Prevalence and Outcomes of COVID-19 among Hematology/Oncology Patients and Providers of a Community-Facing Health System during the B1.1.529 (“Omicron”) SARS-CoV-2 Variant Wave
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. COVID Data Tracker; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2022. Available online: https://covid.cdc.gov/covid-data-tracker (accessed on 22 August 2022).
- Hippisley-Cox, J.; Coupland, C.A.; Mehta, N.; Diaz-Ordaz, K.; Lyons, R.A.; Sheikh, A.; Rahman, S.; Valabhji, J.; Sellen, P.; Haq, N.; et al. Risk prediction of covid-19 related death and hospital admission in adults after covid-19 vaccination: National prospective cohort study. BMJ 2021, 374, n2244. [Google Scholar] [CrossRef] [PubMed]
- American Association for Cancer Research. AACR Report on the Impact of COVID-19 on Cancer Research and Patient Care; American Association for Cancer Research: Philadelphia, PA, USA, 2022; Available online: https://www.AACR.org/COVIDReport (accessed on 8 August 2022).
- Ladoire, S.; Goussot, V.; Redersdorff, E.; Cueff, A.; Ballot, E.; Truntzer, C.; Ayati, S.; Bengrine-Lefevre, L.; Bremaud, N.; Coudert, B.; et al. Seroprevalence of SARS-CoV-2 among the staff and patients of a French cancer centre after first lockdown: The canSEROcov study. Eur. J. Cancer 2021, 148, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Miami-Dade County. COVID-19 Dashboard; Miami-Dade County: Miami, FL, USA, 2022. Available online: https://miamidade.gov/information/library/2022-08-19-covid-dashboard.pdf (accessed on 19 August 2022).
- Van Dam, P.; Huizing, M.; Roelant, E.; Hotterbeekx, A.; De Winter, F.H.R.; Kumar-Singh, S.; Moons, P.; Amajoud, Z.; Vulsteke, C.; Croes, L.; et al. Immunoglobin G/total antibody testing for SARS-CoV-2: A prospective cohort study of ambulatory patients and health care workers in two Belgian oncology units comparing three commercial tests. Eur. J. Cancer 2021, 148, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Mair, M.J.; Mitterer, M.; Gattinger, P.; Berger, J.M.; Trutschnig, W.; Bathke, A.C.; Gansterer, M.; Berghoff, A.S.; Laengle, S.; Gottmann, L.; et al. Enhanced SARS-CoV-2 breakthrough infections in patients with hematologic and solid cancers due to Omicron. Cancer Cell 2022, 40, 444–446. [Google Scholar] [CrossRef]
- Ladoire, S.; Rederstorff, E.; Goussot, V.; Parnalland, S.; Briot, N.; Ballot, E.; Truntzer, C.; Ayati, S.; Bengrine-Lefevre, L.; Bremaud, N.; et al. Parallel evolution and differences in seroprevalence of SARS-CoV-2 antibody between patients with cancer and health care workers in a tertiary cancer centre during the first and second wave of COVID-19 pandemic: canSEROcov-II cross-sectional study. Eur. J. Cancer 2022, 165, 13–24. [Google Scholar] [CrossRef]
- Billock, R.M.; Groenewold, M.R.; Sweeney, M.H.; de Perio, M.A.; Gaughan, D.M.; Luckhaupt, S.E. Reported exposure trends among healthcare personnel COVID-19 cases, USA, March 2020–March 2021. Am. J. Infect. Control 2022, 50, 548–554. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. In Science Brief: Indicators for Monitoring COVID-19 Community Levels and Making Public Health Recommendations; US Department of Health and Human Services: CDC Atlanta, GA, USA, 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/indicators-monitoring-community-levels.html (accessed on 27 August 2022).
