Targeted Depletion of Hyaluronic Acid Mitigates Murine Breast Cancer Growth
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Cell Lines
2.2. YS1646 and Generation of YS-HAse
2.3. Bacterial Growth, Viability, and Analysis of HAse Expression
2.4. Gel Electrophoresis and Hyaluronic Acid-BSA LB (LB-HA/BSA) Plate Assays
2.5. Cell Proliferation and Scratch Test Assays
2.6. Orthotopic Tumor Implantation and Detection of Tumor Colonization by YS1646
2.7. Immunohistochemistry/Immunofluorescence (IHC/IF)
2.8. Administration and Induction of YS-HAse in Tumor-Bearing Mice
2.9. Flow Cytometry
2.10. Statistics
3. Results
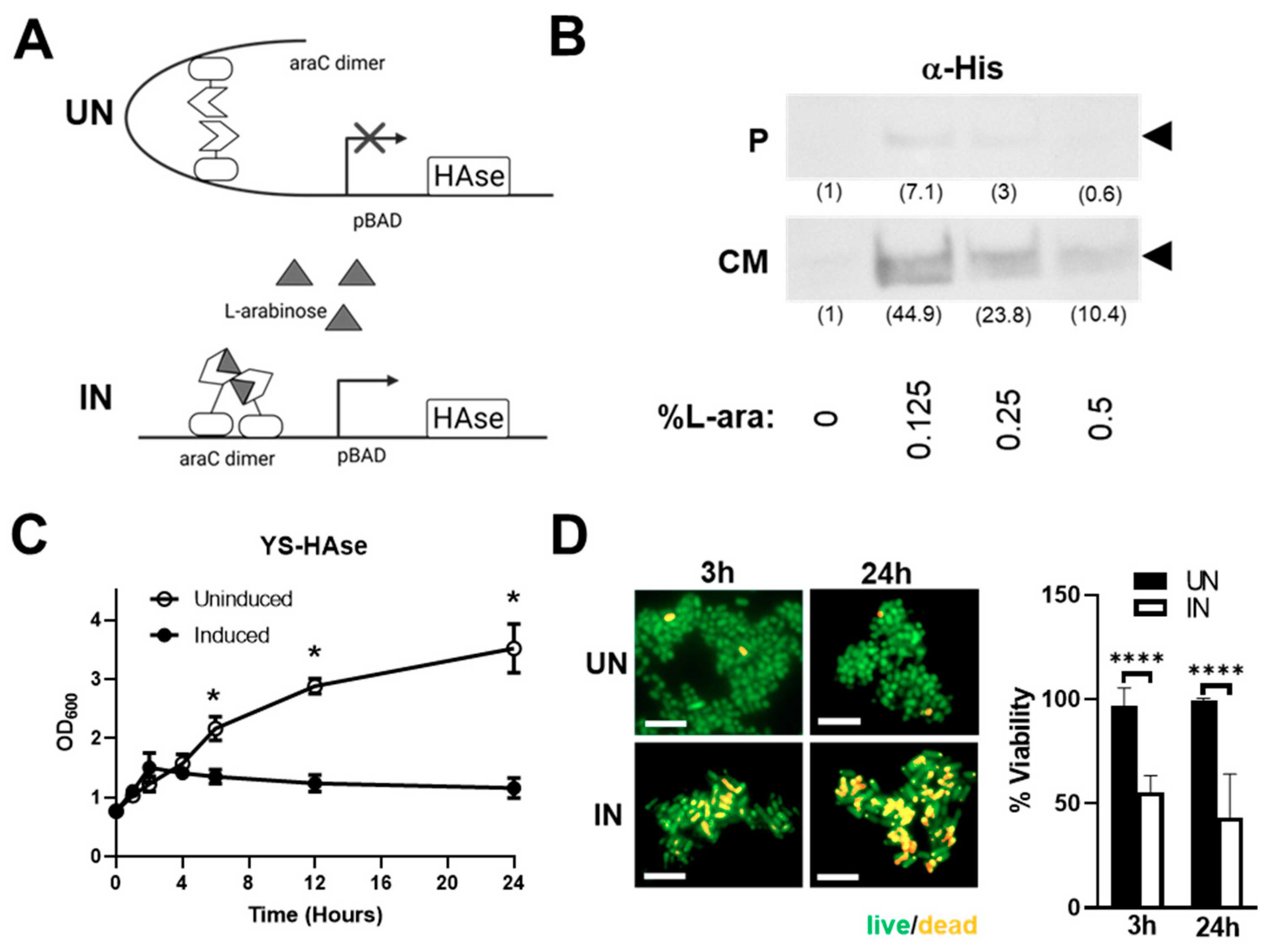
3.1. Construction and In Vitro Characterization of YS-HAse
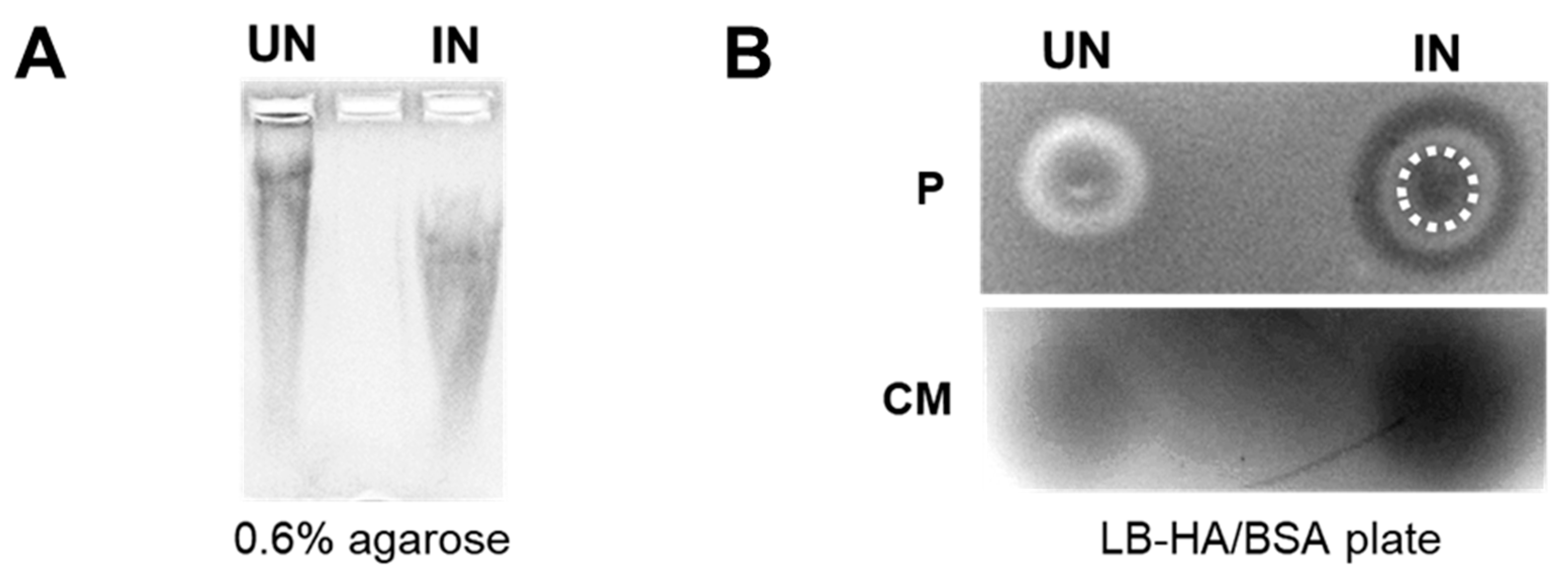
3.2. YS-HAse Secretes Functional HAse following L-Arabinose Induction
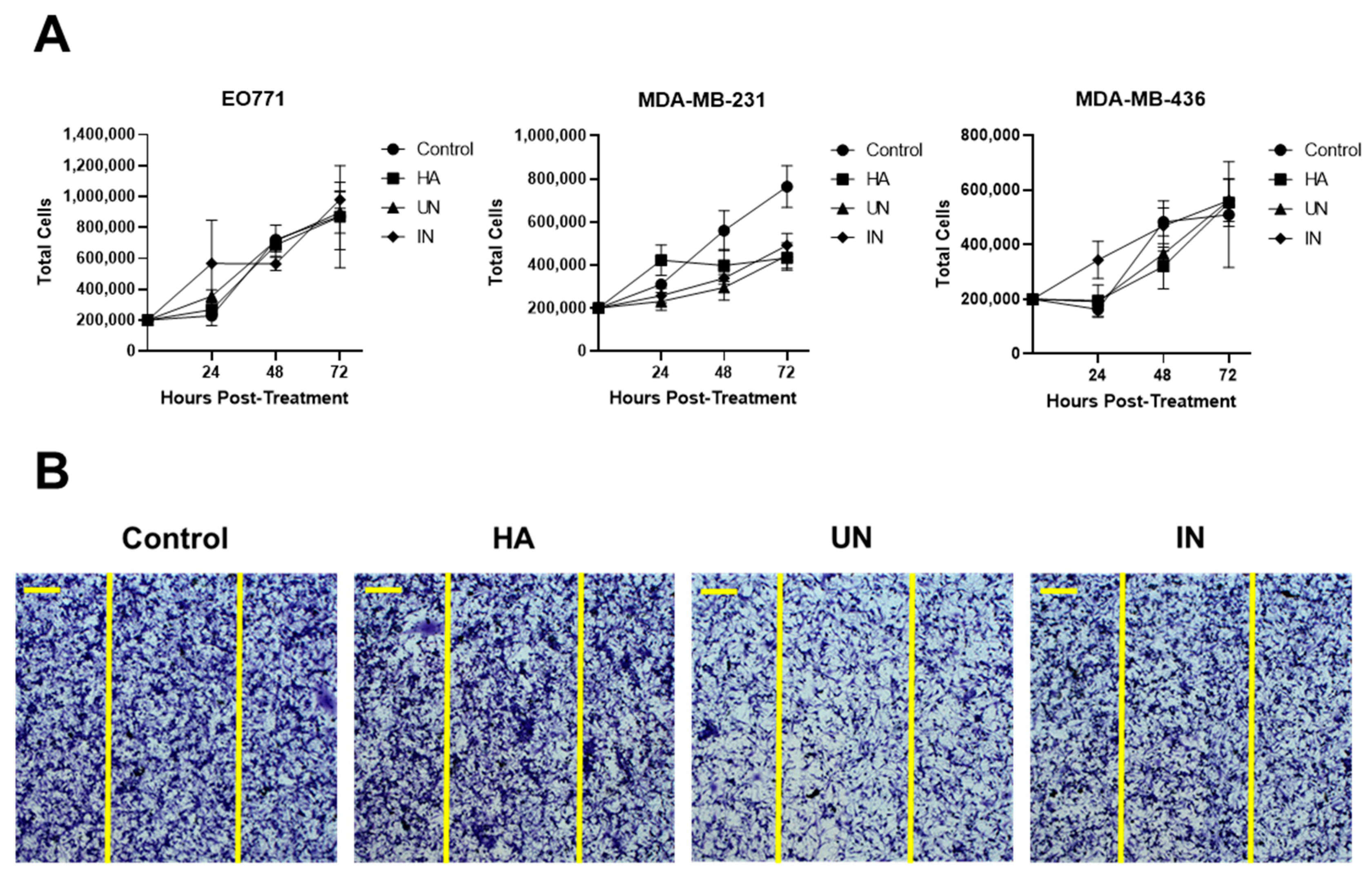
3.3. YS-HAse Degradation Products Do Not Affect BC Cell Proliferation and Migration
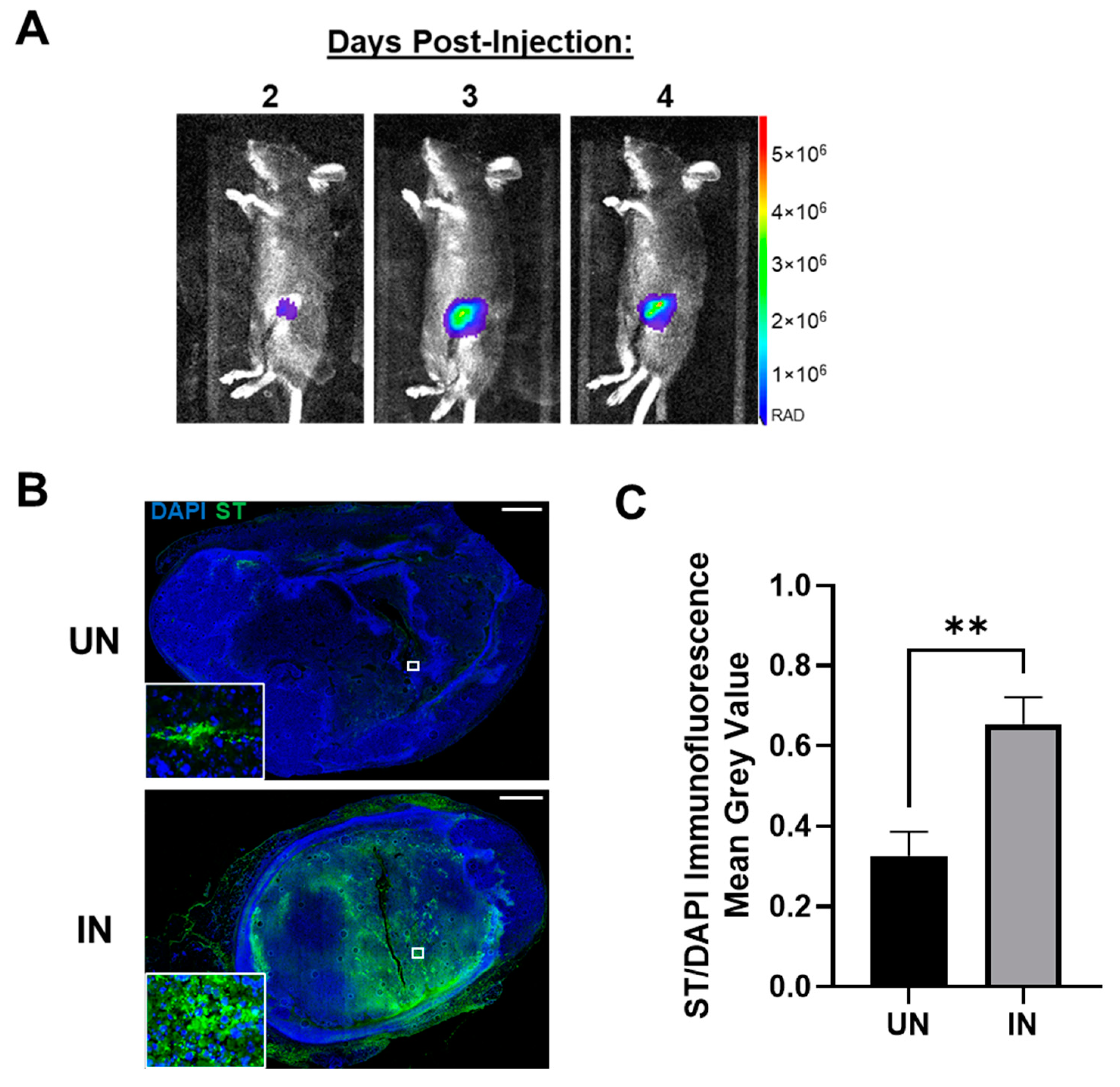
3.4. YS-HAse Specifically and Preferentially Colonizes Orthotopic BC Tumors
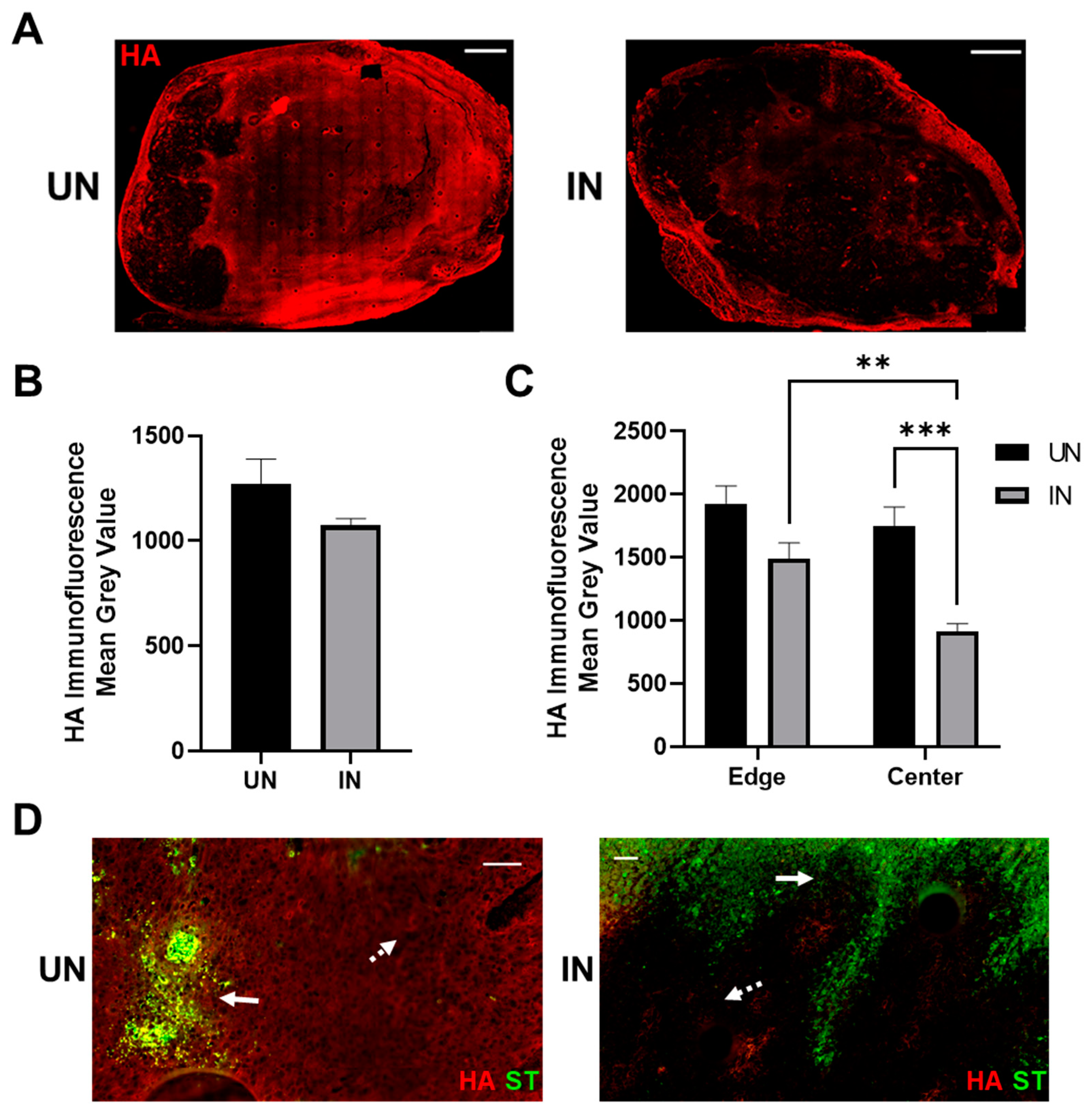
3.5. YS-HAse Degrades Intratumoral HA in Murine BC Tumors
3.6. Systemically Delivered YS-HAse Controls Orthotopic BC Tumor Growth in Immunocompetent Mice
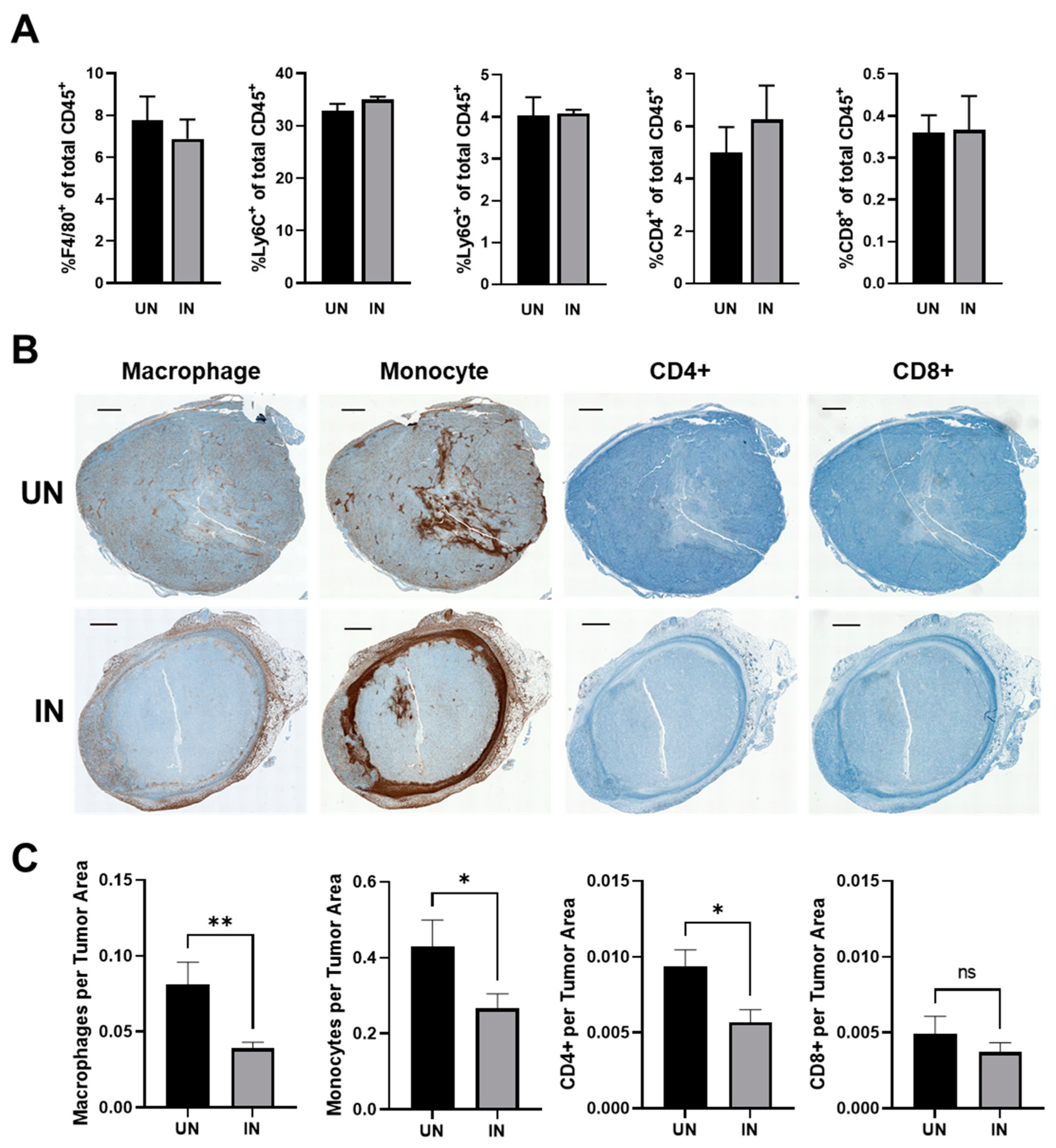
3.7. YS-HAse Treatment Decreases Immune Subsets in Areas of HA Degradation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, H.; Song, X.; Yang, Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin. Cancer Biol. 2020, 60, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Abyaneh, H.S.; Regenold, M.; McKee, T.D.; Allen, C.; Gauthier, M.A. Towards extracellular matrix normalization for improved treatment of solid tumors. Theranostics 2020, 10, 1960–1980. [Google Scholar] [CrossRef] [PubMed]
