris, R.L.; Psyrri, A.; Haddad, R.I.; Tahara, M.; Bourhis, J.; Harrington, K.; Chang, P.M.-H.; Lin, J.-C.; Razaq, M.A.; et al. Avelumab plus Standard-of-Care Chemoradiotherapy versus Chemoradiotherapy Alone in Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck: A Randomised, Double-Blind, Placebo-Controlled, Multicentre, Phase 3 Trial. Lancet Oncol. 2021, 22, 450–462. [Google Scholar] [CrossRef]
- Jiang, Z.; Hsu, J.L.; Li, Y.; Hortobagyi, G.N.; Hung, M.-C. Cancer Cell Metabolism Bolsters Immunotherapy Resistance by Promoting an Immunosuppressive Tumor Microenvironment. Front. Oncol. 2020, 10, 1197. [Google Scholar] [CrossRef]
- Economopoulou, P.; Kladi-Skandali, A.; Strati, A.; Koytsodontis, G.; Kirodimos, E.; Giotakis, E.; Maragoudakis, P.; Gagari, E.; Maratou, E.; Dimitriadis, G.; et al. Prognostic Impact of Indoleamine 2,3-Dioxygenase 1 (IDO1) MRNA Expression on Circulating Tumour Cells of Patients with Head and Neck Squamous Cell Carcinoma. ESMO Open 2020, 5, e000646. [Google Scholar] [CrossRef]
- Katz, J.B.; Muller, A.J.; Prendergast, G.C. Indoleamine 2,3-Dioxygenase in T-Cell Tolerance and Tumoral Immune Escape. Immunol. Rev. 2008, 222, 206–221. [Google Scholar] [CrossRef]
- Ye, J.; Liu, H.; Hu, Y.; Li, P.; Zhang, G.; Li, Y. Tumoral Indoleamine 2,3-Dioxygenase Expression Predicts Poor Outcome in Laryngeal Squamous Cell Carcinoma. Virchows Arch. 2013, 462, 73–81. [Google Scholar] [CrossRef]
- Hou, A.; Hou, K.; Huang, Q.; Lei, Y.; Chen, W. Targeting Myeloid-Derived Suppressor Cell, a Promising Strategy to Overcome Resistance to Immune Checkpoint Inhibitors. Front. Immunol. 2020, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer Immunoediting and Resistance to T Cell-Based Immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of Resistance to Immune Checkpoint Inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiang, Y.; Li, F.; Yin, C.; Li, B.; Ke, X. WNT/β-Catenin Signaling Pathway Regulating T Cell-Inflammation in the Tumor Microenvironment. Front. Immunol. 2019, 10, 2293. [Google Scholar] [CrossRef]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-Intrinsic β-Catenin Signalling Prevents Anti-Tumour Immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef]
- Peng, W.; Chen, J.Q.; Liu, C.; Malu, S.; Creasy, C.; Tetzlaff, M.T.; Xu, C.; McKenzie, J.A.; Zhang, C.; Liang, X.; et al. Loss of PTEN Promotes Resistance to T Cell–Mediated Immunotherapy. Cancer Discov. 2016, 6, 202–216. [Google Scholar] [CrossRef]
- Hutchinson, L. Evading Immune Escape: Synergy of COX and Immune-Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2015, 12, 622. [Google Scholar] [CrossRef]
- Pu, D.; Yin, L.; Huang, L.; Qin, C.; Zhou, Y.; Wu, Q.; Li, Y.; Zhou, Q.; Li, L. Cyclooxygenase-2 Inhibitor: A Potential Combination Strategy With Immunotherapy in Cancer. Front Oncol. 2021, 11, 637504. [Google Scholar] [CrossRef]
- Ma, G.; Li, C.; Zhang, Z.; Liang, Y.; Liang, Z.; Chen, Y.; Wang, L.; Li, D.; Zeng, M.; Shan, W.; et al. Targeted Glucose or Glutamine Metabolic Therapy Combined With PD-1/PD-L1 Checkpoint Blockade Immunotherapy for the Treatment of Tumors—Mechanisms and Strategies. Front. Oncol. 2021, 11, 697894. [Google Scholar] [CrossRef]
- Angevin, E.; Groenland, S.L.; Lim, A.M.L.; Martin-Liberal, J.; Moreno, V.; Trigo, J.M.; Le Tourneau, C.; Mathew, M.; Cho, D.C.; Hansen, A.R.; et al. Updated Analysis of the Inducible T-Cell Co-Stimulatory Receptor (ICOS) Agonist, GSK3359609 (GSK609), Combination with Pembrolizumab (PE) in Patients (Pts) with Anti-PD-1/L1 Treatment-Naïve Head and Neck Squamous Cell Carcinoma (HNSCC). J. Clin. Oncol. 2020, 38, 6517. [Google Scholar] [CrossRef]
