Safety of Inhomogeneous Dose Distribution IMRT for High-Grade Glioma Reirradiation: A Prospective Phase I/II Trial (GLIORAD TRIAL)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Study Design
- –
- Step 1 included a sequential treatment and evaluation of the first 3 subjects;
- –
- Step 2 included a sequential treatment and evaluation of 3 other patients;
- –
- Step 3 included a sequential treatment and evaluation of the last 6 patients.
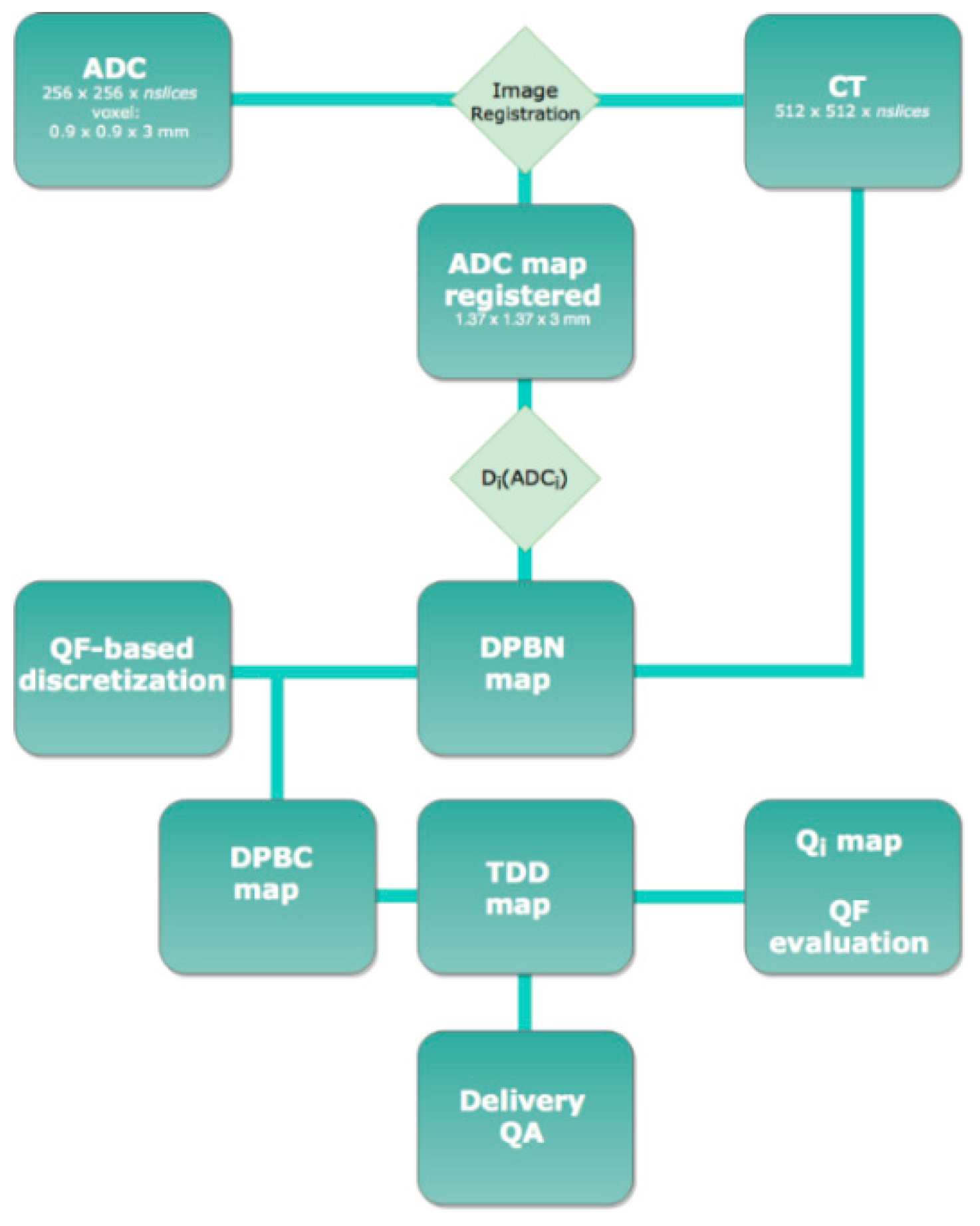
2.4. Imaging
2.5. Planning
2.6. Treatment
2.7. Follow-Up
2.8. Statistical Analysis
2.9. Ethics Approval and Consent to Participate
3. Results
3.1. Treatment Characteristics
3.2. Outcomes and Toxicities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.G.; Freels, S.; Grutsch, J.; Barlas, S.; Brem, S. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: An analysis based on Surveillance, Epidemiology, and End Results (SEER) data, 1973–1991. J. Neurosurg. 1998, 88, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McLendon, R.E.; Halperin, E.C. Is the long-term survival of patients with intracranial glioblastoma multiforme overstated? Cancer 2003, 98, 1745–1748. [Google Scholar] [CrossRef]
- Halperin, E.C.; Burger, P.C.; Bullard, D.E. The fallacy of the localized supratentorial malignant glioma. Int. J. Radiat. Oncol. Biol. Phys. 1988, 15, 505–509. [Google Scholar] [CrossRef]
- Lee, S.W.; A Fraass, B.; Marsh, L.H.; Herbort, K.; Gebarski, S.S.; Martel, M.K.; Radany, E.H.; Lichter, A.S.; Sandler, H.M. Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas: A quantitative dosimetric study. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 79–88. [Google Scholar] [CrossRef]
- Loeffler, J.; Alexander, E.; Hochberg, F.H.; Wen, P.Y.; Morris, J.H.; Schoene, W.C.; Siddon, R.L.; Morse, R.H.; Black, P.M. Clinical patterns of failure following stereotactic interstitial irradiation for malignant gliomas. Int. J. Radiat. Oncol. Biol. Phys. 1990, 19, 1455–1462. [Google Scholar] [CrossRef]
- Choucair, A.K.; Levin, V.A.; Gutin, P.H.; Davis, R.L.; Silver, P.; Edwards, M.S.B.; Wilson, C.B. Development of multiple lesions during radiation therapy and chemotherapy in patients with gliomas. J. Neurosurg. 1986, 65, 654–658. [Google Scholar] [CrossRef]
- Gaspar, L.E.; Fisher, B.J.; Macdonald, D.R.; Leber, D.V.; Halperin, E.C.; Schold, S.C., Jr.; Cairncross, J. Supratentorial malignant glioma: Patterns of recurrence and implications for external beam local treatment. Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 55–57. [Google Scholar] [CrossRef]
- Rossi, J.; Giaccherini, L.; Cavallieri, F.; Napoli, M.; Moratti, C.; Froio, E.; Serra, S.; Fraternali, A.; Ghadirpour, R.; Cozzi, S.; et al. Extracranial metastases in secondary glioblastoma multiforme: A case report. BMC Neurol. 2020, 20, 382. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; DeGroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef]
- Brandes, A.A.; Franceschi, E.; Tosoni, A.; Blatt, V.; Pession, A.; Tallini, G.; Bertorelle, R.; Bartolini, S.; Calbucci, F.; Andreoli, A.; et al. MGMT Promoter Methylation Status Can Predict the Incidence and Outcome of Pseudoprogression After Concomitant Radiochemotherapy in Newly Diagnosed Glioblastoma Patients. J. Clin. Oncol. 2008, 26, 2192–2197. [Google Scholar] [CrossRef]
- Taal, W.; Brandsma, D.; De Bruin, H.G.; Bromberg, J.E.C.; Swaak-Kragten, A.T.; Smitt, P.A.E.S.; Van Es, C.A.; Van Den Bent, M. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 2008, 113, 405–410. [Google Scholar] [CrossRef]