- Itano, N.; Zhuo, L.; Kimata, K. Impact of the hyaluronan-rich tumor microenvironment on cancer initiation and progression. Cancer Sci. 2008, 99, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Shepard, H.M. Breaching the Castle Walls: Hyaluronan Depletion as a Therapeutic Approach to Cancer Therapy. Front. Oncol. 2015, 5, 192. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, P.; Tammi, R.; Parkkinen, J.; Tammi, M.; Agren, U.; Johansson, R.; Hirvikoski, P.; Eskelinen, M.; Kosma, V.M. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am. J. Pathol. 2000, 156, 529–536. [Google Scholar] [CrossRef]
- Wu, W.; Chen, L.; Wang, Y.; Jin, J.; Xie, X.; Zhang, J. Hyaluronic acid predicts poor prognosis in breast cancer patients: A protocol for systematic review and meta analysis. Medicine 2020, 99, e20438. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cong, Y.; Wang, D.; Sun, Y.; Deng, L.; Liu, Y.; Martin-Trevino, R.; Shang, L.; McDermott, S.P.; Landis, M.D.; et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2014, 2, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Miletti-Gonzalez, K.E.; Murphy, K.; Kumaran, M.N.; Ravindranath, A.K.; Wernyj, R.P.; Kaur, S.; Miles, G.D.; Lim, E.; Chan, R.; Chekmareva, M.; et al. Identification of function for CD44 intracytoplasmic domain (CD44-ICD): Modulation of matrix metalloproteinase 9 (MMP-9) transcription via novel promoter response element. J. Biol. Chem. 2012, 287, 18995–19007. [Google Scholar] [CrossRef] [PubMed]