- Forster, M.; Felip, E.; Doger, B.; Lopez Pousa, A.; Carcereny, E.; Bajaj, P.; Church, M.; Peguero, J.; Roxburgh, P.; Triebel, F. 927P Initial Results from a Phase II Study (TACTI-002) of Eftilagimod Alpha (Soluble LAG-3 Protein) and Pembrolizumab as 2nd Line Treatment for PD-L1 Unselected Metastatic Head and Neck Cancer Patients. Ann. Oncol. 2020, 31, S667. [Google Scholar] [CrossRef]
- Chen, J.-T.; Chen, C.-H.; Ku, K.-L.; Hsiao, M.; Chiang, C.-P.; Hsu, T.-L.; Chen, M.-H.; Wong, C.-H. Glycoprotein B7-H3 Overexpression and Aberrant Glycosylation in Oral Cancer and Immune Response. Proc. Natl. Acad. Sci. USA 2015, 112, 13057–13062. [Google Scholar] [CrossRef]
- Katayama, A.; Takahara, M.; Kishibe, K.; Nagato, T.; Kunibe, I.; Katada, A.; Hayashi, T.; Harabuchi, Y. Expression of B7-H3 in Hypopharyngeal Squamous Cell Carcinoma as a Predictive Indicator for Tumor Metastasis and Prognosis. Int. J. Oncol. 2011, 38, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Loo, D.; Baughman, J.E.; Yun, S.; Chen, F.; Moore, P.A.; Bonvini, E.; Vasselli, J.R.; Wigginton, J.M.; Cohen, R.B.; et al. A Phase 1 Study of Enoblituzumab in Combination with Pembrolizumab in Patients with Advanced B7-H3-Expressing Cancers. J. Clin. Oncol. 2016, 34, TPS3104. [Google Scholar] [CrossRef]
- Creelan, B.C.; Antonia, S.J. The NKG2A Immune Checkpoint—A New Direction in Cancer Immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.B.; Lefebvre, G.; Posner, M.R.; Bauman, J.R.; Salas, S.; Even, C.; Saada-Bouzid, E.; Seiwert, T.; Colevas, D.; Calmels, F.; et al. 1134P—Monalizumab in Combination with Cetuximab in Patients (Pts) with Recurrent or Metastatic (R/M) Head and Neck Cancer (SCCHN) Previously Treated or Not with PD-(L)1 Inhibitors (IO): 1-Year Survival Data. Ann. Oncol. 2019, 30, v460. [Google Scholar] [CrossRef]
- Colevas, D.A.; Misiukiewicz, K.; Pearson, A.T.; Fayette, J.; Bauman, J.R.; Cupissol, D.; Saada-Bouzid, E.; Adkins, D.R.; Marie, D.B.; Cornen, S.L.; et al. 123MO Monalizumab, Cetuximab and Durvalumab in First-Line Treatment of Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (R/M SCCHN): A Phase II Trial. Ann. Oncol. 2021, 32, S1432. [Google Scholar] [CrossRef]
- Reilley, M.J.; Morrow, B.; Ager, C.R.; Liu, A.; Hong, D.S.; Curran, M.A. TLR9 Activation Cooperates with T Cell Checkpoint Blockade to Regress Poorly Immunogenic Melanoma. J. ImmunoTherapy Cancer 2019, 7, 323. [Google Scholar] [CrossRef]
- Cohen, E.E.; Hong, D.S.; Wise Draper, T.; Nassib William, W.; Schrijvers, D.; Mesia Nin, R.; Scott, M.L.; Lyne, P.; Mugundu, G.; McCoon, P.; et al. 1135O—Phase 1b/2 Study (SCORES) Assessing Safety, Tolerability, and Preliminary Anti-Tumor Activity of Durvalumab plus AZD9150 or AZD5069 in Patients with Advanced Solid Malignancies and Squamous Cell Carcinoma of the Head and Neck (SCCHN). Ann. Oncol. 2017, 28, v403. [Google Scholar] [CrossRef]
- Chamie, K.; Chang, S.; Gonzalgo, M.L.; Kramolowsky, E.V.; Sexton, W.J.; Reddy, S.K.; Bhar, P.; Garner, C.; Soon-Shiong, P. Phase II/III Clinical Results of IL-15RαFc Superagonist N-803 with BCG in BCG-Unresponsive Non-Muscle Invasive Bladder Cancer (NMIBC) Carcinoma in Situ (CIS) Patients. J. Clin. Oncol. 2021, 39, 510. [Google Scholar] [CrossRef]