- Macdonald, D.R.; Cascino, T.L.; Schold, S.C.; Cairncross, J.G. Response criteria for phase II studies of supratentorial malignant glioma. J. Clin. Oncol. 1990, 8, 1277–1280. [Google Scholar] [CrossRef]
- Zikou, A.; Sioka, C.; Alexiou, G.A.; Fotopoulos, A.; Voulgaris, S.; Argyropoulou, M.I. Radiation Necrosis, Pseudoprogression, Pseudoresponse, and Tumor Recurrence: Imaging Challenges for the Evaluation of Treated Gliomas. Contrast Media Mol. Imaging 2018, 2018, 6828396. [Google Scholar] [CrossRef]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- A Reardon, D.; Weller, M. Pseudoprogression: Fact or wishful thinking in neuro-oncology? Lancet Oncol. 2018, 19, 1561–1563. [Google Scholar] [CrossRef]
- Omuro, A.; Vlahovic, G.; Lim, M.; Sahebjam, S.; Baehring, J.; Cloughesy, T.; Voloschin, A.; Ramkissoon, S.H.; Ligon, K.L.; Latek, R.; et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: Results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018, 20, 674–686. [Google Scholar] [CrossRef]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; la Fougère, C.; Langen, K.-J.; Lopci, E.; Lowe, V.; et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: Version 1.0. Eur. J. Pediatr. 2018, 46, 540–557. [Google Scholar] [CrossRef]
- Weller, M.; Cloughesy, T.; Perry, J.R.; Wick, W. Standards of care for treatment of recurrent glioblastoma—Are we there yet? Neuro-Oncology 2013, 15, 4–27. [Google Scholar] [CrossRef]
- Okada, H.; Weller, M.; Huang, R.; Finocchiaro, G.; Gilbert, M.R.; Wick, W.; Ellingson, B.M.; Hashimoto, N.; Pollack, I.F.; Brandes, A.A.; et al. Immunotherapy response assessment in neuro-oncology: A report of the RANO working group. Lancet Oncol. 2015, 16, e534–e542. [Google Scholar] [CrossRef]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; van den Bent, M.J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef]
- Shah, R.; Vattoth, S.; Jacob, R.; Manzil, F.F.P.; O’Malley, J.P.; Borghei, P.; Patel, B.N.; Curé, J.K. Radiation Necrosis in the Brain: Imaging Features and Differentiation from Tumor Recurrence. RadioGraphics 2012, 32, 1343–1359. [Google Scholar] [CrossRef]
- Kumar, A.J.; Leeds, N.E.; Fuller, G.N.; Van Tassel, P.; Maor, M.H.; Sawaya, R.E.; Levin, V.A. Malignant Gliomas: MR Imaging Spectrum of Radiation Therapy- and Chemotherapy-induced Necrosis of the Brain after Treatment. Radiology 2000, 217, 377–384. [Google Scholar] [CrossRef]
- Mullins, M.E.; Barest, G.D.; Schaefer, P.W.; Hochberg, F.H.; Gonzalez, R.G.; Lev, M.H. Radiation necrosis versus glioma recurrence: Conventional MR imaging clues to diagnosis. Am. J. Neuroradiol. 2005, 26, 1967–1972. [Google Scholar]
- Hein, P.A.; Eskey, C.J.; Dunn, J.F.; Hug, E.B. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: Tumor recurrence versus radiation injury. Am. J. Neuroradiol. 2004, 25, 201–209. [Google Scholar]
- Xu, J.-L.; Li, Y.-L.; Lian, J.-M.; Dou, S.-W.; Yan, F.-S.; Wu, H.; Shi, D.-P. Distinction between postoperative recurrent glioma and radiation injury using MR diffusion tensor imaging. Neuroradiology 2010, 52, 1193–1199. [Google Scholar] [CrossRef]
- Barajas, R.F., Jr.; Chang, J.S.; Segal, M.R.; Parsa, A.T.; McDermott, M.W.; Berger, M.S.; Cha, S. Differentiation of Recurrent Glioblastoma Multiforme from Radiation Necrosis after External Beam Radiation Therapy with Dynamic Susceptibility-weighted Contrast-enhanced Perfusion MR Imaging. Radiology 2009, 253, 486–496. [Google Scholar] [CrossRef]