- Witschen, P.M.; Chaffee, T.S.; Brady, N.J.; Huggins, D.N.; Knutson, T.P.; LaRue, R.S.; Munro, S.A.; Tiegs, L.; McCarthy, J.B.; Nelson, A.C.; et al. Tumor Cell Associated Hyaluronan-CD44 Signaling Promotes Pro-Tumor Inflammation in Breast Cancer. Cancers 2020, 12, 1325. [Google Scholar] [CrossRef] [PubMed]
- Karalis, T.; Skandalis, S.S. Hyaluronan network: A driving force in cancer progression. Am. J. Physiol. Cell Physiol. 2022, 323, C145–C158. [Google Scholar] [CrossRef] [PubMed]
- Turley, E.A.; Noble, P.W.; Bourguignon, L.Y. Signaling properties of hyaluronan receptors. J. Biol. Chem. 2002, 277, 4589–4592. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin. Cancer Biol. 2008, 18, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.; Peyrollier, K.; Xia, W.; Gilad, E. Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J. Biol. Chem. 2008, 283, 17635–17651. [Google Scholar] [CrossRef]
- Jung, H. Hyaluronidase: An overview of its properties, applications, and side effects. Arch. Plast. Surg. 2020, 47, 297–300. [Google Scholar] [CrossRef]
- Weber, G.C.; Buhren, B.A.; Schrumpf, H.; Wohlrab, J.; Gerber, P.A. Clinical Applications of Hyaluronidase. Adv. Exp. Med. Biol. 2019, 1148, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Knudson, W.; Gundlach, M.W.; Schmid, T.M.; Conrad, H.E. Selective hydrolysis of chondroitin sulfates by hyaluronidase. Biochemistry 1984, 23, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Whatcott, C.J.; Han, H.; Posner, R.G.; Hostetter, G.; Von Hoff, D.D. Targeting the tumor microenvironment in cancer: Why hyaluronidase deserves a second look. Cancer Discov. 