TREM2 Is Associated with Advanced Stages and Inferior Prognosis in Oral Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Immunohistochemistry
2.3. Blood Samples
2.4. Peripheral Blood Mononuclear Cell Preparation
2.5. Preparation of Single-Cell Suspension
2.6. Flow Cytometry
2.7. Enzyme-Linked Immunosorbent Assay
2.8. Statistical Analysis
3. Results
3.1. Patient Cohort for Tissue Microarrays
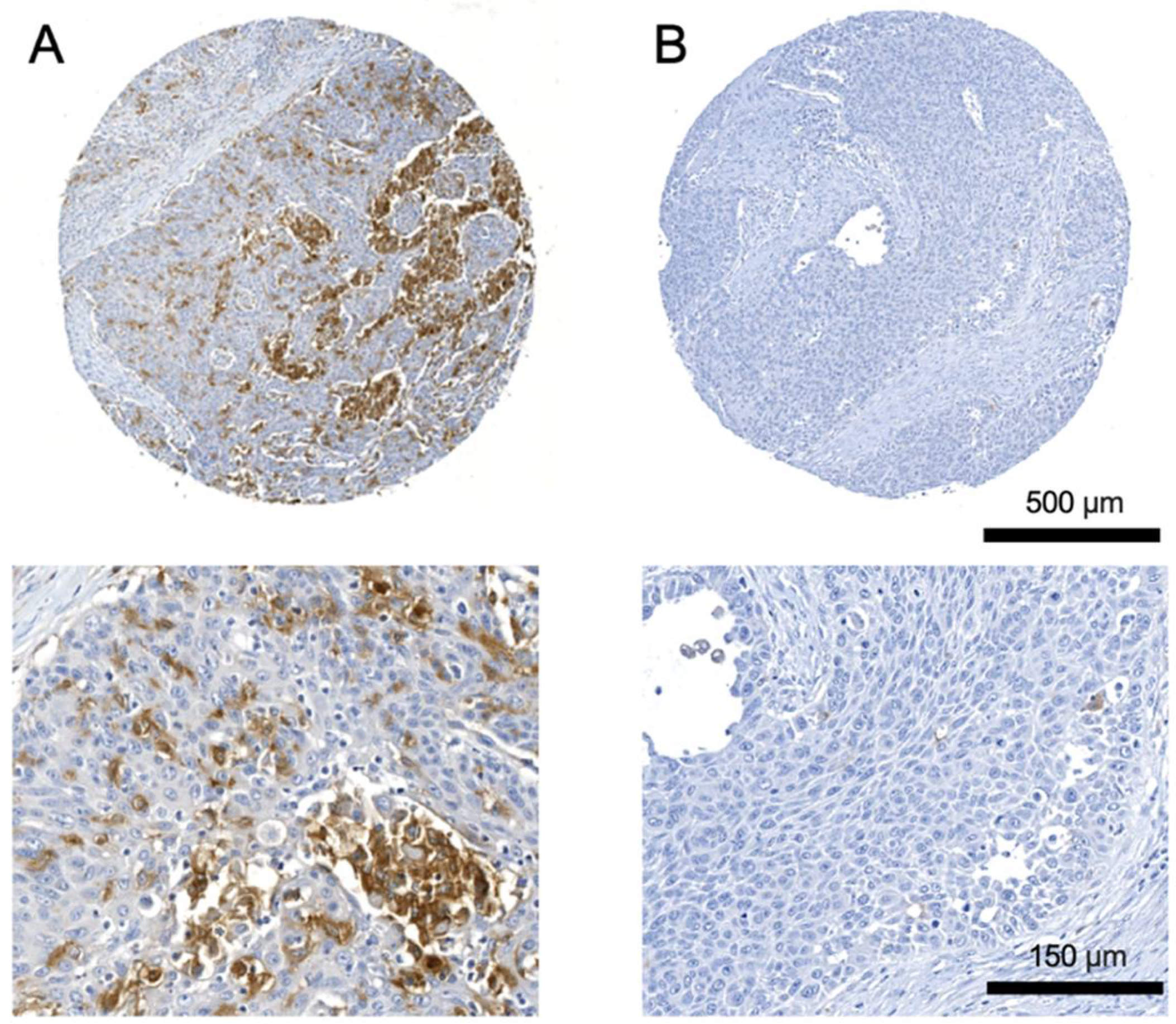
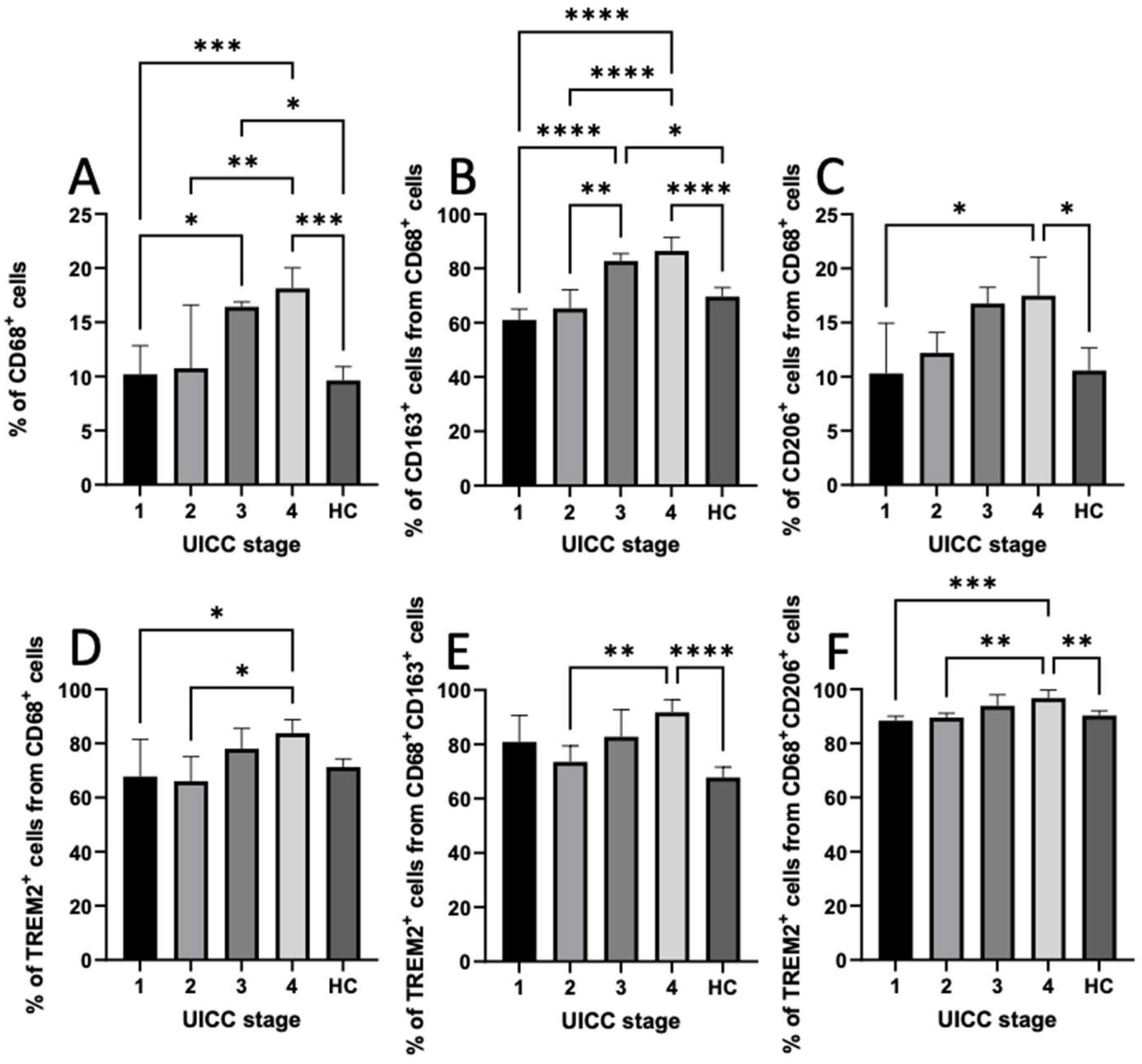
3.2. Correlation of TREM2 Expression in Primary Tumors with Clinicopathological Characteristics
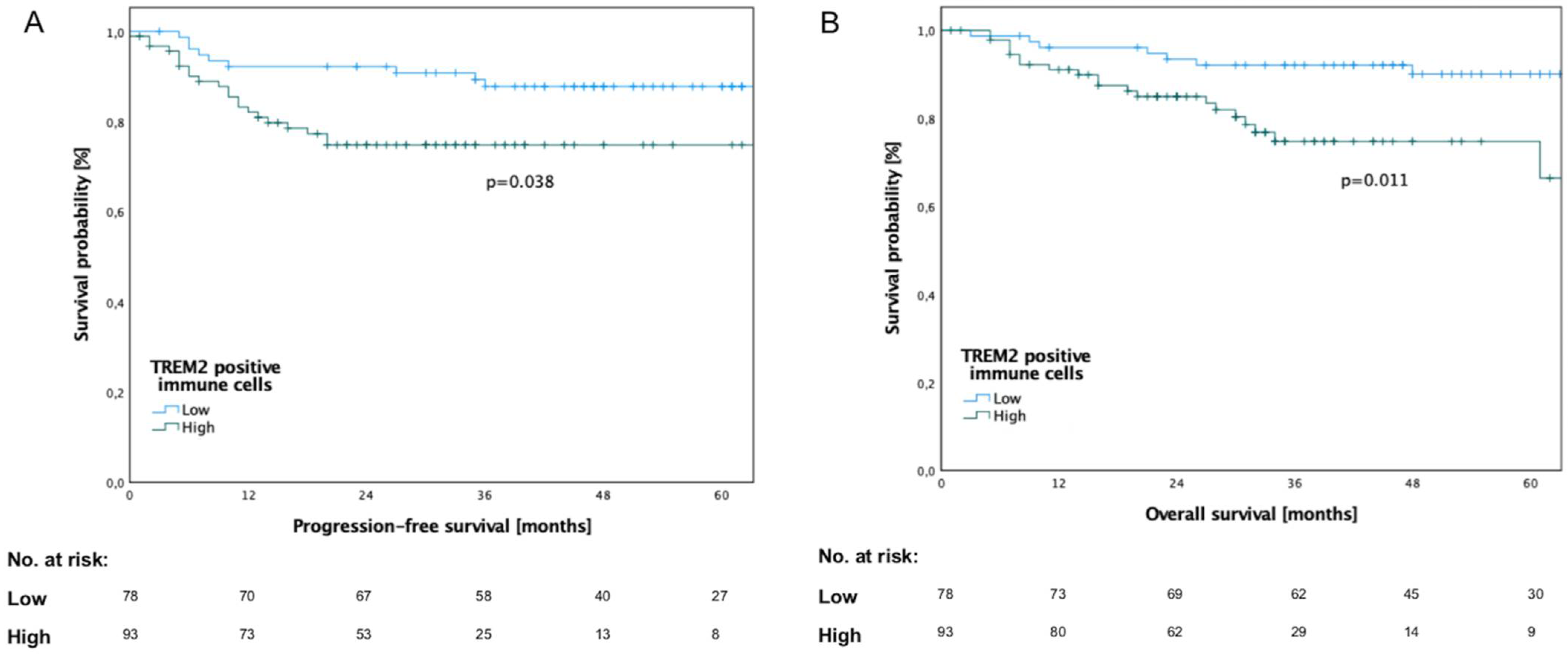
3.3. Survival Analysis in Relation to TREM2 Expression
3.4. Expression of TREM2 in Lymph-Node Metastases
3.5. Patient Cohort for Flow Cytometric Analysis and Enzyme-Linked Immunosorbent Assay
3.6. Expression of CD68, CD163, CD206, and TREM2 in PBMCs
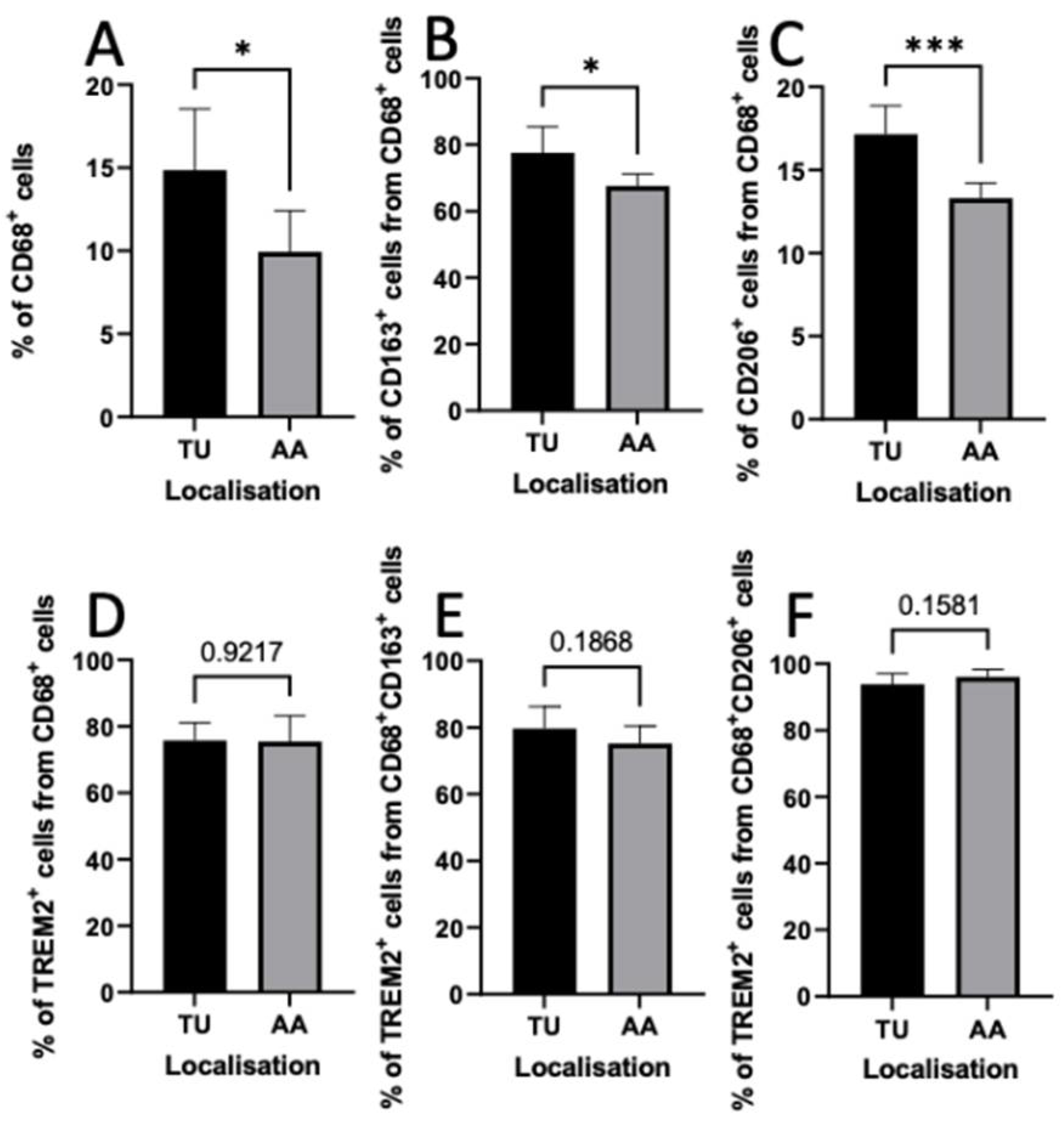
3.7. Distribution of TAMs in Intratumoral and Peritumoral Regions
3.8. Serum Levels of Soluble TREM2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Massano, J.; Regateiro, F.S.; Januário, G.; Ferreira, A. Oral squamous cell carcinoma: Review of prognostic and predictive factors. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Blatt, S.; Krüger, M.; Ziebart, T.; Sagheb, K.; Schiegnitz, E.; Goetze, E.; Al-Nawas, B.; Pabst, A.M. Biomarkers in diagnosis and therapy of oral squamous cell carcinoma: A review of the literature. J. Craniomaxillofacial Surg. 2017, 45, 722–730. [Google Scholar] [CrossRef] [PubMed]
- McCusker, M.G.; Orkoulas-Razis, D.; Mehra, R. Potential of Pembrolizumab in Metastatic or Recurrent Head and Neck Cancer: Evidence to Date. OncoTargets Ther. 2020, 13, 3047–3059. [Google Scholar] [CrossRef] [PubMed]