- Rabinov, J.D.; Lee, P.L.; Barker, F.G.; Louis, D.N.; Harsh, G.R.; Cosgrove, G.R.; Chiocca, E.A.; Thornton, A.F.; Loeffler, J.S.; Henson, J.W.; et al. In Vivo 3-T MR Spectroscopy in the Distinction of Recurrent Glioma versus Radiation Effects: Initial Experience. Radiology 2022, 225, 871–879. [Google Scholar] [CrossRef]
- Zhou, J.; Tryggestad, E.; Wen, Z.-B.; Lal, B.; Zhou, T.; Grossman, R.; Wang, S.; Yan, K.; Fu, D.-X.; Ford, E.; et al. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat. Med. 2011, 17, 130–134. [Google Scholar] [CrossRef]
- Kim, Y.H.; Oh, S.W.; Lim, Y.J.; Park, C.-K.; Lee, S.-H.; Kang, K.W.; Jung, H.-W.; Chang, K.H. Differentiating radiation necrosis from tumor recurrence in high-grade gliomas: Assessing the efficacy of 18F-FDG PET, 11C-methionine PET and perfusion MRI. Clin. Neurol. Neurosurg. 2010, 112, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Woodroffe, R.W.; Zanaty, M.; Soni, N.; Mott, S.L.; Helland, L.C.; Pasha, A.; Maley, J.; Dhungana, N.; A Jones, K.; Monga, V.; et al. Survival after reoperation for recurrent glioblastoma. J. Clin. Neurosci. 2020, 73, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Helseth, R.; Helseth, E.; Johannesen, T.B.; Langberg, C.W.; Lote, K.; Rønning, P.; Scheie, D.; Vik, A.; Meling, T.R. Overall survival, prognostic factors, and repeated surgery in a consecutive series of 516 patients with glioblastoma multiforme: Survival, prognostic factors, and repeat surgery in GBM patients. Acta Neurol. Scand. 2010, 122, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, G.M.V.; Jenkinson, M.D.; Brodbelt, A.R. ‘Recurrent’ glioblastoma multiforme, when should we reoperate? Br. J. Neurosurg. 2008, 22, 452–455. [Google Scholar] [CrossRef]
- Ringel, F.; Pape, H.; Sabel, M.; Krex, D.; Bock, H.C.; Misch, M.; Weyerbrock, A.; Westermaier, T.; Senft, C.; Schucht, P.; et al. Clinical benefit from resection of recurrent glioblastomas: Results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro-Oncology 2016, 18, 96–104. [Google Scholar] [CrossRef]
- Cozzi, S.; Jamal, D.N.; Slocker, A.; Laplana, M.; Tejedor, A.G.; Krengli, M.; Guedea, F.; Gutierrez, C. Second breast-conserving therapy with interstitial brachytherapy (APBI) as a salvage treatment in ipsilateral breast tumor recurrence: A retrospective study of 40 patients. J. Contemp. Brachytherapy 2019, 11, 101–107. [Google Scholar] [CrossRef]
- Laplana, M.; Cozzi, S.; Najjari, D.; Martín, M.I.; Rodríguez, G.; Slocker, A.; Sancho, I.; Pla, M.J.; García, M.; Gracia, R.; et al. Five-year results of accelerated partial breast irradiation: A single-institution retrospective review of 289 cases. Brachytherapy 2021, 20, 807–817. [Google Scholar] [CrossRef]
- Maddalo, M.; D’Angelo, E.; Fiorica, F.; Argenone, A.; Scricciolo, M.; Cozzi, S.; Nardangeli, A.; Dionisi, F.; Costantino, G.; Vagge, F.; et al. Thoracic re-irradiation with 3D-conformal or more advanced techniques: A systematic review of treatment safety by the Re-irradiation Study Group of the Italian Association of Radiation and Oncology AIRO. Crit. Rev. Oncol. 2021, 167, 103500. [Google Scholar] [CrossRef]
- Cozzi, S.; Augugliaro, M.; Ciammella, P.; Botti, A.; Trojani, V.; Najafi, M.; Blandino, G.; Ruggieri, M.P.; Giaccherini, L.; Alì, E.; et al. The Role of Interstitial Brachytherapy for Breast Cancer Treatment: An Overview of Indications, Applications, and Technical Notes. Cancers 2022, 14, 2564. [Google Scholar] [CrossRef]