2011, 1, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Messina, L.; Gavira, J.A.; Pernagallo, S.; Unciti-Broceta, J.D.; Sanchez Martin, R.M.; Diaz-Mochon, J.J.; Vaccaro, S.; Conejero-Muriel, M.; Pineda-Molina, E.; Caruso, S.; et al. Identification and characterization of a bacterial hyaluronidase and its production in recombinant form. FEBS Lett. 2016, 590, 2180–2189. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.K.; McDonough, S.L.; Philip, P.A.; Hingorani, S.R.; Lacy, J.; Kortmansky, J.S.; Thumar, J.; Chiorean, E.G.; Shields, A.F.; Behl, D.; et al. Phase IB/II Randomized Study of FOLFIRINOX Plus Pegylated Recombinant Human Hyaluronidase Versus FOLFIRINOX Alone in Patients With Metastatic Pancreatic Adenocarcinoma: SWOG S1313. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.R.; Korn, R.L.; Rosen, L.S.; LoRusso, P.; Dychter, S.S.; Zhu, J.; Maneval, D.C.; Jiang, P.; Shepard, H.M.; Frost, G.; et al. Phase 1 trials of PEGylated recombinant human hyaluronidase PH20 in patients with advanced solid tumours. Br. J. Cancer 2018, 118, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Pavan, M.; Beninatto, R.; Galesso, D.; Panfilo, S.; Vaccaro, S.; Messina, L.; Guarise, C. A new potential spreading factor: Streptomyces koganeiensis hyaluronidase. A comparative study with bovine testes hyaluronidase and recombinant human hyaluronidase of the HA degradation in ECM. Biochim. Biophys. Acta 2016, 1860, 661–668. [Google Scholar] [CrossRef]
