Simple Summary
Spine metastases are a common life-threatening complication of advanced-stage malignancies and often result in poor prognosis. Symptomatic spine metastases develop in the course of about 10% of malignant neoplasms. Therefore, it is essential for contemporary medicine to understand metastatic processes in order to find appropriate, targeted therapeutic options. Our literature review aimed to describe the up-to-date knowledge about the molecular pathways and biomarkers engaged in the spine’s metastatic processes. Moreover, we described current data regarding bone-targeted treatment, the emerging targeted therapies, radiotherapy, and immunotherapy used for the treatment of spine metastases. We hope that knowledge comprehensively presented in our review will contribute to the development of novel drugs targeting specific biomarkers and pathways. The more we learn about the molecular aspects of cancer metastasis, the easier it will be to look for treatment methods that will allow us to precisely kill tumor cells.
Abstract
Spine metastases are a common life-threatening complication of advanced-stage malignancies and often result in poor prognosis. Symptomatic spine metastases develop in the course of about 10% of malignant neoplasms. Therefore, it is essential for contemporary medicine to understand metastatic processes in order to find appropriate, targeted therapeutic options. Thanks to continuous research, there appears more and more detailed knowledge about cancer and metastasis, but these transformations are extremely complicated, e.g., due to the complexity of reactions, the variety of places where they occur, or the participation of both tumor cells and host cells in these transitions. The right target points in tumor metastasis mechanisms are still being researched; that will help us in the proper diagnosis as well as in finding the right treatment. In this literature review, we described the current knowledge about the molecular pathways and biomarkers engaged in metastatic processes involving the spine. We also presented a current bone-targeted treatment for spine metastases and the emerging therapies targeting the discussed molecular mechanisms.
1. Introduction
Spine metastases are a common life-threatening complication of advanced-stage malignancies and often result in poor prognosis. Symptomatic spine metastases develop in the course of about 10% of malignant neoplasms [1].
The spine is the most frequent localization among the bone metastatic lesions and the third most common site of metastases after lungs and liver [2]. The thoracic region is the site of metastatic spine tumors in 60–70% of the cases, the lumbosacral spine (20–25%) and the cervical spine (10–15%) are less common [3]. In the analysis of CT scans, it has been observed that metastatic lesions typically occur in the posterior part of the vertebral body at first and then penetrate the pedicles [4]. Bone metastases most frequently develop from primary solid tumors (such as breast (70%), prostate (85%), lung (40%), and kidney (40%)) [5] (Figure 1). Women suffering from breast cancer with overexpression of the estrogen receptor or the progesterone-one receptor or HER2 triple-positive women and men with castration-resistant prostate cancer are most vulnerable to bone metastases [6].
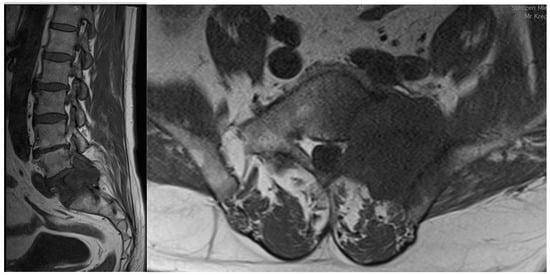
Figure 1.
Sagittal and coronal T1-weighted MRI views of a patient diagnosed with sacral metastasis of a pulmonary squamous cell carcinoma.
Bone metastases can be osteolytic, osteoblastic, or mixed (osteolytic and osteoblastic components). According to Constans et al., osteolytic lesions constitute more than 70% of spinal metastases, 8% are osteoblastic, and mixed metastases (osteolytic and osteoblastic) account for 21% [7]. Osteolytic lesions are characteristic of metastases from breast cancer, lung cancer, and renal cancer, whereas metastases of prostate cancer most often form osteoblastic lesions. Causes of mixed lesions include many tumor types, but most frequently are observed in breast cancer [8].
Metastatic cells can reach the spine through different ways of dissemination—hematogenous (intravenous and arterial), by contiguity, and lymphatic. The intravenous route is the most common way of propagation and is carried out through the paravertebral venous plexus of Batson [9,10]. This venous system communicates the spine with intercostal veins of the pulmonary, caval, or portal systems and provides a direct route of dissemination for breast and prostate cancer to the spine [11].