- Shi, W.; Bryan, M.S.; Gilbert, M.R.; Mehta, M.P.; Blumenthal, D.T.; Brown, P.D.; Valeinis, E.; Hopkins, K.; Souhami, L.; Andrews, D.W.; et al. Investigating the Effect of Reirradiation or Systemic Therapy in Patients with Glioblastoma after Tumor Progression: A Secondary Analysis of NRG Oncology/Radiation Therapy Oncology Group Trial 0525. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 38–44. [Google Scholar] [CrossRef]
- Cozzi, S.; Najafi, M.; Gomar, M.; Ciammella, P.; Iotti, C.; Iaccarino, C.; Dominici, M.; Pavesi, G.; Chiavelli, C.; Kazemian, A.; et al. Delayed Effect of Dendritic Cells Vaccination on Survival in Glioblastoma: A Systematic Review and Meta-Analysis. Curr. Oncol. 2022, 29, 75. [Google Scholar] [CrossRef]
- Kazmi, F.; Soon, Y.Y.; Leong, Y.H.; Koh, W.Y.; Vellayappan, B. Re-irradiation for recurrent glioblastoma (GBM): A systematic review and meta-analysis. J. Neuro-Oncol. 2019, 142, 79–90. [Google Scholar] [CrossRef]
- Paoletti, L.; Ceccarelli, C.; Menichelli, C.; Aristei, C.; Borghesi, S.; Tucci, E.; Bastiani, P.; Cozzi, S. Special stereotactic radiotherapy techniques: Procedures and equipment for treatment simulation and dose delivery. Rep. Pract. Oncol. Radiother. 2022, 27, 1–9. [Google Scholar] [CrossRef]
- Mayer, R.; Sminia, P. Reirradiation Tolerance of the Human Brain. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1350–1360. [Google Scholar] [CrossRef]
- Scoccianti, S.; Francolini, G.; Carta, G.A.; Greto, D.; Detti, B.; Simontacchi, G.; Visani, L.; Baki, M.; Poggesi, L.; Bonomo, P.; et al. Re-irradiation as salvage treatment in recurrent glioblastoma: A comprehensive literature review to provide practical answers to frequently asked questions. Crit. Rev. Oncol. Hematol. 2018, 126, 80–91. [Google Scholar] [CrossRef]
- Shapiro, L.Q.; Beal, K.; Goenka, A.; Karimi, S.; Iwamoto, F.M.; Yamada, Y.; Zhang, Z.; Lassman, A.B.; Abrey, L.E.; Gutin, P.H. Patterns of Failure After Concurrent Bevacizumab and Hypofractionated Stereotactic Radiation Therapy for Recurrent High-Grade Glioma. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 636–642. [Google Scholar] [CrossRef]
- Orlandi, M.; Botti, A.; Sghedoni, R.; Cagni, E.; Ciammella, P.; Iotti, C.; Iori, M. Feasibility of voxel-based Dose Painting for recurrent Glioblastoma guided by ADC values of Diffusion-Weighted MR imaging. Phys. Med. 2016, 32, 1651–1658. [Google Scholar] [CrossRef]
- Thorwarth, D.; Eschmann, S.-M.; Paulsen, F.; Alber, M. Hypoxia Dose Painting by Numbers: A Planning Study. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 291–300. [Google Scholar] [CrossRef]
- Meijer, G.; Steenhuijsen, J.; Bal, M.; De Jaeger, K.; Schuring, D.; Theuws, J. Dose painting by contours versus dose painting by numbers for stage II/III lung cancer: Practical implications of using a broad or sharp brush. Radiother. Oncol. 2011, 100, 396–401. [Google Scholar] [CrossRef]
- Bowen, S.R.; Flynn, R.; Bentzen, S.M.; Jeraj, R. On the sensitivity of IMRT dose optimization to the mathematical form of a biological imaging-based prescription function. Phys. Med. Biol. 2009, 54, 1483–1501. [Google Scholar] [CrossRef]
- Vanderstraeten, B.; De Gersem, W.; Duthoy, W.; De Neve, W.; Thierens, H. Implementation of biologically conformal radiation therapy (BCRT) in an algorithmic segmentation-based inverse planning approach. Phys. Med. Biol. 2006, 51, N277–N286. [Google Scholar] [CrossRef]
- National Cancer Institute. Common Terminology Criteria for Adverse Events v.3.0and v.4.0 (CTCAE). Available online: http://ctep.cancer.gov/protocol (accessed on 25 May 2022).