- Heimann, D.M.; Rosenberg, S.A. Continuous intravenous administration of live genetically modified salmonella typhimurium in patients with metastatic melanoma. J. Immunother. 2003, 26, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Toso, J.F.; Gill, V.J.; Hwu, P.; Marincola, F.M.; Restifo, N.P.; Schwartzentruber, D.J.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Stock, F.; et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Kasinskas, R.W.; Forbes, N.S. Salmonella typhimurium specifically chemotax and proliferate in heterogeneous tumor tissue in vitro. Biotechnol. Bioeng. 2006, 94, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Kazmierczak, R.A.; Eisenstark, A. Strains, Mechanism, and Perspective: Salmonella-Based Cancer Therapy. Int. J. Microbiol. 2016, 2016, 5678702. [Google Scholar] [CrossRef] [PubMed]
- Guzman, L.M.; Belin, D.; Carson, M.J.; Beckwith, J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1995, 177, 4121–4130. [Google Scholar] [CrossRef]
- Ebelt, N.D.; Zamloot, V.; Zuniga, E.; Passi, K.B.; Sobocinski, L.J.; Young, C.A.; Blazar, B.R.; Manuel, E.R. Collagenase-Expressing Salmonella Targets Major Collagens in Pancreatic Cancer Leading to Reductions in Immunosuppressive Subsets and Tumor Growth. Cancers 2021, 13, 3565. [Google Scholar] [CrossRef]
- Park, C.; Kim, Y.H.; Lee, S.R.; Park, S.; Jung, Y.; Lee, Y.; Kim, J.S.; Eom, T.; Kim, J.S.; Lee, D.M.; et al. Characterization of Recombinant Bovine Sperm Hyaluronidase and Identification of an Important Asn-X-Ser/Thr Motif for Its Activity. J. Microbiol. Biotechnol. 2018, 28, 1547–1553. [Google Scholar] [CrossRef]