- Stevanović, S.; Helman, S.R.; Wunderlich, J.R.; Langhan, M.M.; Doran, S.L.; Kwong, M.L.M.; Somerville, R.P.T.; Klebanoff, C.A.; Kammula, U.S.; Sherry, R.M.; et al. A Phase II Study of Tumor-Infiltrating Lymphocyte Therapy for Human Papillomavirus–Associated Epithelial Cancers. Clin. Cancer Res. 2019, 25, 1486–1493. [Google Scholar] [CrossRef]
- Mitchell, T.C.; Hamid, O.; Smith, D.C.; Bauer, T.M.; Wasser, J.S.; Olszanski, A.J.; Luke, J.J.; Balmanoukian, A.S.; Schmidt, E.V.; Zhao, Y.; et al. Epacadostat Plus Pembrolizumab in Patients With Advanced Solid Tumors: Phase I Results From a Multicenter, Open-Label Phase I/II Trial (ECHO-202/KEYNOTE-037). J. Clin. Oncol. 2018, 36, 3223–3230. [Google Scholar] [CrossRef]
- Hamid, O.; Bauer, T.M.; Spira, A.I.; Olszanski, A.J.; Patel, S.P.; Wasser, J.S.; Smith, D.C.; Balmanoukian, A.S.; Aggarwal, C.; Schmidt, E.V.; et al. Epacadostat plus Pembrolizumab in Patients with SCCHN: Preliminary Phase I/II Results from ECHO-202/KEYNOTE-037. J. Clin. Oncol. 2017, 35, 6010. [Google Scholar] [CrossRef]
- Blank, C.U.; Enk, A. Therapeutic Use of Anti-CTLA-4 Antibodies. Int. Immunol. 2015, 27, 3–10. [Google Scholar] [CrossRef]
- Huang, C.-T.; Workman, C.J.; Flies, D.; Pan, X.; Marson, A.L.; Zhou, G.; Hipkiss, E.L.; Ravi, S.; Kowalski, J.; Levitsky, H.I.; et al. Role of LAG-3 in Regulatory T Cells. Immunity 2004, 21, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a Novel Lymphocyte Activation Gene Closely Related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.-P.; Wang, L.-F.; Chiang, F.-Y.; Lee, K.-W.; Kuo, P.-L.; Liang, C.-H. IL-8 Promotes HNSCC Progression on CXCR1/2-Meidated NOD1/RIP2 Signaling Pathway. Oncotarget 2016, 7, 61820–61831. [Google Scholar] [CrossRef]
- Nicholls, D.J.; Wiley, K.; Dainty, I.; MacIntosh, F.; Phillips, C.; Gaw, A.; Mårdh, C.K. Pharmacological Characterization of AZD5069, a Slowly Reversible CXC Chemokine Receptor 2 Antagonist. J. Pharm. Exp. Ther. 2015, 353, 340–350. [Google Scholar] [CrossRef]
- Hong, D.; Falchook, G.; Cook, C.E.; Harb, W.; Lyne, P.; McCoon, P.; Mehta, M.; Mitchell, P.; Mugundu, G.M.; Scott, M.; et al. A Phase 1b Study (SCORES) Assessing Safety, Tolerability, Pharmacokinetics, and Preliminary Anti-Tumor Activity of Durvalumab Combined with AZD9150 or AZD5069 in Patients with Advanced Solid Malignancies and SCCHN. Ann. Oncol. 2016, 27, vi360. [Google Scholar] [CrossRef]
- Adachi, M.; Cui, C.; Dodge, C.T.; Bhayani, M.K.; Lai, S.Y. Targeting STAT3 Inhibits Growth and Enhances Radiosensitivity in Head and Neck Squamous Cell Carcinoma. Oral Oncol. 2012, 48, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhou, F.; Zhang, R.; Claret, F.X. Stat3 Inhibitor Stattic Exhibits Potent Antitumor Activity and Induces Chemo- and Radio-Sensitivity in Nasopharyngeal Carcinoma. PLoS ONE 2013, 8, e54565. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.M.; Lee, S.C.; Andrade Filho, P.A.; Lord, C.A.; Jie, H.-B.; Davidson, H.C.; López-Albaitero, A.; Gibson, S.P.; Gooding, W.E.; Ferrone, S.; et al. Cetuximab-Activated Natural Killer and Dendritic Cells Collaborate to Trigger Tumor Antigen–Specific T-Cell Immunity in Head and Neck Cancer Patients. Clin. Cancer Res. 2013, 19, 1858–1872. [Google Scholar] [CrossRef]
- Sacco, A.G.; Chen, R.; Worden, F.P.; Wong, D.J.L.; Adkins, D.; Swiecicki, P.; Chai-Ho, W.; Oppelt, P.; Ghosh, D.; Bykowski, J.; et al. Pembrolizumab plus Cetuximab in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: An Open-Label, Multi-Arm, Non-Randomised, Multicentre, Phase 2 Trial. Lancet Oncol. 2021, 22, 883–892. [Google Scholar] [CrossRef]