- Twomey, J.D.; Zhang, B. Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics. AAPS J. 2021, 23, 39. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, K.; Hana, D.; Chou, J.T.-T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar] [CrossRef]
- Wu, P.; Gao, W.; Su, M.; Nice, E.C.; Zhang, W.; Lin, J.; Xie, N. Adaptive Mechanisms of Tumor Therapy Resistance Driven by Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 641469. [Google Scholar] [CrossRef]
- Molgora, M.; Esaulova, E.; Vermi, W.; Hou, J.; Chen, Y.; Luo, J.; Brioschi, S.; Bugatti, M.; Omodei, A.S.; Ricci, B.; et al. TREM2 Modulation Remodels the Tumor Myeloid Landscape Enhancing Anti-PD-1 Immunotherapy. Cell 2020, 182, 886–900.e17. [Google Scholar] [CrossRef]
- Coelho, I.; Duarte, N.; Barros, A.; Macedo, M.P.; Penha-Gonçalves, C. Trem-2 Promotes Emergence of Restorative Macrophages and Endothelial Cells During Recovery From Hepatic Tissue Damage. Front. Immunol. 2021, 11, 616044. [Google Scholar] [CrossRef]
- Jay, T.R.; Von Saucken, V.E.; Landreth, G.E. TREM2 in Neurodegenerative Diseases. Mol. Neurodegener. 2017, 12, 56. [Google Scholar] [CrossRef]
- Jaitin, D.A.; Adlung, L.; Thaiss, C.A.; Weiner, A.; Li, B.; Descamps, H.; Lundgren, P.; Bleriot, C.; Liu, Z.; Deczkowska, A.; et al. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell 2019, 178, 686–698.e14. [Google Scholar] [CrossRef] [PubMed]
- Cochain, C.; Vafadarnejad, E.; Arampatzi, P.; Pelisek, J.; Winkels, H.; Ley, K.; Wolf, D.; Saliba, A.-E.; Zernecke, A. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ. Res. 2018, 122, 1661–1674. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, J.; Cui, P.; Zhou, Y.; Liu, C.; Wu, X.; Ji, Y.; Wang, S.; Cheng, B.; Ye, H.; et al. TREM2 sustains macrophage-hepatocyte metabolic coordination in nonalcoholic fatty liver disease and sepsis. J. Clin. Investig. 2021, 131, e135197. [Google Scholar] [CrossRef] [PubMed]