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Tosoni, A.; Franceschi, E.; Poggi, R.; Brandes, A.A. Relapsed Glioblastoma: Treatment Strategies for Initial and Subsequent Recurrences. Curr. Treat. Options Oncol. 2016, 17, 49. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Combs, S.E.; Edler, L.; Rausch, R.; Welzel, T.; Wick, W.; Debus, J. Generation and validation of a prognostic score to predict outcome after re-irradiation of recurrent glioma. Acta Oncol. 2013, 52, 147–152. [Google Scholar] [CrossRef]
- Niyazi, M.; Adeberg, S.; Kaul, D.; Boulesteix, A.-L.; Bougatf, N.; Fleischmann, D.F.; Grün, A.; Krämer, A.; Rödel, C.; Eckert, F.; et al. Independent validation of a new reirradiation risk score (RRRS) for glioma patients predicting post-recurrence survival: A multicenter DKTK/ROG analysis. Radiother. Oncol. 2018, 127, 121–127. [Google Scholar] [CrossRef]
- Sminia, P.; Mayer, R. External Beam Radiotherapy of Recurrent Glioma: Radiation Tolerance of the Human Brain. Cancers 2012, 4, 379–399. [Google Scholar] [CrossRef]
- Ciammella, P.; Podgornii, A.; Galeandro, M.; D’Abbiero, N.; Pisanello, A.; Botti, A.; Cagni, E.; Iori, M.; Iotti, C. Hypofractionated stereotactic radiation therapy for recurrent glioblastoma: Single institutional experience. Radiat. Oncol. 2013, 8, 222. [Google Scholar] [CrossRef]
- Cabrera, A.R.; Cuneo, K.C.; Desjardins, A.; Sampson, J.H.; McSherry, F.; Herndon, J.E.; Peters, K.B.; Allen, K.; Hoang, J.K.; Chang, Z.; et al. Concurrent Stereotactic Radiosurgery and Bevacizumab in Recurrent Malignant Gliomas: A Prospective Trial. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 873–879. [Google Scholar] [CrossRef]
- Clarke, J.; Neil, E.; Terziev, R.; Gutin, P.; Barani, I.; Kaley, T.; Lassman, A.B.; Chan, T.A.; Yamada, J.; DeAngelis, L.; et al. Multicenter, Phase 1, Dose Escalation Study of Hypofractionated Stereotactic Radiation Therapy with Bevacizumab for Recurrent Glioblastoma and Anaplastic Astrocytoma. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 797–804. [Google Scholar] [CrossRef]
- Ernst-Stecken, A.; Ganslandt, O.; Lambrecht, U.; Sauer, R.; Grabenbauer, G. Survival and quality of life after hypofractionated stereotactic radiotherapy for recurrent malignant glioma. J. Neurooncol. 2007, 81, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Fields, E.C.; Damek, D.; Gaspar, L.E.; Liu, A.K.; Kavanagh, B.D.; Waziri, A.; Lillehei, K.; Chen, C. Phase I Dose Escalation Trial of Vandetanib with Fractionated Radiosurgery in Patients with Recurrent Malignant Gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Gutin, P.H.; Iwamoto, F.M.; Beal, K.; Mohile, N.A.; Karimi, S.; Hou, B.L.; Lymberis, S.; Yamada, Y.; Chang, J.; Abrey, L.E. Safety and Efficacy of Bevacizumab with Hypofractionated Stereotactic Irradiation for Recurrent Malignant Gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.-S.; Lee, J.-I.; Park, K.; Kim, J.H.; Lim, D.-H.; Nam, D.-H. Efficacy of stereotactic radiosurgery as a salvage treatment for recurrent malignant gliomas. Cancer 2008, 112, 2046–2051. [Google Scholar] [CrossRef]