- Kao, H.-F.; Liao, B.-C.; Huang, Y.-L.; Huang, H.-C.; Chen, C.-N.; Chen, T.-C.; Hong, Y.-J.; Chan, C.-Y.; Chia, J.-S.; Hong, R.-L. Afatinib and Pembrolizumab for Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma (ALPHA Study): A Phase II Study with Biomarker Analysis. Clin. Cancer Res. 2022, 28, 1560–1571. [Google Scholar] [CrossRef]
- Chung, C.H.; Bonomi, M.; Steuer, C.E.; Li, J.; Bhateja, P.; Johnson, M.; Masannat, J.; Song, F.; Hernandez-Prera, J.C.; Wenig, B.M.; et al. Concurrent Cetuximab and Nivolumab as a Second-Line or beyond Treatment of Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results of Phase I/II Study. Cancers 2021, 13, 1180. [Google Scholar] [CrossRef]
- Glazar, D.J.; Johnson, M.; Farinhas, J.; Steuer, C.E.; Saba, N.F.; Bonomi, M.; Chung, C.H.; Enderling, H. Early Response Dynamics Predict Treatment Failure in Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma Treated with Cetuximab and Nivolumab. Oral Oncol. 2022, 127, 105787. [Google Scholar] [CrossRef]
- Forster, M.; Metcalf, R.; Sacco, J.; Kong, A.; Wheeler, G.; Forsyth, S.; Bhat, R.; Blair, K.; Ward, J.; Lowe, H.; et al. 922P EACH: A Phase II Study Evaluating the Safety and Anti-Tumour Activity of Avelumab and Cetuximab in Recurrent/Metastatic Squamous Cell Carcinomas. Ann. Oncol. 2020, 31, S665. [Google Scholar] [CrossRef]
- Gulati, S.; Vachon, R.; Desai, S.; Steele, A.; Palackdharry, S.; Takiar, V.; Wise-Draper, T.M. Preliminary Effects on the Innate Immune System with the Combination of Cetuximab and Durvalumab in Metastatic/ Relapsed Head and Neck Squamous Cell Carcinoma in a Phase II Trial. J. Clin. Oncol. 2019, 37, e14273. [Google Scholar] [CrossRef]
- Carla, C.; Daris, F.; Cecilia, B.; Francesca, B.; Francesca, C.; Paolo, F. Angiogenesis in Head and Neck Cancer: A Review of the Literature. J. Oncol. 2011, 2012, e358472. [Google Scholar] [CrossRef][Green Version]
- Grünwald, V.; Powles, T.; Choueiri, T.K.; Hutson, T.E.; Porta, C.; Eto, M.; Sternberg, C.N.; Rha, S.Y.; He, C.S.; Dutcus, C.E.; et al. Lenvatinib plus Everolimus or Pembrolizumab versus Sunitinib in Advanced Renal Cell Carcinoma: Study Design and Rationale. Future Oncol. 2019, 15, 929–941. [Google Scholar] [CrossRef]
- Dunn, L.; Ho, A.L.; Eng, J.; Michel, L.S.; Fetten, J.V.; Warner, E.; Kriplani, A.; Zhi, W.I.; Ng, K.K.; Haque, S.; et al. A Phase I/Ib Study of Lenvatinib and Cetuximab in Patients with Recurrent/Metastatic (R/M) Head and Neck Squamous Cell Carcinoma (HNSCC). J. Clin. Oncol. 2020, 38, 6541. [Google Scholar] [CrossRef]
- Taylor, M.H.; Lee, C.-H.; Makker, V.; Rasco, D.; Dutcus, C.E.; Wu, J.; Stepan, D.E.; Shumaker, R.C.; Motzer, R.J. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients With Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J. Clin. Oncol. 2020, 38, 1154–1163. [Google Scholar] [CrossRef]
- Siu, L.L.; Burtness, B.; Cohen, E.E.W.; Harrington, K.J.; Licitra, L.F.; Rischin, D.; Zhu, Y.; Lee, C.P.; Pinheiro, C.; Swaby, R.F.; et al. Phase III LEAP-010 Study: First-Line Pembrolizumab with or without Lenvatinib in Recurrent/Metastatic (R/M) Head and Neck Squamous Cell Carcinoma (HNSCC). J. Clin. Oncol. 2020, 38, TPS6589. [Google Scholar] [CrossRef]
- Research, C. FDA Approves Lenvatinib plus Pembrolizumab for Advanced Renal Cell Carcinoma. FDA. 2021. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lenvatinib-plus-pembrolizumab-advanced-renal-cell-carcinoma (accessed on 21 September 2022).