- Joung, S.Y.; Ebinger, J.E.; Sun, N.; Liu, Y.; Wu, M.; Tang, A.B.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Sobhani, K.; et al. Awareness of SARS-CoV-2 Omicron Variant Infection Among Adults With Recent COVID-19 Seropositivity. JAMA Netw. Open 2022, 5, e2227241. [Google Scholar] [CrossRef]
- Pinato, D.J.; Aguilar-Company, J.; Ferrante, D.; Hanbury, G.; Bower, M.; Salazar, R.; Mirallas, O.; Sureda, A.; Plaja, A.; Cucurull, M.; et al. Outcomes of the SARS-CoV-2 omicron (B.1.1.529) variant outbreak among vaccinated and unvaccinated patients with cancer in Europe: Results from the retrospective, multicentre, OnCovid registry study. Lancet Oncol. 2022, 23, 865–875. [Google Scholar] [CrossRef]
- Metzger, K.; Mrosek, J.; Zittel, S.; Pilz, M.; Held, T.; Adeberg, S.; Ristow, O.; Hoffmann, J.; Engel, M.; Freudlsperger, C.; et al. Treatment delay and tumor size in patients with oral cancer during the first year of the COVID-19 pandemic. Head Neck 2021, 43, 3493–3497. [Google Scholar] [CrossRef]
- Bardet, A.; Fraslin, A.M.; Marghadi, J.; Borget, I.; Faron, M.; Honoré, C.; Delaloge, S.; Albiges, L.; Planchard, D.; Ducreux, M.; et al. Impact of COVID-19 on healthcare organisation and cancer outcomes. Eur. J. Cancer 2021, 153, 123–132. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, J.; Cai, S.; Fu, X.; Ji, Y. Psychological distress of cancer patients caused by treatment delay during the COVID-19 pandemic in China: A cross-sectional study. Psychooncology 2022, 31, 1067–1615. [Google Scholar] [CrossRef] [PubMed]
- Sud, A.; Jones, M.E.; Broggio, J.; Loveday, C.; Torr, B.; Garrett, A.; Nicol, D.L.; Jhanji, S.; Boyce, S.A.; Gronthoud, F.; et al. Collateral damage: The impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann. Oncol. 2020, 31, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. COVID-19 and Cancer Consortium. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918, Erratum in Lancet 2020, 396, 758. [Google Scholar] [CrossRef]
- Ullrich, F.; Hanoun, C.; Turki, A.T.; Liebregts, T.; Breuckmann, K.; Alashkar, F.; Reinhardt, H.C.; von Tresckow, B.; von Tresckow, J. Early report on the severity of COVID-19 in hematologic patients infected with the SARS-CoV2 omicron variant. Eur. J. Haematol. 2022; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.; Lyman, G.H.; Bokemeyer, C.; Rapoport, B.L.; Mathieson, N.; Koptelova, N.; Cornes, P.; Anderson, R.; Gascón, P.; Kuderer, N.M. Supportive care in patients with cancer during the COVID-19 pandemic. ESMO Open 2021, 6, 100038. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Sze, S.; Minhas, J.S.; Bangash, M.N.; Pareek, N.; Divall, P.; Williams, C.M.; Oggioni, M.R.; Squire, I.B.; Nellums, L.B.; et al. The impact of ethnicity on clinical outcomes in COVID-19: A systematic review. eClinicalMedicine 2020, 23, 100404. [Google Scholar] [CrossRef]
- Mullangi, S.; Aviki, E.M.; Chen, Y.; Robson, M.; Hershman, D.L. Factors Associated With Cancer Treatment Delay Among Patients Diagnosed With COVID-19. JAMA Netw. Open 2022, 5, e2224296. [Google Scholar] [CrossRef]