- A Larson, D.; Prados, M.; Lamborn, K.R.; Smith, V.; Sneed, P.K.; Chang, S.; Nicholas, K.M.; Wara, W.M.; Devriendt, D.; Kunwar, S.; et al. Phase II study of high central dose Gamma Knife radiosurgery and marimastat in patients with recurrent malignant glioma. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 1397–1404. [Google Scholar] [CrossRef]
- Lee, J.; Ahn, S.S.; Chang, J.H.; Suh, C.-O. Hypofractionated Re-irradiation after Maximal Surgical Resection for Recurrent Glioblastoma: Therapeutic Adequacy and Its Prognosticators of Survival. Yonsei Med. J. 2018, 59, 194–201. [Google Scholar] [CrossRef]
- Maranzano, E.; Anselmo, P.; Casale, M.; Trippa, F.; Carletti, S.; Principi, M.; Loreti, F.; Italiani, M.; Caserta, C.; Giorgi, C. Treatment of recurrent glioblastoma with stereotactic radiotherapy: Long-term results of a mono-institutional trial. Tumori 2011, 97, 56–61. [Google Scholar] [CrossRef]
- Møller, S.; Rosenschold, P.M.A.; Costa, J.; Law, I.; Poulsen, H.S.; Engelholm, S.A.; Engelholm, S. Toxicity and efficacy of re-irradiation of high-grade glioma in a phase I dose- and volume escalation trial. Radiother. Oncol. 2017, 125, 223–227. [Google Scholar] [CrossRef]
- Schwer, A.L.; Damek, D.M.; Kavanagh, B.D.; Gaspar, L.E.; Lillehei, K.; Stuhr, K.; Chen, C. A Phase I Dose-Escalation Study of Fractionated Stereotactic Radiosurgery in Combination with Gefitinib in Patients With Recurrent Malignant Gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 993–1001. [Google Scholar] [CrossRef]
- Shi, W.; Palmer, J.D.; Werner-Wasik, M.; Andrews, D.W.; Evans, J.J.; Glass, J.; Kim, L.; Bar-Ad, V.; Judy, K.; Farrell, C.; et al. Phase I trial of panobinostat and fractionated stereotactic re-irradiation therapy for recurrent high grade gliomas. J. Neurooncol. 2016, 127, 535–539. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Saito, K.; Kajiwara, K.; Nomura, S.; Ishihara, H.; Suzuki, M. CyberKnife Stereotactic Radiotherapy for Patients with Malignant Glioma. Minim. Invasive Neurosurg. 2006, 49, 110–115. [Google Scholar] [CrossRef]
- Cho, K.H.; A Hall, W.; Gerbi, B.J.; Higgins, P.D.; A McGuire, W.; Clark, H. Single dose versus fractionated stereotactic radiotherapy for recurrent high-grade gliomas. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 1133–1141. [Google Scholar] [CrossRef]
- Kim, B.; Soisson, E.; Duma, C.; Chen, P.; Hafer, R.; Cox, C.; Cubellis, J.; Minion, A.; Plunkett, M.; Mackintosh, R. Treatment of recurrent high grade gliomas with hypofractionated stereotactic image-guided helical tomotherapy. Clin. Neurol. Neurosurg. 2011, 113, 509–512. [Google Scholar] [CrossRef]
- Gregucci, F.; Surgo, A.; Carbonara, R.; Laera, L.; Ciliberti, M.P.; Gentile, M.A.; Caliandro, M.; Sasso, N.; Bonaparte, I.; Fanelli, V.; et al. Radiosurgery and Stereotactic Brain Radiotherapy with Systemic Therapy in Recurrent High-Grade Gliomas: Is It Feasible? Therapeutic Strategies in Recurrent High-Grade Gliomas. J. Pers. Med. 2022, 12, 1336. [Google Scholar] [CrossRef]

| Study Population (n = 12) | |
|---|---|
| Primary Treatment | |
TYPE OF SURGERY, n (%)
| 8 (66.7) 3 (25) 1 (8.3) |
HISTOLOGICAL TYPE, n (%)
| 10 (83.4) 1 (8.3) 1 (8.3) |
IDH MUTATION, n (%)
| 4 (33.3) 1 (8.3) 7 (58.4) |
MGMT metylation, n (%)
| 7 (58.4) 5 (41.6) |