- Katzenelenbogen, Y.; Sheban, F.; Yalin, A.; Yofe, I.; Svetlichnyy, D.; Jaitin, D.A.; Bornstein, C.; Moshe, A.; Keren-Shaul, H.; Cohen, M.; et al. Coupled scRNA-Seq and Intracellular Protein Activity Reveal an Immunosuppressive Role of TREM2 in Cancer. Cell 2020, 182, 872–885.e19. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Colegio, O.R.; Chu, N.-Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor Metastasis: Molecular Insights and Evolving Paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef]
- Frafjord, A.; Skarshaug, R.; Hammarström, C.; Stankovic, B.; Dorg, L.T.; Aamodt, H.; Woldbaek, P.R.; Helland, Å.; Brustugun, O.T.; Øynebråten, I.; et al. Antibody combinations for optimized staining of macrophages in human lung tumours. Scand. J. Immunol. 2020, 92, e12889. [Google Scholar] [CrossRef]
- Filipello, F.; Goldsbury, C.; You, S.F.; Locca, A.; Karch, C.M.; Piccio, L. Soluble TREM2: Innocent bystander or active player in neurological diseases? Neurobiol. Dis. 2022, 165, 105630. [Google Scholar] [CrossRef]
- Piccio, L.; Buonsanti, C.; Cella, M.; Tassi, I.; Schmidt, R.E.; Fenoglio, C.; Rinker, J., 2nd; Naismith, R.T.; Panina-Bordignon, P.; Passini, N.; et al. Identification of soluble TREM-2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain 2008, 131, 3081–3091. [Google Scholar] [CrossRef]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, E.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.; Younkin, S.; et al. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Freier, K.; Joos, S.; Flechtenmacher, C.; Devens, F.; Benner, A.; Bosch, F.X.; Lichter, P.; Hofele, C. Tissue microarray analysis reveals site-specific prevalence of oncogene amplifications in head and neck squamous cell carcinoma. Cancer Res. 2003, 63, 1179–1182. [Google Scholar] [PubMed]