The most common clinical symptom caused by metastatic lesions in spine is being refractory to severe treatment pain. Other symptoms include hypercalcemia, pathological fractures, and spinal cord or nerve root compression, which are referred to as skeletal-related events (SREs). The current treatment of spine metastases is mostly palliative. It focuses on the improvement of health-related quality of life (HRQoL) through the control of pain, protection, or improvement of neurological functions and maintenance of spinal stability [1]. To prevent SREs and increase the quality of life of patients with spinal metastases as a result, antiresorptive agents such as aminobisphosphonates and anti-RANKL monoclonal antibody denosumab have been approved by the FDA. These drugs act through inhibition of the osteoclast function, which leads to the interruption of the vicious cycle of bone metastases and increase of bone mass [12]. However, improvement of the overall survival (OS) and progression-free survival (PFS) by these agents suggested by some studies is highly questionable. Recently investigated novel therapeutic agents targeting specific molecular mechanisms in the bone microenvironment represent a promising way of treatment. The bone microenvironment constitutes a pivotal role in the process of spine metastases. Multiple molecular interactions between metastatic tumor cells and bone tissue result in the alteration of many molecular pathways, which leads to the development of osteolytic or osteoblastic lesions. Improving the understanding of these mechanisms may help in the development of novel drugs targeting specific biomarkers and pathways. Currently, multiple substances are being investigated in preclinical and clinical studies with regard to inhibition of the bone metastatic process.
In this literature review, we described the current knowledge about the molecular pathways and biomarkers engaged in metastatic processes involving the spine. We also presented a current bone-targeted treatment for spine metastases and the emerging therapies targeting the discussed molecular mechanisms.
2. Molecular Basis of Spine Metastases
2.1. Escape from the Primary Site
Tumor is a type of malignant cell growth where abnormal cells multiply uncontrollably with an ability for close or distant tissue invasion. Cells within a tumor may differ in many ways, e.g., in their proliferative potential or the ability to undergo apoptosis or metastasize [13,14,15,16]. The route that cancer cells must surmount to reach other organs is burdensome, with escape from the primary site being the first obstacle encountered [17,18,19]. Another essential step in the process of metastasis is the entrance of neoplastic cells into blood or lymphatic vessels through which they reach even remotely located organs [20]. Increased activity of the master regulator of angiogenesis—oxygen-sensitive transcriptional activator HIF (hypoxia-inducible factor-1)—contributes to both provision of the path utilized for metastatic spread as well as tumor cells’ nutrition via modulation of proangiogenic factors that include, e.g., VEGF (vascular endothelial growth factor), Ang-1, Ang-2 (angiopoietins), or PlGF (placental growth factor) [21,22,23].
A hallmark of metastasis, epithelial–mesenchymal transition (EMT) is a biological process where epithelial cells forfeit their adhesive properties and apical–basal polarity and acquire migratory as well as invasive features in order to transform into mesenchymal stem cells [24,25,26]. EMT is involved in both pathological (e.g., cancer formation) and physiological (embryogenesis, organ development, wound healing) processes [27,28] conditioned by biologically different EMT subtypes [29,30,31,32]. Reversibility of this process (MET—mesenchymal–epithelial transition) enables cancer cells to return to their original form in another organ [33,34].
Both EMT and MET comprise series of biochemical reactions regulated by a plethora of transcriptional factors including Snail/Slug, Twist, Six1, Cripto, TGF-β, and Wnt/β-catenin that, when activated, reprogram gene expression [35,36,37] and, in consequence, alter tumor cell properties. While the cytoskeleton undergoes remodeling, modified protein expression into proteins distinctive for mesenchymal cells, e.g., N-cadherin, FSP-1, α-SMA, α5β1 integrin, αvβ6 integrin, vimentin, type I collagen, laminin 5, and fibrotin lead to the termination of connection with the basal membrane [38,39,40,41]. As a result, cancer cells acquire enhanced capability for relocation. Additionally, enzymes produced by tumor cells that break down the extracellular matrix, MMP (matrix metalloproteinase) and ADAM (A disintegrin and metalloproteinase), enable vascular wall penetration [42,43]. EMT also provides resistance to apoptosis due to diminished cell adhesion. The same mechanism facilitates migration and, in turn, evasion of various factors that lead to cell destruction.