1p19q-codeletion, n (%)
| 1 (8.3) 10 (83.4) 1 (8.3) |
Radiotherapy dose, n (%)
| 11 (91.7) 1 (8.3) |
Re-surgery, n (%)
| 2 (16.7) 10 (83.3) |
Second-line chemotherapy, n (%)
| 3 (25) 9 (75) |
| At Recurrence | |
GENDER, n (%)
| 7 (58.3) 5 (41.7) |
| Median age, years (range) | 60.5 (51–70) |
KPS, n (%)
| 9 (75) 1(8.3) 1 (8.3) 1 (8.3) |
GTV, cc
| 22.95 5–69.6 |
PTV, cc
| 45.7 15–97.13 |
| Patient | 3-m MR I Evaluation | 3-m Tox (Grade) | 6-m MRI Evaluation | 6-m Tox (Grade) | 12-m Tox (Grade) | RN | PFS2 (m) | OS2 (m) | Treatment to Progression |
|---|---|---|---|---|---|---|---|---|---|
| 1 | SD | G1 (headache) | PD | G1 (eye disorders—other, headache, fatigue) | G1 (cognitive disturbance) | Yes | 6.9 | 13.2 | Fotemustine |
| 2 | NA | G2 (dysphasia), G2 (muscle weakness lower limb), G3 (muscle weakness, fatigue) | NA | NA | NA | 1.2 | 2.8 | BSC | |
| 3 | NA | NA | NA | NA | NA | 5.5 | 11.6 | NA | |
| 4 | PD | G1 (fatigue, muscle weakness lower limb, nausea, eye disorders—other) | NA | NA | NA | 2.2 | 5.3 | Fotemustine | |
| 5 | NA | G1 (ataxia), G2 (nervous system disorders—other, muscle weakness lower limb) | NA | NA | NA | 5.3 (date of death) | 5.3 | BSC | |
| 6 | SD | G1 (irritability) | SD | G0 | G1 (irritability) | Yes | 9.3 | 12.6 | BSC |
| 7 | PD | G1 (generalized muscle weakness, dysesthesia), G2 (headache) | PD | G1 (ataxia), G2 (nervous system disorders—other) | NA | 3.5 | 6.8 | Fotemustine | |
| 8 | SD | G2 (fatigue, gynecomastia) | SD | G1 (fatigue) | G1 (fatigue) | 10.9 | 19.3 | Fotemustine | |
| 9 | PsP | G1 (headache, fatigue, seizure, conjunctivitis), G2 (eye disorders-other, urinary incontinence, generalized muscle weakness | PD | G2 (seizure, nervous system disorders—other) | NA | 2.6 | 9.2 | BSC | |
| 10 | PD | G1 (dysesthesia, headache) | NA | NA | NA | 3.3 | 6.1 | BSC | |
| 11 | PD | G1 (dysphasia, cognitive disturbance, headache) | PD | G0 | G1 (nervous system disorders—other) | 4.1 | 16.5 | Fotemustine | |
| 12 | SD | G1 (fatigue, irritability) | SD | G0 | G2 (headache) | 11.5 | 13.5 | Surgery |
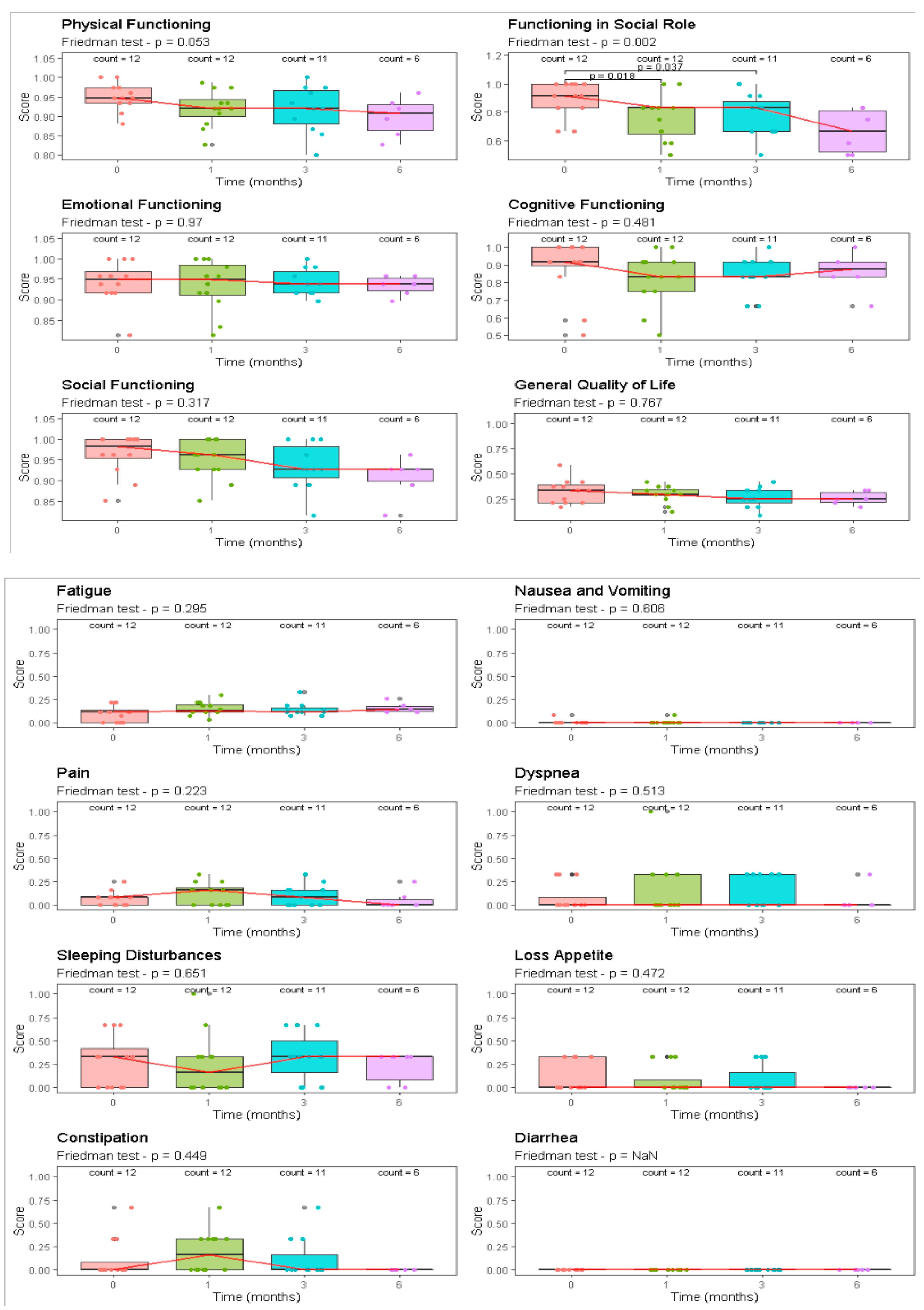
| EORTC QLO-C30 | Pre Treat | 30 Days | 90 Days | 120 Days | |||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 12) | (n = 12) | (n = 12) | (n = 12) | p | |||||
| Component | Median [Range] | Median [Range] | Median [Range] | Median [Range] | Friedman | ||||
| Functional scale | |||||||||
| Physical functioning | 95 | [88–100] | 92 | [83–99] | 92 | [80–100] | 91 | [83–96] | 0.05 |
| Functioning in social role | 92 | [67–100] | 83 | [50–100] | 83 | [50–100] | 67 | [50–83] | <0.01 |
| Emotional functioning | 95 | [81–100] | 95 | [81–100] | 94 | [90–100] | 94 | [90–96] | 0.97 |
| Cognitive functioning | 92 | [50–100] | 83 | [50–100] | 83 | [67–100] | 88 | [67–100] | 0.48 |
| Social functioning | 98 | [85–100] | 96 | [85–100] | 93 | [81–100] | 93 | [81–96] | 0.32 |
| General quality of life | 33 | [17–58] | 29 | [13–42] | 25 | [8–42] | 25 | [17–33] | 0.77 |
| Symptom scale | |||||||||
| Fatigue | 11 | [0–22] | 13 | [4–30] | 11 | [7–33] | 15 | [11–26] | 0.29 |
| Nausea and vomiting | 0 | [0–8] | 0 | [0–8] | 0 | [0–0] | 0 | [0–0] | 0.61 |
| Pain | 8 | [0–25] | 17 | [0–33] | 8 | [0–33] | 0 | [0–25] | 0.22 |