- Califano, J.A.; Khan, Z.; Noonan, K.A.; Rudraraju, L.; Zhang, Z.; Wang, H.; Goodman, S.; Gourin, C.G.; Ha, P.K.; Fakhry, C.; et al. Tadalafil Augments Tumor Specific Immunity in Patients with Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2015, 21, 30–38. [Google Scholar] [CrossRef]
- Kong, J.H.; Siebold, C.; Rohatgi, R. Biochemical Mechanisms of Vertebrate Hedgehog Signaling. Development 2019, 146, dev166892. [Google Scholar] [CrossRef] [PubMed]
- Richtig, G.; Aigelsreiter, A.M.; Asslaber, M.; Weiland, T.; Pichler, M.; Eberhard, K.; Sygulla, S.; Schauer, S.; Hoefler, G.; Aigelsreiter, A. Hedgehog Pathway Proteins SMO and GLI Expression as Prognostic Markers in Head and Neck Squamous Cell Carcinoma. Histopathology 2019, 75, 118–127. [Google Scholar] [CrossRef]
- Ruiz-Gómez, A.; Molnar, C.; Holguín, H.; Mayor, F.; de Celis, J.F. The Cell Biology of Smo Signalling and Its Relationships with GPCRs. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2007, 1768, 901–912. [Google Scholar] [CrossRef]
- Rodon, J.; Tawbi, H.A.; Thomas, A.L.; Stoller, R.G.; Turtschi, C.P.; Baselga, J.; Sarantopoulos, J.; Mahalingam, D.; Shou, Y.; Moles, M.A.; et al. A Phase I, Multicenter, Open-Label, First-in-Human, Dose-Escalation Study of the Oral Smoothened Inhibitor Sonidegib (LDE225) in Patients with Advanced Solid Tumors. Clin. Cancer. Res. 2014, 20, 1900–1909. [Google Scholar] [CrossRef]
- Tang, A.; Gao, K.; Chu, L.; Zhang, R.; Yang, J.; Zheng, J. Aurora Kinases: Novel Therapy Targets in Cancers. Oncotarget 2017, 8, 23937–23954. [Google Scholar] [CrossRef]
- Du, R.; Huang, C.; Liu, K.; Li, X.; Dong, Z. Targeting AURKA in Cancer: Molecular Mechanisms and Opportunities for Cancer Therapy. Mol. Cancer 2021, 20, 15. [Google Scholar] [CrossRef]
- Dar, A.A.; Goff, L.W.; Majid, S.; Berlin, J.; El-Rifai, W. Aurora Kinases’ Inhibitors—Rising Stars in Cancer Therapeutics? Mol. Cancer 2010, 9, 268. [Google Scholar] [CrossRef]
- O’Connor, O.A.; Özcan, M.; Jacobsen, E.D.; Roncero, J.M.; Trotman, J.; Demeter, J.; Masszi, T.; Pereira, J.; Ramchandren, R.; Beaven, A.; et al. Randomized Phase III Study of Alisertib or Investigator’s Choice (Selected Single Agent) in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma. J. Clin. Oncol. 2019, 37, 613–623. [Google Scholar] [CrossRef] [PubMed]
- A Phase I-II, Open Label Study Evaluating the Safety and Efficacy of Alisertib and Pembrolizumab in Patients With Rb-Deficient Head and Neck Squamous Cell Carcinomas. Available online: https://www.medifind.com/articles/clinical-trial/229742430 (accessed on 14 September 2022).
- Morales, J.C.; Li, L.; Fattah, F.J.; Dong, Y.; Bey, E.A.; Patel, M.; Gao, J.; Boothman, D.A. Review of Poly (ADP-Ribose) Polymerase (PARP) Mechanisms of Action and Rationale for Targeting in Cancer and Other Diseases. Crit. Rev. Eukaryot Gene Expr. 2014, 24, 15–28. [Google Scholar] [CrossRef]
- Moutafi, M.; Economopoulou, P.; Rimm, D.; Psyrri, A. PARP Inhibitors in Head and Neck Cancer: Molecular Mechanisms, Preclinical and Clinical Data. Oral Oncol. 2021, 117, 105292. [Google Scholar] [CrossRef]
- Duan, R.; Du, W.; Guo, W. EZH2: A Novel Target for Cancer Treatment. J. Hematol. Oncol. 2020, 13, 104. [Google Scholar] [CrossRef]
- Chang, J.W.; Gwak, S.Y.; Shim, G.-A.; Liu, L.; Lim, Y.C.; Kim, J.M.; Jung, M.G.; Koo, B.S. EZH2 Is Associated with Poor Prognosis in Head-and-Neck Squamous Cell Carcinoma via Regulating the Epithelial-to-Mesenchymal Transition and Chemosensitivity. Oral Oncol. 2016, 52, 66–74. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, M.; Yang, K.; Chi, T.; Liao, Z.; Wei, P. PPAR-α Modulators as Current and Potential Cancer Treatments. Front Oncol. 2021, 11, 599995. [Google Scholar] [CrossRef]
- Yarchoan, M.; Powderly, J.D.; Bastos, B.R.; Karasic, T.B.; Crysler, O.V.; Munster, P.N.; McKean, M.; Emens, L.A.; Saenger, Y.M.; Ged, Y.; et al. A Phase 1 Study of TPST-1120 as a Single Agent and in Combination with Nivolumab in Subjects with Advanced Solid Tumors. J. Clin. Oncol. 2022, 40, 3005. [Google Scholar] [CrossRef]
- Hering, L.; Katkeviciute, E.; Schwarzfischer, M.; Busenhart, P.; Gottier, C.; Mrdjen, D.; Komuczki, J.; Wawrzyniak, M.; Lang, S.; Atrott, K.; et al. Protein Tyrosine Phosphatase Non-Receptor Type 2 Function in Dendritic Cells Is Crucial to Maintain Tissue Tolerance. Front. Immunol. 2020, 11, 1856. [Google Scholar] [CrossRef]