2.2. Cancer Cells Dissemination
Cancers can spread to bones through various pathways, both venous and arterial, as well as through the lymphatic route or by direct contact [44]. Although the lymphatic system is mentioned as a potential route for spread, the main routes to enter the spinal column are comprised of venous and arterial vessels [45]. The Batson plexus, a network of veins devoid of valves that connect pelvic and thoracic with intraspinal veins contributes to spinal metastasis. Due to the absence of valves, any increase in vena cava pressure is followed by increased blood flow within the plexus, leading to cancer cells dissemination. In turn, neoplastic metastases reach the vertebral body directly through nutritional arteries [46,47]. Less frequently, neoplastic lesions metastasize through direct contact, e.g., prostate cancer that metastasizes to the lumbosacral spine [48]. Additionally, tumor cells have the ability to adhere to blood cells, including platelets, which serves as protection from the detrimental effects of hemodynamic forces during flow [49,50,51], as well as to bone marrow cells produced by tumor mimic precursors of immune cells, which aids to avoid innate immune response [52].
2.3. Bone Invasion
There are two main theories of metastasis. One of them is the so called “seed and soil” theory proposed by Paget over 100 years ago. It says that cancers anchor where they find convenient conditions, similarly to seeds in fertile soil, a process not dependent on anatomical relations [52,53]. Ewing, in turn, said that metastases are only the result of the structure of the circulatory system and are strictly related to anatomical conditions, such as the diameter of the vessels or connections between organs. In the 1980s, the work by Hart and Fiedler on melanoma metastases confirmed that theory [54,55].
However, looking at the development of tumors, it seems that both theories are relevant. Bone marrow, due to abundant vascularization, constitutes a part of the bone tissue of relatively high affinity for tumor metastases. The bones of the axial skeleton (skull, spine, sternum, ribs, hips, shoulders) contain a substantial amount of red bone marrow and therefore are a frequent target of tumor spread [56]. Low velocity of the blood flow enables easier adhesion to endothelial cells and, in consequence, quicker integration with endosteum. Bone marrow and bone cells also produce cytokines, hormones, enzymes, as well as growth factors that regulate the immune system and affect the colonization of bone tissue by cancer cells [57,58].
Tumor cells release a plethora of factors [59,60], e.g., VEGFR1+—bone marrow-derived progenitor cells that activate VLA-4 that via binding to fibronectin [61,62,63,64] enables entrance to the potential metastatic site and create an appropriate environment, a premetastatic niche that facilitates tumor cells implantation [65,66,67,68,69].
The abovementioned mechanisms are best understood in an example of metastatic breast cancer. It has been found that CXCR4, also known as fusin, is necessary for breast cancer cells migration towards tissues that present a high quantity of its specific ligand—cytokine SDF1 (CXCL12). Among the organs that express high levels of SDF1 are lungs, liver, bone marrow, and brain, which explains the high affinity of breast cancer cells to these tissues [70,71,72,73]. Tyrosine kinase Src is activated through the binding of CXCL12 to CXCR4, and downstream effector AKT improves the survival of cancer cells that occupy bone tissues [74].
Primary tumor cells also produce substances that modify the extracellular matrix at sites of metastasis, e.g., lysyl oxidates. Conversely, exosomes and miRNAs are able to influence bone remodeling. Metalloproteinases (MMPs) and bone sialoproteins (BSPs) destroy basal membranes at the site of metastasis, stimulate angiogenesis, and activate various elements involved in the destruction of bone tissue and the spread of tumor cells [70,75,76,77].
Once cancer cells reach the bone marrow, their growth depends on multiple factors, including in situ vascularization, available space, type of bone remodeling, or proliferating potential of neoplastic cells [78].