- Moratin, J.; Metzger, K.; Safaltin, A.; Herpel, E.; Hoffmann, J.; Freier, K.; Hess, J.; Horn, D. Upregulation of PD-L1 and PD-L2 in neck node metastases of head and neck squamous cell carcinoma. Head Neck 2019, 41, 2484–2491. [Google Scholar] [CrossRef] [PubMed]
- Kühn, J.P.; Schmid, W.; Körner, S.; Bochen, F.; Wemmert, S.; Rimbach, H.; Smola, S.; Radosa, J.C.; Wagner, M.; Morris, L.G.T.; et al. HPV Status as Prognostic Biomarker in Head and Neck Cancer-Which Method Fits the Best for Outcome Prediction? Cancers 2021, 13, 4730. [Google Scholar] [CrossRef]
- Linxweiler, M.; Bochen, F.; Wemmert, S.; Lerner, C.; Hasenfus, A.; Bohle, R.M.; Al-Kadah, B.; Takacs, Z.F.; Smola, S.; Schick, B. Combination of p16(INK4a) /Ki67 immunocytology and HPV polymerase chain reaction for the noninvasive analysis of HPV involvement in head and neck cancer. Cancer Cytopathol. 2015, 123, 219–229. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Li, P.; Wang, X.; Ni, K. High TREM2 expression correlates with poor prognosis in gastric cancer. Hum. Pathol. 2018, 72, 91–99. [Google Scholar] [CrossRef]
- Zhang, H.; Sheng, L.; Tao, J.; Chen, R.; Li, Y.; Sun, Z.; Qian, W. Depletion of the triggering receptor expressed on myeloid cells 2 inhibits progression of renal cell carcinoma via regulating related protein expression and PTEN-PI3K/Akt pathway. Int. J. Oncol. 2016, 49, 2498–2506. [Google Scholar] [CrossRef]
- Tang, W.; Lv, B.; Yang, B.; Chen, Y.; Yuan, F.; Ma, L.; Chen, S.; Zhang, S.; Xia, J. TREM2 acts as a tumor suppressor in hepatocellular carcinoma by targeting the PI3K/Akt/β-catenin pathway. Oncogenesis 2019, 8, 2498–2506. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, E.M.; Ji, K.Y.; Lee, H.Y.; Yee, S.M.; Woo, S.M.; Yi, J.W.; Yun, C.H.; Choi, H.; Kang, H.S. TREM2 Acts as a Tumor Suppressor in Colorectal Carcinoma through Wnt1/β-catenin and Erk Signaling. Cancers 2019, 11, 1315. [Google Scholar] [CrossRef]