- LaFleur, M.W.; Nguyen, T.H.; Coxe, M.A.; Miller, B.C.; Yates, K.B.; Gillis, J.E.; Sen, D.R.; Gaudiano, E.F.; Al Abosy, R.; Freeman, G.J.; et al. PTPN2 Regulates the Generation of Exhausted CD8+ T Cell Subpopulations and Restrains Tumor Immunity. Nat. Immunol. 2019, 20, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Manguso, R.T.; Pope, H.W.; Zimmer, M.D.; Brown, F.D.; Yates, K.B.; Miller, B.C.; Collins, N.B.; Bi, K.; LaFleur, M.W.; Juneja, V.R.; et al. In Vivo CRISPR Screening Identifies Ptpn2 as a Cancer Immunotherapy Target. Nature 2017, 547, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Zhang, D.; Xu, C.; Hance, K.W.; Marelli, B.; Qi, J.; Yu, H.; Qin, G.; Sircar, A.; Hernández, V.M.; et al. Enhanced Preclinical Antitumor Activity of M7824, a Bifunctional Fusion Protein Simultaneously Targeting PD-L1 and TGF-β. Sci. Transl. Med. 2018, 10, eaan5488. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.; Gatti-Mays, M.E.; Cho, B.C.; Hill, A.; Salas, S.; McClay, E.; Redman, J.M.; Sater, H.A.; Donahue, R.N.; Jochems, C.; et al. Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGF-β and PD-L1, in Patients with Human Papillomavirus-Associated Malignancies. J. Immunother. Cancer 2020, 8, e001395. [Google Scholar] [CrossRef] [PubMed]
- Massarelli, E.; William, W.; Johnson, F.; Kies, M.; Ferrarotto, R.; Guo, M.; Feng, L.; Lee, J.J.; Tran, H.; Kim, Y.U.; et al. Combining Immune Checkpoint Blockade and Tumor-Specific Vaccine for Patients With Incurable Human Papillomavirus 16-Related Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 67–73. [Google Scholar] [CrossRef]
- de Sousa, L.G.; Rajapakshe, K.; Rodriguez Canales, J.; Chin, R.L.; Feng, L.; Wang, Q.; Barrese, T.Z.; Massarelli, E.; William, W.; Johnson, F.M.; et al. ISA101 and Nivolumab for HPV-16+ Cancer: Updated Clinical Efficacy and Immune Correlates of Response. J. Immunother. Cancer 2022, 10, e004232. [Google Scholar] [CrossRef]
- Smalley Rumfield, C.; Roller, N.; Pellom, S.T.; Schlom, J.; Jochems, C. Therapeutic Vaccines for HPV-Associated Malignancies. Immunotargets 2020, 9, 167–200. [Google Scholar] [CrossRef]
- Quayle, S.N.; Girgis, N.; Thapa, D.R.; Merazga, Z.; Kemp, M.M.; Histed, A.; Zhao, F.; Moreta, M.; Ruthardt, P.; Hulot, S.; et al. CUE-101, a Novel E7-PHLA-IL2-Fc Fusion Protein, Enhances Tumor Antigen-Specific T-Cell Activation for the Treatment of HPV16-Driven Malignancies. Clin. Cancer Res. 2020, 26, 1953–1964. [Google Scholar] [CrossRef]
- Aggarwal, C.; Cohen, R.B.; Morrow, M.P.; Kraynyak, K.A.; Sylvester, A.J.; Knoblock, D.M.; Bauml, J.M.; Weinstein, G.S.; Lin, A.; Boyer, J.; et al. Immunotherapy Targeting HPV16/18 Generates Potent Immune Responses in HPV-Associated Head and Neck Cancer. Clin. Cancer Res. 2019, 25, 110–124. [Google Scholar] [CrossRef]
- Aggarwal, C.; Saba, N.F.; Algazi, A.P.; Sukari, A.; Seiwert, T.; Haigentz, M.; Porosnicu, M.; Bonomi, M.; Boyer, J.; Durham, N.; et al. 916MO Safety and Efficacy of MEDI0457 plus Durvalumab in Patients (Pts) with Human Papillomavirus-Associated Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma (HPV+ R/M HNSCC). Ann. Oncol. 2020, 31, S661–S662. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and Clinical Activity of Pembrolizumab for Treatment of Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-012): An Open-Label, Multicentre, Phase 1b Trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired Resistance to Fractionated Radiotherapy Can Be Overcome by Concurrent PD-L1 Blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Li, M.; Zhu, Z.; Xiong, H.; Shen, H.; Zhang, H.; Du, Q.; Li, Q. Vincristine Upregulates PD-L1 and Increases the Efficacy of PD-L1 Blockade Therapy in Diffuse Large B-Cell Lymphoma. J. Cancer Res. Clin. Oncol. 2021, 147, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Julian, R.; Savani, M.; Bauman, J.E. Immunotherapy Approaches in HPV-Associated Head and Neck Cancer. Cancers 2021, 13, 5889. [Google Scholar] [CrossRef]
- Powel, S.F.; Gitau, M.M.; Sumey, C.J.; Reynolds, J.T.; Lohr, M.; McGraw, S.; Nowak, R.K.; Terrell, A.M.; Jensen, A.W.; Blanchard, M.J.; et al. Safety of Pembrolizumab with Chemoradiation (CRT) in Locally Advanced Squamous Cell Carcinoma of the Head and Neck (LA-SCCHN). J. Clin. Oncol. 2017, 35, 6011. [Google Scholar] [CrossRef]