- Guo, X.; Shi, Y.; Sheng, J.; Wang, F. A novel hyaluronidase produced by Bacillus sp. A50. PLoS ONE 2014, 9, e94156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.L.; Zhang, Y.; Cao, K.X.; Wang, X.M. Orthotopic Injection of Breast Cancer Cells into the Mice Mammary Fat Pad. J. Vis. Exp. JoVE 2019, 143, e58604. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Burdette, L.A.; Leach, S.A.; Wong, H.T.; Tullman-Ercek, D. Developing Gram-negative bacteria for the secretion of heterologous proteins. Microb. Cell Fact. 2018, 17, 196. [Google Scholar] [CrossRef]
- Gniadek, T.J.; Augustin, L.; Schottel, J.; Leonard, A.; Saltzman, D.; Greeno, E.; Batist, G. A Phase I, Dose Escalation, Single Dose Trial of Oral Attenuated Salmonella typhimurium Containing Human IL-2 in Patients with Metastatic Gastrointestinal Cancers. J. Immunother. 2020, 43, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Tolg, C.; Turley, E. Dissecting the Dual Nature of Hyaluronan in the Tumor Microenvironment. Front. Immunol. 2019, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.X.; Wang, X.Y.; Su, X.L.; Li, H.Y.; Shi, Y.; Wang, L.; Ren, G.S. Upregulation of HYAL1 expression in breast cancer promoted tumor cell proliferation, migration, invasion and angiogenesis. PLoS ONE 2011, 6, e22836. [Google Scholar] [CrossRef] [PubMed]
- Karsi, A.; Lawrence, M.L. Broad host range fluorescence and bioluminescence expression vectors for Gram-negative bacteria. Plasmid 2007, 57, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef] [PubMed]
- Schwertfeger, K.L.; Cowman, M.K.; Telmer, P.G.; Turley, E.A.; McCarthy, J.B. Hyaluronan, Inflammation, and Breast Cancer Progression. Front. Immunol. 2015, 6, 236. [Google Scholar] [CrossRef]