Bone tissue is made up of three main types of cells: osteoblasts, osteoclasts, and osteocytes. Osteoclasts are cells that have the ability to dissolve and resorb bone tissue, while osteoblasts are responsible for the growth and remodeling of bone tissue. Processes that lead to the formation of bone metastases may have different mechanisms: osteolytic and osteoblastic. Sometimes both of these mechanisms work simultaneously. In a healthy organism, the activity of osteoclasts and osteoblasts corresponds to the RANK-RANKL/OPG system [79,80,81].
RANKL (receptor activator for nuclear factor κB ligand) is produced by the osteoblastic line and activated T lymphocytes [80,82]. It is responsible for activating the process of creating mature osteoclasts. While RANK (receptor activator for nuclear factor κB) is located on osteoclasts and serves as the main regulator during the formation of osteoclasts, RANK combines with RANKL, ligand of the receptor activator of nuclear factor kappa B (NF-κB), which at the same time causes upregulation of nuclear factor of activated T cells 1 (NFATc1) [83,84]. NFATc1 is the major regulator of cytokine expression in the process of osteoclastogenesis [85,86]. As a result of these changes, mature osteoblasts are formed. Their main task is old bone reabsorption, which causes the release of nutrients and creates space for osteoblasts. Osteoprotegrin (OPG) binds to RANK and blocks the formation of the RANK–RANKL complex, thereby inhibiting the maturation process of osteoclasts [87,88,89].
2.4. Osteocyte Physiology and Pathology
Osteocytes have an impressive lifespan of up to 25 years, during which they undertake several important physiological functions. They differentiate from osteoblasts with four stages of formation, type I preosteocytes (osteoblastic osteocytes), type II preosteocytes (osteoid osteocytes), and type III preosteocytes (young and old osteocytes) [90]. During the process of bone formation, the osteoblastic cell body reduces in size, and its cytoplasm expands, springing processes from out of the cell’s wall. The Golgi apparatus during the type I and II cycles has to be well-developed to efficiently synthesize type I collagen essential for maintaining the bone matrix. Entering the type III preosteocyte phase, the Golgi apparatus is reduced in size, and the osteocyte matrix proceeds from the incompletely mineralized phase to the formation of old osteocytes with high mineral density [90]. Mature osteocytes express such markers as DMP1, Sost, as well as the cx43 protein, which is believed to be critical in the role of keeping the cell from entering apoptosis [90,91]. It is theorized that cx43 influences bone cell activity by regulating the osteoprotegerin and sclerostin levels [91].
2.5. Osteoblast Physiology and Pathology
The aforementioned osteoblasts are generated from pluripotent mesenchymal stem cells that take on the crucial role of bone matrix synthesis by firstly establishing the collagen, OCN, osteonectin, BSP II, and osteopontin proteins alongside decorin and biglycan to create osteoids, which would be further mineralized [90].
Osteoblasts tend to communicate with osteocytes using the RANK–RANKL pathway as a way to order growth factor release from the bone matrix [92].
The aforementioned processes summarize the bone homeostasis. It has been established that the regions of the bone with the largest amount of turnover (trabecular bone) tend to become sites for metastatic cell growth. One very prominent factor of this cancer cell homing is CXC motif chemokine 12, also known as CXCL12 or SDF-1, produced by bone marrow stromal cells and osteoblasts, which was proven to be crucial to metastases [93,94]. Cancer cells interact with osteoblasts and osteoclasts, as well as the cytokines released by the bone in an otherwise physiological process [95].
2.6. Osteoblastic Metastasis Pathogenesis
Bone tissue is considered to be the third place in the aspect of frequency of metastatic changes. Most of these metastatic changes are the result of oncological diseases, primarily breast and prostate cancer [8,96]. The statistics analyzed in previous years showed bone metastasis to be the effect of up to 70–75% of breast and prostate cancers [17].
A factor that one has to take into consideration is the bone’s extreme metabolic activity derived from the three main cell types: osteocytes, osteoblasts, and osteoclasts. Osteoblasts account for 4–6% of total cells, osteocytes—90–95%, osteoclasts—about 1–4% [90].