- Pahl, J.; Ruslan, E.; Buddingh, E.; Santos, S.; Szuhai, K.; Serra, M.; Gelderblom, H.; Hogendoorn, P.; Egeler, R.; Schilham, M.; et al. Anti-EGFR Antibody Cetuximab Enhances the Cytolytic Activity of Natural Killer Cells toward Osteosarcoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 18, 432–441. [Google Scholar] [CrossRef]
- Jie, H.-B.; Schuler, P.J.; Lee, S.C.; Srivastava, R.M.; Argiris, A.; Ferrone, S.; Whiteside, T.L.; Ferris, R.L. CTLA-4+ Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer Res. 2015, 75, 2200–2210. [Google Scholar] [CrossRef]
- Jie, H.-B.; Srivastava, R.M.; Argiris, A.; Bauman, J.E.; Kane, L.P.; Ferris, R.L. Increased PD-1+ and TIM-3+ TILs during Cetuximab Therapy Inversely Correlate with Response in Head and Neck Cancer Patients. Cancer Immunol. Res. 2017, 5, 408–416. [Google Scholar] [CrossRef]
- Boyerinas, B.; Jochems, C.; Fantini, M.; Heery, C.R.; Gulley, J.L.; Tsang, K.Y.; Schlom, J. Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti–PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunol. Res. 2015, 3, 1148–1157. [Google Scholar] [CrossRef]
- Prestwich, R.J.; Errington, F.; Ilett, E.J.; Morgan, R.S.M.; Scott, K.J.; Kottke, T.; Thompson, J.; Morrison, E.E.; Harrington, K.J.; Pandha, H.S.; et al. Tumor Infection by Oncolytic Reovirus Primes Adaptive Antitumor Immunity. Clin. Cancer Res. 2008, 14, 7358–7366. [Google Scholar] [CrossRef]
- Woller, N.; Knocke, S.; Mundt, B.; Gürlevik, E.; Strüver, N.; Kloos, A.; Boozari, B.; Schache, P.; Manns, M.P.; Malek, N.P.; et al. Virus-Induced Tumor Inflammation Facilitates Effective DC Cancer Immunotherapy in a Treg-Dependent Manner in Mice. J. Clin. Investig. 2011, 121, 2570–2582. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.I.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Tal Imogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Greig, S.L. Talimogene Laherparepvec: First Global Approval. Drugs 2016, 76, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Hodi, F.S.; Wolchok, J.D.; Topalian, S.L.; Schadendorf, D.; Larkin, J.; Sznol, M.; Long, G.V.; Li, H.; Waxman, I.M.; et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J. Clin. Oncol. 2017, 35, 785–792. [Google Scholar] [CrossRef] [PubMed]

| Treatment Setting | Trial | Description | Objective | Results |
|---|---|---|---|---|
| ICI–chemo | KEYNOTE 048 (NCT02358031) | Pembrolizumab monotherapy vs. pembrolizumab + platinum-based CT + 5-FU vs. cetuximab + platinum-based CT + 5-FU | Pembrolizumab as a first-line treatment of R/M HNSCC. | OS over SOC was improved with pembrolizumab alone in populations with PD-L1 CPS ≥ 20 (p = 0.0007) and CPS ≥ 1 (p = 0.0086). Pembrolizumab + CT significantly improved OS in the total population (p = 0.0034) [52]. |
| Dual checkpoint blockade | CheckMate 651 (NCT02741570) | Nivolumab + ipilimumab vs. SOC (EXTREME study regimen) as first-line treatment in patients with R/M HNSCC | Combination nivolumab + ipilimumab has shown significant promise in patients with NSCLC, advanced melanoma, and advanced RCC. | Trial failed end point. OS for dual immune checkpoint blockade 13.9 months vs. 13.5 months for the EXTREME group. Higher OS for double immune blockade when CPS > 20 (17.6 months), but also n.s., ORR 34%, and DOR 32.6 months. No single nivolumab arm for comparison [53]. |
| Dual checkpoint blockade | CheckMate 714 (NCT02823574) | Nivolumab + ipilimumab vs. nivolumab + ipilimumab placebo in R/M HNSCC | R/M HNSCC ORR, DOR, TTR. | Study aim failed: OS for nivolumab plus ipilimumab 10.0 months vs. 12.0 for nivolumab plus placebo. ORR: 13.2 for nivolumab plus ipilimumab vs. 18.3 for nivolumab plus placebo. |
Dual checkpoint blockade | KESTREL (NCT02551159) | Durvalumab + tremelimumab vs. durvalumab monotherapy vs. SOC CT in treatment-naive R/M HNSCC patients | First-line treatment for R/M HNSCC targeting both PD-L1 and CTLA-4 pathways has potential for synergistic anti-tumor effects. | Results ongoing [54]. |
| Dual checkpoint blockade | EAGLE (NCT02369874) | Durvalumab monotherapy vs. durvalumab + tremelimumab vs. SOC in R/M HNSCC with progress on platinum therapy | Second-line treatment for R/M HNSCC targeting both PD-1 and CTLA-4 pathways may induce synergistic anti-tumor effects. | Did not meet primary endpoint of improved OS [55,56]. |
| Single ICI adjuvant | WO40242 (NCT03452137) | Atezolizumab vs. placebo for high-risk stage IV HPV- or stage III HPV+ HNSCC after definitive local therapy | To evaluate the efficacy and safety of atezolizumab as an adjuvant therapy. | Primary outcomes include independently assessed event-free survival (IRF assessed EFS) and OS. |