- Kareff, S.; Diaz, C.; Zeigler, A.; Faulkenberry, J.G.; Utter, B.F.; Barber, C.M.; Symes, S. Characterization of the Demographics and Psychiatric Co-Morbidites Among Clients of a Human Rights Clinic in Miami-Dade County, Florida, United States. Cureus 2020, 12, e8944. [Google Scholar] [CrossRef]
- Hayward, S.E.; Deal, A.; Cheng, C.; Crawshaw, A.; Orcutt, M.; Vandrevala, T.F.; Norredam, M.; Carballo, M.; Ciftci, Y.; Requena-Méndez, A.; et al. Clinical outcomes and risk factors for COVID-19 among migrant populations in high-income countries: A systematic review. J. Migr. Health 2021, 3, 100041. [Google Scholar] [CrossRef]
- Zarifkar, P.; Kamath, A.; Robinson, C.; Morgulchik, N.; Shah, S.F.H.; Cheng, T.K.M.; Dominic, C.; Fehintola, A.O.; Bhalla, G.; Ahillan, T.; et al. Clinical Characteristics and Outcomes in Patients with COVID-19 and Cancer: A Systematic Review and Meta-analysis. Clin. Oncol. (R Coll Radiol.) 2021, 33, e180–e191. [Google Scholar] [CrossRef]
- Klompas, M.; Rhee, C.; Baker, M.A. Universal Use of N95 Respirators in Healthcare Settings When Community Coronavirus Disease 2019 Rates Are High. Clin. Infect. Dis. 2022, 74, 529–531. [Google Scholar] [CrossRef]
- Passaro, A.; Addeo, A.; Von Garnier, C.; Blackhall, F.; Planchard, D.; Felip, E.; Dziadziuszko, R.; de Marinis, F.; Reck, M.; Bouchaab, H.; et al. ESMO Management and treatment adapted recommendations in the COVID-19 era: Lung cancer. ESMO Open 2020, 5 (Suppl. 3), e000820. [Google Scholar] [CrossRef] [PubMed]
- Vecchione, L.; Stintzing, S.; Pentheroudakis, G.; Douillard, J.Y.; Lordick, F. ESMO management and treatment adapted recommendations in the COVID-19 era: Colorectal cancer. ESMO Open 2020, 5 (Suppl. 3), e000826. [Google Scholar] [CrossRef] [PubMed]
- Catanese, S.; Pentheroudakis, G.; Douillard, J.Y.; Lordick, F. ESMO Management and treatment adapted recommendations in the COVID-19 era: Pancreatic Cancer. ESMO Open 2020, 5 (Suppl. 3), e000804. [Google Scholar] [CrossRef] [PubMed]
- Shiels, M.S.; Haque, A.T.; Berrington de González, A.; Freedman, N.D. Leading Causes of Death in the US During the COVID-19 Pandemic, March 2020 to October 2021. JAMA Intern. Med. 2022, 182, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Galvani, A.P.; Parpia, A.S.; Pandey, A.; Sah, P.; Colón, K.; Friedman, G.; Campbell, T.; Kahn, J.G.; Singer, B.H.; Fitzpatrick, M.C. Universal healthcare as pandemic preparedness: The lives and costs that could have been saved during the COVID-19 pandemic. Proc. Natl. Acad. Sci. USA 2022, 119, e2200536119. [Google Scholar] [CrossRef]
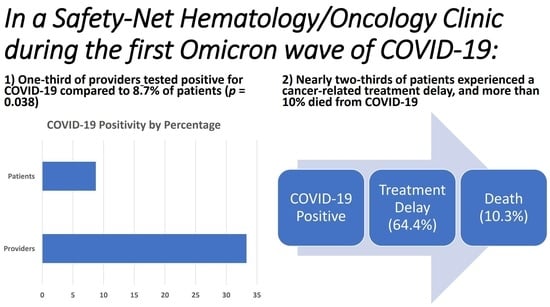
| Variable | n (% Total) | Variable | n (% Total) | p-Value * |
|---|---|---|---|---|
| Total Providers | 18 | Total Patients | 1031 | 0.038 |
| Providers testing positive for COVID-19 | 6 (33.3%) | Patients testing positive for COVID-19 | 90 (8.73%) | |
| Providers not testing positive for COVID-19 | 12 (66.7%) | Patients not testing positive for COVID-19 | 941 (91.27%) | |
| Total Deaths | 39 | 0.77 | ||
| Deaths due to COVID-19 | 4 (10.3%) 1 | |||
| Deaths due to Cancer or Other Causes | 35 (89.7%) | |||
| Cancer Treatment Delays due to COVID-19 | Median Days of Treatment Delay | Not applicable | ||
| Yes | 58 (64.4%) | 20 days (range 7–65 days) (IQR 15) | ||
| No | 32 (35.6%) |
| Demographic Category | Sub-Category | n (% Total) |