Osteoblastic metastasis is characterized by the deposition of new bone rather than lysis of the already existing structures. It is most notably present in prostate cancers, carcinoids, small-cell lung cancers, Hodgkin lymphomas, and medulloblastomas [17]. Tumor cells invading the bone tend to produce growth factors such as bone morphogenic proteins, epidermal growth factors, and platelet-derived growth factors. There have been consistent data proving that the physical contact of osteoblasts and prostate cancer cells promote tumor growth in vitro via protein ECM components, proteoglycans (PGs), and junction-related molecules [97]. Some of the more prominent factors of this process are BMPSs, TGF beta, and endothelin-1. BMP4 has been proven to stimulate osteoblast differentiation after being secreted from PCa-118b prostate cancer cells via the pSmad1–Notch–Hey1 and GSK3 β–β-catenin–Slug pathways [98]. The aforementioned TGF beta, specifically, TGF beta 2, is secreted from the prostate cancer metastasized cells [98] to foster the progression of tumor growth [99]. Another mechanism of growth is the secretion of endothelin-1 which downregulates DKK-1 and stimulates the secretion of the Wnt signaling pathways proven to be associated with lytic lesions and suppressing the growth of bone tissue in myelomas [100]. This occurs because DDK1 inhibits the production of osteoblasts by preventing the binding of low-density lipoprotein receptor-related proteins 5 and 6 (LRP5/6) in osteoblast precursors [101].
The role of PTHrP fragments in the process must not be underestimated. The parathyroid hormone-related protein increases calcium absorption and bone resorption [102], but it greatly increases the metastatic growth of cancer cells [103]. It has been theorized that NH2-terminal fragments of this protein stimulate bone formation via the ETA receptor because of the shared sequence homology to ET-1, which was proven to increase metastatic growth [104].
Other research has proven that PTHrP acts as a mediator in osteoblastogenesis, increasing early osteoblast differentiation and proliferation of bone marrow cells [105].
One other crucial aspect of bone metastasis that has to be taken into consideration is the so-called vicious cycle. Prostate cancer cells that have been freshly metastasized tend to produce PDGF, ET1, and BMPs that activate osteoblastic differentiation and bone matrix formation. As mentioned before, bone turnover marks the sites where tumors tend to grow, as the freshly synthesized structures are rich with growth factors such as IGF, FGF, and TGF-β that attract prostate cancer cells [95]. The physical contact of tumor cells and osteoblasts further promotes the secretion of growth factors—the vicious cycle continues to propel itself until it reaches the physical limits of the metastatic site.
2.7. Osteolytic Bone Metastasis
When discussing osteolytic metastases, we must look at the research on breast cancer. The majority of breast cancer metastases are lytic; breast cancer cells produce TNF-A, IL-8, IL-11 [106], IGF1, LIF (leukemia inhibitory factor), lysyl oxidase [107], RANK ligands, and PTHrP (Figure 2) [108].
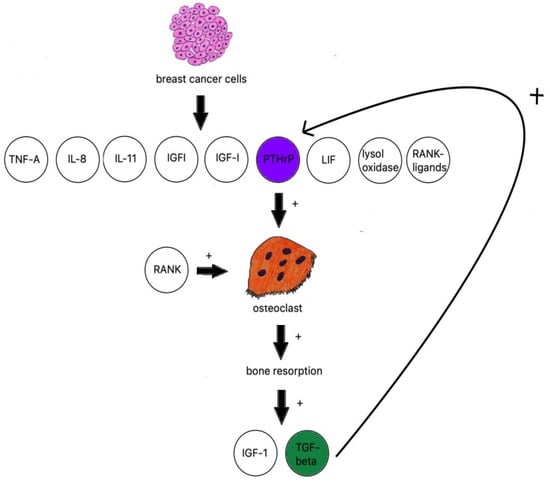
Figure 2.
Breast cancer influence on osteoclastogenesis.
The receptor activator of nuclear factor kappa B ligand (RANKL) and the macrophage colony-stimulating factor (M-CSF) are considered essential for proliferation and differentiation of osteoclast progenitor cells [109].
PTHrP is an osteoclast-activating factor; it stimulates osteoblasts to produce RANKL, which binds to RANK-stimulating osteoclasts to increase bone resorption [110]. IGF-1 and TGF beta are then released from bones and further fuel PTHrP production [111].