- Spinelli, F.M.; Vitale, D.L.; Icardi, A.; Caon, I.; Brandone, A.; Giannoni, P.; Saturno, V.; Passi, A.; Garcia, M.; Sevic, I.; et al. Hyaluronan preconditioning of monocytes/macrophages affects their angiogenic behavior and regulation of TSG-6 expression in a tumor type-specific manner. FEBS J. 2019, 286, 3433–3449. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, G.; Gomar-Hoss, C.; Sakr, L.; Ulsperger, E.; Wogritsch, C. The impact of extracellular matrix on the chemoresistance of solid tumors—Experimental and clinical results of hyaluronidase as additive to cytostatic chemotherapy. Cancer Lett. 1998, 131, 85–99. [Google Scholar] [CrossRef]
- Ebelt, N.D.; Zuniga, E.; Passi, K.B.; Sobocinski, L.J.; Manuel, E.R. Hyaluronidase-Expressing Salmonella Effectively Targets Tumor-Associated Hyaluronic Acid in Pancreatic Ductal Adenocarcinoma. Mol. Cancer Ther. 2020, 19, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, P.; Girard, N.; Delpech, B.; Duval, C.; d’Anjou, J.; Dauce, J.P. Hyaluronan (hyaluronic acid) and hyaluronectin in the extracellular matrix of human breast carcinomas: Comparison between invasive and non-invasive areas. Int. J. Cancer 1992, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.N.; Hirata, T.; Hayasaka, H.; Stern, R.; Murai, T.; Miyasaka, M. Tumor cells enhance their own CD44 cleavage and motility by generating hyaluronan fragments. J. Biol. Chem. 2006, 281, 5861–5868. [Google Scholar] [CrossRef]
- Franzmann, E.J.; Schroeder, G.L.; Goodwin, W.J.; Weed, D.T.; Fisher, P.; Lokeshwar, V.B. Expression of tumor markers hyaluronic acid and hyaluronidase (HYAL1) in head and neck tumors. Int. J. Cancer 2003, 106, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Kuang, D.M.; Wu, Y.; Chen, N.; Cheng, J.; Zhuang, S.M.; Zheng, L. Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood 2007, 110, 587–595. [Google Scholar] [CrossRef]