- Yao, Y.; Li, H.; Chen, J.; Xu, W.; Yang, G.; Bao, Z.; Xia, D.; Lu, G.; Hu, S.; Zhou, J. TREM-2 serves as a negative immune regulator through Syk pathway in an IL-10 dependent manner in lung cancer. Oncotarget 2016, 7, 29620–29634. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, X.; Nie, K.; Cheng, L.; Zhang, Z.; Hu, Y.; Peng, W. Systematic Pan-Cancer Analysis Identifies TREM2 as an Immunological and Prognostic Biomarker. Front. Immunol. 2021, 12, 646523. [Google Scholar] [CrossRef] [PubMed]
- Komohara, Y.; Jinushi, M.; Takeya, M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2013, 105, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kaku, Y.; Imaoka, H.; Morimatsu, Y.; Komohara, Y.; Ohnishi, K.; Oda, H.; Takenaka, S.; Matsuoka, M.; Kawayama, T.; Takeya, M.; et al. Overexpression of CD163, CD204 and CD206 on Alveolar Macrophages in the Lungs of Patients with Severe Chronic Obstructive Pulmonary Disease. PLoS ONE 2014, 9, e87400. [Google Scholar] [CrossRef]
- Komohara, Y.; Hirahara, J.; Horikawa, T.; Kawamura, K.; Kiyota, E.; Sakashita, N.; Araki, N.; Takeya, M. AM-3K, an Anti-macrophage Antibody, Recognizes CD163, a Molecule Associated with an Anti-inflammatory Macrophage Phenotype. J. Histochem. Cytochem. 2006, 54, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Skytthe, M.K.; Graversen, J.H.; Moestrup, S.K. Targeting of CD163+ Macrophages in Inflammatory and Malignant Diseases. Int. J. Mol. Sci. 2020, 21, 5497. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Sánchez, F.J.; Lequerica-Fernández, P.; Suárez-Canto, J.; Rodrigo, J.P.; Rodríguez, J.C.D.V.; Domínguez-Iglesias, F.; García-Pedrero, J.M.; De Vicente, J.C. Macrophages in Oral Carcinomas: Relationship with Cancer Stem Cell Markers and PD-L1 Expression. Cancers 2020, 12, 1764. [Google Scholar] [CrossRef]