TGF-β is produced by active osteoclasts and increases the production of PTHrP. The research on samples of MDA-MB-231 breast cancer cells with neutralized TGF-β shows a significant decrease in osteolytic lesions [112].
TGF-β has also been reported to activate Notch ligand Jagged1 [113] proven to be an important tumor growth stimulant and COX-2 expression [114] which, in turn, stimulates the production of PGE2 in MDA-MB-231 breast cancer cells, a proven factor in bone resorption. It is worth noting that binding to EP4 receptor PGE2 increases the number of RANK ligands produced by osteoblasts [115]. It has been proven to be prominent in estrogen receptor-negative breast cancer cells and their metastatic tumors [107]. Another way of initializing osteoclastogenesis independent from RANKL is the aforementioned lysyl oxidase, a mediator of HIF-1 and the cause of metastasis in hypoxic cells [116].
Leukemia inhibitory factor (LIF), which is part of the IL-6 family of cytokines, is a known breast epithelial growth suppressor [117] that also promotes breast cancer metastasis utilizing the AKT–mTOR signaling pathway in the absence of the lncRNA-CTP-210809.1 gene [118], which is, unlike the aforementioned lysyl oxidase, independent from the estrogen receptor status [117].
Bone-derived insulin-like growth factors are a family of abundant mediators generated by the bone. The IGF-1 receptor was proven to be present in up to 86.7% of the cases where metastatic cancer cells were analyzed. The studies conducted on IGF-IR-disabled mice models have shown decreased mitosis and increased apoptosis of metastatic sites formed by breast cancer, multiple myeloma, neuroblastoma, and prostate cancer [94]. IGFs tend to promote bone metastases utilizing the IGF-IR, Akt, and NF-kappa B pathways [119].
Tumor necrosis factor alpha is a well-studied proinflammatory cytokine and a very strong bone resorption inducer. TNF-α activates the AP-1 and NF-kappa B transcription factors, leading to NFATc1-mediated expression of osteoclast-specific genes. TNF-α can also activate RANKL expression directly, alongside IL-6 and IL-1, to increase bone resorption or, working via the DDK-1 protein, downregulate bone formation [120]. Other interleukins 8 and 11 are also essential to the process of osteolysis by stimulating osteoclast formation independently from the RANKL pathway by utilizing JAK/STAT3 osteoclastogenesis (Figure 3) [106].
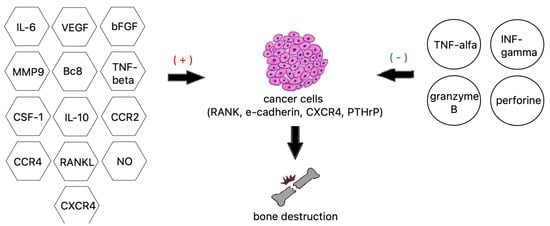
Figure 3.
Factors contributing to bone destruction induced by metastatic cancer cells.
3. Role of the Immune System
Due to the complexity of the processes involved in the formation of bone metastases, the part of the immunology which investigates, inter alia, the mechanisms of metastasis formation has been termed osteoimmunology and it still remains the subject of multilateral scientific research [121,122].
Many cells of the immune system participate in the formation of metastases, including macrophages, T cells, NK cells, dendritic cells, myeloid-derived suppressor cells (MDSCs) [57,121,122,123].
Bone marrow is a space rich in cells of the immune system, for instance, T CD4+ or T CD8++ lymphocytes. In osteoclastogenesis, T CD4+ lymphocytes play an important role, especially Th17 cells which interact with osteoclasts mainly through osteoprotegerin (OPG)/RANKL/RANK, resulting in an increased activity of osteoclasts [57,122]. Likewise, the cells of the tumor located in the primary site produce many factors, such as IL-1, IL-6, IL-11, PDGF, MIP-1α, TNF, M-CFS, RANKL, and PTHrP, directly stimulating osteoclasts to form osteoclastic-type lesions [57]. Among the T CD4+ cell population, there are regulatory T cells (Treg) responsible for the maintenance of homeostasis of the immune system, but their increased amount reduces the immune response to cancer [57]. In turn, T CD8 + lymphocytes play the opposite role, with the cytotoxic substances TNF-α and IFN-α destroying cancer cells. Hence, it can be concluded that maintaining the balance between lymphocyte populations may play a key role in preventing cancer [57,122].