- Tiainen, S.; Tumelius, R.; Rilla, K.; Hamalainen, K.; Tammi, M.; Tammi, R.; Kosma, V.M.; Oikari, S.; Auvinen, P. High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology 2015, 66, 873–883. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, C.; Zhang, Q.; Ye, J.; Wang, F.; Zhang, Y.; Hunborg, P.; Varvares, M.A.; Hoft, D.F.; Hsueh, E.C.; et al. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. Oncotarget 2015, 6, 17462–17478. [Google Scholar] [CrossRef] [PubMed]
- Zenobia, C.; Hajishengallis, G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontol 2000 2015, 69, 142–159. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamloot, V.; Ebelt, N.D.; Soo, C.; Jinka, S.; Manuel, E.R. Targeted Depletion of Hyaluronic Acid Mitigates Murine Breast Cancer Growth. Cancers 2022, 14, 4614. https://doi.org/10.3390/cancers14194614
Zamloot V, Ebelt ND, Soo C, Jinka S, Manuel ER. Targeted Depletion of Hyaluronic Acid Mitigates Murine Breast Cancer Growth. Cancers. 2022; 14(19):4614. https://doi.org/10.3390/cancers14194614
Chicago/Turabian StyleZamloot, Vic, Nancy Danielle Ebelt, Catherine Soo, Shweta Jinka, and Edwin R. Manuel. 2022. "Targeted Depletion of Hyaluronic Acid Mitigates Murine Breast Cancer Growth" Cancers 14, no. 19: 4614. https://doi.org/10.3390/cancers14194614
APA StyleZamloot, V., Ebelt, N. D., Soo, C., Jinka, S., & Manuel, E. R. (2022). Targeted Depletion of Hyaluronic Acid Mitigates Murine Breast Cancer Growth. Cancers, 14(19), 4614. https://doi.org/10.3390/cancers14194614