| Dyspnea | 0 | [0–33] | 0 | [0–100] | 0 | [0–33] | 0 | [0–33] | 0.51 |
| Sleeping disturbances | 33 | [0–67] | 17 | [0–100] | 33 | [0–67] | 33 | [0–33] | 0.65 |
| Loss of appetite | 0 | [0–33] | 0 | [0–33] | 0 | [0–33] | 0 | [0–0] | 0.47 |
| Constipation | 0 | [0–67] | 17 | [0–67] | 0 | [0–67] | 0 | [0–0] | 0.45 |
| Diarrhea | 0 | [0–0] | 0 | [0–0] | 0 | [0–0] | 0 | [0–0] | - |
| Author | Number of pts | Re-RT Total Dose (Gy) | No. of Fractions | Median PTV (cc) | Cumulative BED 2 Dose (Gy) | Severe Toxicities | Median OS (m) | PFS (m) |
|---|---|---|---|---|---|---|---|---|
| Cabrera et al. (2013) [61] | 15 | 18–25 | 1–5 | n.r. | n.r. | 6.3 | 14.4 | 3.9 |
| Clarke et al. (2017) [62] | 15 | 27–33 | 3 | n.r. | 197.78 | n.r. | 13 | 7 |
| Ernst-Stecken et al. (2007) [63] | 15 | 35 | 5 | 22.40 | 157.50 | 0 | 12 | 12 |
| Fields et al. (2012) [64] | 10 | 36 | 3 | 54.30 | n.r. | n.r. | 6 | 3 |
| Gutin et al. (2009) [65] | 25 | 30 | 5 | 34 | 120 | 4% | 12.5 | n.r. |
| Konget al. (2008) [66] | 114 | 16 | 1 | n.r. | 544 | 0 | 13 | n.r. |
| Larson et al. (2002) [67] | 26 | 12–20 | 1 | 17.2 | n.r. | 8 | 9.5 | n.r. |
| Lee et al. (2018) [68] | 25 | 45 | 15 | n.r. | n.r. | 32 | 16 | n.r. |
| Marzano et al. (2011) [69] | 22 | 14–22 | 1 | n.r. | 197.28 | 0 | 11 | n.r. |
| Møller et al. (2017) [70] | 31 | 29, 5–42 | 5–10 | 67 | n.r. | 9.7 | 2.8 | |
| Schwer et al. (2008) [71] | 15 | 18–36 | 3 | 41.3 | n.r. | 0 | 10 | 7 |
| Shi et al. (2016) [72] | 17 | 35 | 5 | n.r. | n.r. | n.r. | n.r. | n.r. |
| Yoshikawa et al. (2006) [73] | 25 | 13, 9–26, 4 | n.r. | 19.5 | n.r. | n.r. | n.r. | n.r. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciammella, P.; Cozzi, S.; Botti, A.; Giaccherini, L.; Sghedoni, R.; Orlandi, M.; Napoli, M.; Pascarella, R.; Pisanello, A.; Russo, M.; et al. Safety of Inhomogeneous Dose Distribution IMRT for High-Grade Glioma Reirradiation: A Prospective Phase I/II Trial (GLIORAD TRIAL). Cancers 2022, 14, 4604. https://doi.org/10.3390/cancers14194604
Ciammella P, Cozzi S, Botti A, Giaccherini L, Sghedoni R, Orlandi M, Napoli M, Pascarella R, Pisanello A, Russo M, et al. Safety of Inhomogeneous Dose Distribution IMRT for High-Grade Glioma Reirradiation: A Prospective Phase I/II Trial (GLIORAD TRIAL). Cancers. 2022; 14(19):4604. https://doi.org/10.3390/cancers14194604
Chicago/Turabian StyleCiammella, Patrizia, Salvatore Cozzi, Andrea Botti, Lucia Giaccherini, Roberto Sghedoni, Matteo Orlandi, Manuela Napoli, Rosario Pascarella, Anna Pisanello, Marco Russo, and et al. 2022. "Safety of Inhomogeneous Dose Distribution IMRT for High-Grade Glioma Reirradiation: A Prospective Phase I/II Trial (GLIORAD TRIAL)" Cancers 14, no. 19: 4604. https://doi.org/10.3390/cancers14194604
APA StyleCiammella, P., Cozzi, S., Botti, A., Giaccherini, L., Sghedoni, R., Orlandi, M., Napoli, M., Pascarella, R., Pisanello, A., Russo, M., Cavallieri, F., Ruggieri, M. P., Cavuto, S., Savoldi, L., Iotti, C., & Iori, M. (2022). Safety of Inhomogeneous Dose Distribution IMRT for High-Grade Glioma Reirradiation: A Prospective Phase I/II Trial (GLIORAD TRIAL). Cancers, 14(19), 4604. https://doi.org/10.3390/cancers14194604