After escape from the bone marrow and peripheral blood, monocytes differentiate into macrophages after colonization in multifarious tissues. Macrophages are an important component in tumor progression. First, in the primary site, tumor-associated macrophages (TAMs) are responsible for the evolution of angiogenesis, migration of tumor cells, and escape from the primary focus. In turn, when they reach the metastasis site, they become metastasis-associated macrophages (MAMs) and play the metastasis-promoting role, being in charge of colonization and tumor development [121]. In addition, TAMs can be divided into two lymphocyte populations: M1-like, which represents the tumor-suppressing activity by producing cytokines such as IL-1, IL-6, IL-23, IFN-α, IL-12 which activate cytotoxic T lymphocytes and NK cells to completely remove tumor cells, or M2-like, which leads to tumor promotion. The ratio of these lymphocyte populations may be an important prognostic factor in assessing the course of disease [57,121,123].
NK cells are also involved in the control of bone homeostasis. Cytotoxic cells participating in the tumor cells-killing process, thus reducing the amount of NK cells, can result in cancer progression [57,122]. However, their role in metastasis development is vague, because there is research exposing that NK cells bringing about melanoma cells grow [124].
Dendritic cells (APCs) play a very important role in preventing the development of tumors, insomuch as through the possession of antigen presentation; they have an ability to induce activation and proliferation of Th and Tc lymphocytes, which may be responsible for inducing an antitumor response. Their properties are used in an attempt to create vaccines against cancer. However, cancer cells as well as activation of other cells of the immune system can modify the properties of APCs, causing tumor progression [57].
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous group of immune cells from the myeloid lineage generated in bone marrow that suppress innate and adaptive immunity. Ordinarily, this amounts to the transformation of immature myeloid cells to mature myeloid cells, such as macrophages, dendritic cells, and granulocytes. However, in pathological conditions, such as when a tumor develops in the body, the conversion described above is inhibited (which results in an impaired immune response), as well as further development of the tumor. In addition, the ability of MDSCs to differentiate into osteoclasts has been proven, which can contribute to bone destruction [57].
4. Diagnosis
Regarding clinical practice, the relevant aspect is finding the place of metastasis origin. As in almost any branch of medicine, interview is the most essential tool for a clinical doctor. Patients with bone metastases most often report soreness in the suspected metastatic sites [125]. Additionally, it is worth paying attention to nonspecific symptoms such as weakness, excessive sleepiness, or weight loss often associated with the advancement of the neoplastic process, multiple metastases, and duration of the disease [126]. Abnormalities related to the developing neoplastic process can also be found in basic laboratory tests, such as blood morphology, after determining the level of ions such as calcium or specific tumor markers, for instance, PSA in prostate cancer. Going a step further thanks to the advancements in radiology, a wide variety of imaging tests is available [127].
4.1. X-ray Imaging
The elementary, relatively inexpensive, and most commonly available imaging technology is the X-ray. For the diagnosis of bone metastases, it is important to find the site and the extent of metastatic changes. Since X-ray does not allow for precise three-dimensional imaging or determining the genesis of the lesions, it is not a test that is ultimately used to diagnose metastases. However, an X-ray can be significant as a screening test, e.g., for primary care physicians.
4.2. Computed Tomography—CT
Another method helpful in the diagnosis of neoplastic bone metastases is computed tomography (CT), which is now extensively available and is a noninvasive examination. In comparison with the widespread X-ray, it is more sensitive and allows for better visualization of lesions. This diagnostic method allows us to visualize all types of metastases, both osteolytic (they have a clearer outline than on a regular X-ray image) and osteoblastic (less visible bone trabeculae, blurred border between the cortical bone and cancellous bone) [128]. CT is likely to obtain a spatial image, which allows for a more accurate determination of the extent of metastases. However, it is less sensitive in cases of early metastases to the cortical part of the bone and does not allow adequate visualization of infiltrative bone disease in which bone marrow is involved. CT can be performed to plan a surgical procedure; the imaging helps to select the appropriate access during vertebroplasty [129].