| ICI–chemo- radiation | GORTEC 2017– 01 (REACH) (NCT02999087) | Avelumab + cetuximab and RT vs. SOC in LA HNSCC | The expansion of GORTEC 2015–01, based on the hypothesis of a synergistic benefit when avelumab is combined with cetuximab + RT. | This study demonstrated an acceptable safety profile and was approved for continuation by the Data and Safety Oversight Committee [57]. |
| ICI–radiation | JAVELIN (NCT02952586) | Avelumab + SOC CRT vs. SOC CRT in LA HNSCC patients | The combination of avelumab and CRT may synergistically activate multiple immune-mediated mechanisms and improve long-term disease control [58]. | Currently recruiting. |
| ICI–radiation | KEYNOTE-412 (NCT03040999) | Pembrolizumab or placebo + CRT in LA HNSCC patients | CRT exhibits immunomodulatory effects; preclinical data indicate efficacy may be improved with the addition of pembrolizumab [59]. | Adult patients with newly diagnosed, pathologically proven, untreated LA-HNSCC are being recruited [59]. |
| ICI–radiation | (NCT03349710) | Nivolumab monotherapy vs. nivolumab + cisplatin in combination with RT in cisplatin ineligibility or eligibility will be assessed in LA HNSCC patients | To evaluate whether nivolumab in combination with RT is more efficient compared to cetuximab in combination with RT. | Recruitment completed. n = 74. AE, SAE evaluation. |
| Dual checkpoint blockade, ICI–radiation, adjuvant–neoadjuvant | IMSTAR-HN NCT03700905 | Multicenter randomized controlled study of nivolumab alone or in combination with ipilimumab as an immunotherapy vs. standard follow-up in surgical resectable HNSCC after adjuvant therapy | The combination of anti-PD-1 and anti-CTLA-4 as maintenance therapy may improve DFS due to the anti-tumor effect of immunotherapy by enhancing the cross-presentation of tumor antigens. Primary: DFS at 3 years. | Active, not recruiting, 276 participants estimated. |
| ICI–chemo, ICI–radiation | NCT01810913 | Docetaxel–cetuximab or the addition of an immunotherapy drug, atezolizumab, to the usual chemotherapy and radiation therapy in high-risk HNSCC | DFS, OS. | Active, recruiting, 613 patients estimated. |
| ICI–radiation | NCT03258554 | Radiation therapy with durvalumab or cetuximab in treating patients with locoregionally advanced head and neck cancer who cannot take cisplatin | It is not clear whether radiation therapy with durvalumab is more effective than usual radiation therapy with cetuximab in treating patients with head and neck cancer (DLT, PFS, OS). | Recruitment suspended. |
| ICI–AB–chemo | NCT05063552 | An evaluation of the application of the investigational drugs atezolizumab and/or bevacizumab with or without standard chemotherapy in the second-line treatment of advanced head and neck cancer | To investigate the progression-free survival (PFS) of patients receiving chemotherapy plus cetuximab, chemotherapy plus bevacizumab, and atezolizumab plus bevacizumab (phase II). To assess the overall survival (OS) of patients treated with chemotherapy plus cetuximab versus the superior arm from the phase II portion of the protocol (phase III). | Recruiting. |
| ICI–AB | NCT04199104 | A trial of pembrolizumab with or without lenvatinib (E7080/MK-7902) as a first-line treatment (1 L) in a programmed cell death ligand 1 (PD-L1)-selected population with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M HNSCC). (LEAP-010) (MK-7902-010) (LEAP-10) | ORR, PFS, OS. | Recruiting. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ettl, T.; Grube, M.; Schulz, D.; Bauer, R.J. Checkpoint Inhibitors in Cancer Therapy: Clinical Benefits for Head and Neck Cancers. Cancers 2022, 14, 4985. https://doi.org/10.3390/cancers14204985
Ettl T, Grube M, Schulz D, Bauer RJ. Checkpoint Inhibitors in Cancer Therapy: Clinical Benefits for Head and Neck Cancers. Cancers. 2022; 14(20):4985. https://doi.org/10.3390/cancers14204985
Chicago/Turabian StyleEttl, Tobias, Matthias Grube, Daniela Schulz, and Richard Josef Bauer. 2022. "Checkpoint Inhibitors in Cancer Therapy: Clinical Benefits for Head and Neck Cancers" Cancers 14, no. 20: 4985. https://doi.org/10.3390/cancers14204985
APA StyleEttl, T., Grube, M., Schulz, D., & Bauer, R. J. (2022). Checkpoint Inhibitors in Cancer Therapy: Clinical Benefits for Head and Neck Cancers. Cancers, 14(20), 4985. https://doi.org/10.3390/cancers14204985