|---|---|---|
| Race | Black | 32 (35.6) |
| South Asian | 1 (1.1) | |
| White | 57 (63.3) | |
| Ethnicity | Hispanic/Latinx | 60 (66.7) |
| Not Hispanic/Latinx | 30 (33.3) | |
| Gender | Female | 49 (54.4) |
| Male | 41 (45.6) | |
| Age | 20–29 | 2 (2.2) |
| 30–39 | 9 (11.1) | |
| 40–49 | 11 (12.2) | |
| 50–59 | 31 (34.4) | |
| 60–69 | 27 (30.0) | |
| 70–79 | 7 (7.8) | |
| 80–89 | 2 (2.2) | |
| 90–99 | 1 (1.1) | |
| Condition | Breast Cancer | 23 (25.6%) |
| Lymphoma | 14 (15.6%) | |
| Lung Cancer | 12 (13.3%) | |
| Colorectal Cancer | 6 (6.7%) | |
| Multiple Myeloma | 5 (5.6%) | |
| Prostate Cancer | 4 (4.4%) | |
| Multiple Cancers | 3 (3.3%) | |
| Testicular Cancer | 2 (2.2%) | |
| Renal Cell Carcinoma | 2 (2.2%) | |
| Stomach Cancer | 2 (2.2%) | |
| Leukemia | 2 (2.2%) | |
| Brain Cancer | 2 (2.2%) | |
| Head and Neck Cancer | 2 (2.2%) | |
| Other 1 | 11 (12.3%) | |
| History of Previous COVID-19 | Yes | 6 (6.7) |
| No | 84 (93.3) | |
| Vaccination Status | Unvaccinated | 36 (40.0) |
| Under-vaccinated 2 | 8 (8.9) | |
| Vaccinated 3 | 36 (40.0) | |
| Boosted 4 | 8 (8.9) | |
| Unknown | 2 (2.2) |
| Clinical Outcome | Sub-Category | n (%) |
|---|---|---|
| Symptomatic Disease | Yes | 67 (74.4) |
| No | 23 (25.6) | |
| ED or Urgent Care Visit | Yes | 52 (57.8) |
| No | 38 (42.2) | |
| Admission to Hospital | Yes | 39 (43.3) |
| No | 51 (56.7) | |
| Admission to ICU | Yes | 9 (10.0) |
| No | 81 (90.0) | |
| Advanced Therapeutics | Yes | 25 (27.8) |
| No | 65 (72.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kareff, S.A.; Khan, A.; Barreto-Coelho, P.; Iyer, S.G.; Pico, B.; Stanchina, M.; Dutcher, G.; Monteiro de Oliveira Novaes, J.; Nallagangula, A.; Lopes, G. Prevalence and Outcomes of COVID-19 among Hematology/Oncology Patients and Providers of a Community-Facing Health System during the B1.1.529 (“Omicron”) SARS-CoV-2 Variant Wave. Cancers 2022, 14, 4629. https://doi.org/10.3390/cancers14194629
Kareff SA, Khan A, Barreto-Coelho P, Iyer SG, Pico B, Stanchina M, Dutcher G, Monteiro de Oliveira Novaes J, Nallagangula A, Lopes G. Prevalence and Outcomes of COVID-19 among Hematology/Oncology Patients and Providers of a Community-Facing Health System during the B1.1.529 (“Omicron”) SARS-CoV-2 Variant Wave. Cancers. 2022; 14(19):4629. https://doi.org/10.3390/cancers14194629
Chicago/Turabian StyleKareff, Samuel A., Aliya Khan, Priscila Barreto-Coelho, Sunil Girish Iyer, Brian Pico, Michele Stanchina, Giselle Dutcher, José Monteiro de Oliveira Novaes, Aparna Nallagangula, and Gilberto Lopes. 2022. "Prevalence and Outcomes of COVID-19 among Hematology/Oncology Patients and Providers of a Community-Facing Health System during the B1.1.529 (“Omicron”) SARS-CoV-2 Variant Wave" Cancers 14, no. 19: 4629. https://doi.org/10.3390/cancers14194629
APA StyleKareff, S. A., Khan, A., Barreto-Coelho, P., Iyer, S. G., Pico, B., Stanchina, M., Dutcher, G., Monteiro de Oliveira Novaes, J., Nallagangula, A., & Lopes, G. (2022). Prevalence and Outcomes of COVID-19 among Hematology/Oncology Patients and Providers of a Community-Facing Health System during the B1.1.529 (“Omicron”) SARS-CoV-2 Variant Wave. Cancers, 14(19), 4629. https://doi.org/10.3390/cancers14194629