- Kumar, A.T.; Knops, A.; Swendseid, B.; Martinez-Outschoom, U.; Harshyne, L.; Philp, N.; Rodeck, U.; Luginbuhl, A.; Cognetti, D.; Johnson, J.; et al. Prognostic Significance of Tumor-Associated Macrophage Content in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Front. Oncol. 2019, 9, 656. [Google Scholar] [CrossRef]
- He, K.F.; Zhang, L.; Huang, C.F.; Ma, S.R.; Wang, Y.F.; Wang, W.M.; Zhao, Z.L.; Liu, B.; Zhao, Y.F.; Zhang, W.F.; et al. CD163+ tumor-associated macrophages correlated with poor prognosis and cancer stem cells in oral squamous cell carcinoma. Biomed Res. Int. 2014, 2014, 838632. [Google Scholar] [CrossRef]
- Haque, A.S.M.R.; Moriyama, M.; Kubota, K.; Ishiguro, N.; Sakamoto, M.; Chinju, A.; Mochizuki, K.; Sakamoto, T.; Kaneko, N.; Munemura, R.; et al. CD206+ tumor-associated macrophages promote proliferation and invasion in oral squamous cell carcinoma via EGF production. Sci. Rep. 2019, 9, 14611. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, W.; Zhong, W.Q.; Liu, Z.J.; Li, H.M.; Yu, Z.L.; Zhao, Y.F. Tumor associated macrophages induce epithelial to mesenchymal transition via the EGFR/ERK1/2 pathway in head and neck squamous cell carcinoma. Oncol. Rep. 2018, 40, 2558–2572. [Google Scholar] [CrossRef]
- Bouchon, A.; Hernández-Munain, C.; Cella, M.; Colonna, M. A Dap12-Mediated Pathway Regulates Expression of Cc Chemokine Receptor 7 and Maturation of Human Dendritic Cells. J. Exp. Med. 2001, 194, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Kleinberger, G.; Yamanishi, Y.; Suárez-Calvet, M.; Czirr, E.; Lohmann, E.; Cuyvers, E.; Struyfs, H.; Pettkus, N.; Wenninger-Weinzierl, A.; Mazaheri, F.; et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci. Transl. Med. 2014, 6, 243ra86. [Google Scholar] [CrossRef] [PubMed]
- Efendioglu, M.; Sanli, E.; Turkoglu, C.; Balak, N. Reduced serum sRANKL and sTREM2 levels in high-grade gliomas: Association with prognosis. Arch. Neuropsychiatry 2020, 58, 133–136. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Low TREM2 Expression in Immune Cells (%) | High TREM2 Expression in Immune Cells (%) | p-Value | |
|---|---|---|---|---|
| Sex | Men | 53 (53) | 47 (47) | 0.021 * |
| Women | 25 (35.2) | 46 (64.8) | ||
| Age | ≤ 75 | 66 (48.9) | 69 (51.1) | 0.097 |
| > 75 | 12 (33.3) | 24 (66.7) | ||
| T classification | 1 | 32 (54.2) | 27 (45.8) | 0.030 * |
| 2 | 28 (48.3) | 30 (51.7) | ||
| 3 | 2 (28.6) | 5 (71.4) | ||
| 4 | 16 (34) | 31 (66) | ||
| N classification | 0 | 60 (50.4) | 59 (49.6) | 0.05 |
| 1 | 8 (42.1) | 11 (57.9) | ||
| 2b | 5 (26.3) | 14 (73.7) | ||
| 2c | 5 (35.7) | 9 (64.3) | ||
| UICC stage | I | 28 (53.8) | 24 (46.2) | 0.04 * |
| II | 19 (50) | 19 (50) | ||
| III | 9 (50) | 9 (50) | ||
| IV | 22 (34.9) | 41 (65.1) | ||
| Recurrence | No | 67 (48.5) | 71 (51.5) | 0.116 |
| Yes | 11 (33.3) | 22 (66.7) | ||
| Differentiation grade | 1 | 13 (86.7) | 2 (13.3) | 0.102 |
| 2 | 48 (41) | 69 (59) | ||
| 3 | 15 (44.1) | 19 (55.9) | ||
| Missing | 2 (40) | 3 (60) | ||
| Localization | Floor of the mouth | 26 (55.3) | 21 (44.7) | 0.023 * |
| Tongue | 23 (54.7) | 19 (45.3) | ||
| Lower jaw | 20 (38.5) | 32 (61.5) | ||
| Upper jaw | 1 (33.3) | 2 (66.7) | ||
| Lower lip | 0 (0) | 1 (100) | ||
| Soft palate | 2 (18.2) | 9 (81.8) | ||
| Buccal plane | 6 (50) | 6 (50) | ||
| Missing | 0 (0) | 3 (100) | ||
| PD-L1 | Negative | 33 (73.3) | 12 (26.7) | <0.001 * |
| Positive | 36 (33.3) | 69 (63.9) | ||
| Missing | 9 (34.3) | 12 (65.7) | ||
| PD-L2 | Negative | 15 (62.5) | 9 (37.5) | 0.088 |
| Positive | 53 (43.4) | 69 (56.6) | ||
| Missing | 10 (40) | 15 (60) | ||
| p16 | Negative | 43 (47.7) | 47 (52.3) | 0.7 |
| Positive | 24 (44.4) | 30 (55.6) | ||
| Missing | 11 (40.7) | 16 (59.3) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Struckmeier, A.-K.; Radermacher, A.; Fehrenz, M.; Alansary, D.; Wartenberg, P.; Wagner, M.; Scheller, A.; Hess, J.; Moratin, J.; Freudlsperger, C.; et al. TREM2 Is Associated with Advanced Stages and Inferior Prognosis in Oral Squamous Cell Carcinoma. Cancers 2022, 14, 4635. https://doi.org/10.3390/cancers14194635
Struckmeier A-K, Radermacher A, Fehrenz M, Alansary D, Wartenberg P, Wagner M, Scheller A, Hess J, Moratin J, Freudlsperger C, et al. TREM2 Is Associated with Advanced Stages and Inferior Prognosis in Oral Squamous Cell Carcinoma. Cancers. 2022; 14(19):4635. https://doi.org/10.3390/cancers14194635
Chicago/Turabian StyleStruckmeier, Ann-Kristin, Anne Radermacher, Michael Fehrenz, Dalia Alansary, Philipp Wartenberg, Mathias Wagner, Anja Scheller, Jochen Hess, Julius Moratin, Christian Freudlsperger, and et al. 2022. "TREM2 Is Associated with Advanced Stages and Inferior Prognosis in Oral Squamous Cell Carcinoma" Cancers 14, no. 19: 4635. https://doi.org/10.3390/cancers14194635
APA StyleStruckmeier, A.-K., Radermacher, A., Fehrenz, M., Alansary, D., Wartenberg, P., Wagner, M., Scheller, A., Hess, J., Moratin, J., Freudlsperger, C., Hoffmann, J., Thurner, L., Roemer, K., Freier, K., & Horn, D. (2022). TREM2 Is Associated with Advanced Stages and Inferior Prognosis in Oral Squamous Cell Carcinoma. Cancers, 14(19), 4635. https://doi.org/10.3390/cancers14194635