4.3. Magnetic Resonace Imaging (MRI)
Magnetic resonance imaging is a highly specialized test that uses magnetic water molecules contained in the body and, more specifically, hydrogen atoms. MRI has great importance in the diagnosis of neoplastic metastases, especially in the case of metastases to the spine, in the diagnosis of which it is considered the test of choice. It is worth emphasizing that this is a noninvasive test with high sensitivity and specificity [130].
In the case of whole-body MRI, which images the total physical structure in one examination, the effectiveness of detecting cancer lesions is higher in comparison to CT or even scintigraphy. However, the availability of such MRI is rarer and the examination is associated with higher expenses [131].
4.4. Scintigraphy
Scintigraphy (gamma scan) is a diagnostic method of nuclear medicine that provides means to identify diseases of the skeletal system. This examination relies on introducing radioisotopes attached to pharmaceuticals into the organism, recording the decomposition of these chemical substances, and then presenting them graphically. Among the imaging tests performed to detect metastases, it is the most sensitive. Scintigraphy also enables depicting the entire skeleton in one examination [132]. However, the disadvantage of this method of tissue imaging is its low specificity, which means that the anomalous image acquired does not have to be caused by neoplastic metastases but, for instance, by a healing fracture or an inflammation of a different type [133].
4.5. Biopsy
The biopsy is an invasive method consisting of extraction of the bone tissue for microscopic evaluation and obtaining histopathological confirmation of the etiology of the lesions. This test may be helpful, especially when the patient presents only bone transformations and no tumor foci in other organs are found. By performing a biopsy, we are likely to obtain information about the primary tumor. Performing this type of diagnostics is also important to arrange the treatment because the histopathological examination evaluates the tumor-specific biomarkers for neoplasms, which may be needed to guide therapeutic recommendations. In the case of bone involvement, we perform core needle biopsy. Although biopsy is an invasive method and is associated with the risk of complications, transpedicular biopsy of the spinal column is relatively harmless, as it avoids the most important structures such as nerves, vessels, the lungs, and the spinal cord [134].
A promising method of biopsy is one under the control of CT or MRI, which minimizes the risk of complications and allows for more accurate extraction of material for examination [135].
4.6. Biomarkers
As a result of osteoblastic and osteoclastic transformations in bones, many chemical substances are released, which are termed bone turnover markers (BTMs) [136]. To detect BTMs, samples of blood serum or urine have to be tested. Bone turnover markers include those related to bone resorption and those associated with bone formation [137] (Table 1).

Table 1.
Bone resorption and bone formation markers.
It can be required for the assessment of diagnosis, progression of changes, or qualification for appropriate treatment.
4.7. Gene Expression Profiles
One of the crucial components of metastatic growth is the specific gene expression profile. Studies have shown that metastases are closely related to primary tumors, sharing some of the mutations that start the cancerous process, but with extra mutations nonexistent in primary tumors. Research has shown that this process of obtaining diverging mutations takes place around 87% of the molecular time within a primary tumor [138].
When analyzing breast cancer gene alterations, researchers have discovered that the JAK2 and STAT3 pathway components undergo several nonsense substitutions, splice site mutations, and frameshift indels, resulting in the inactivation of the pathway both in ER+ and triple-negative cancers; even though the primary tumor cells lacked the aforementioned changes, it still led to metastasis [138].
Breast cancer metastases were also examined for genomic copy number imbalances (CNIs). This research showed that the most promising prediction factor was the copy number loss at 8p22. Copy number gains at 1q41 and 1q41.12 and loses at 1p13.3, 8p22, and Xp11.3 increase the risk of metastatic changes appearing specifically in the bone [139].
Another mechanism of activating metastatic traits may come from changes in epigenetic alterations, such as the case of FOXA1 mutations in prostate cancer. Forkhead box A1 is an essential transcription factor, that can undergo class 2 activating mutations to increase DNA affinity, inactivate TLE3, and promote metastasis via the aforementioned WNT pathway [140,141].
A recent study has begun trying to correlate specific locations of metastatic changes in the spine with identifiable differentially expressed genes (DEGs) via analysis of the protein–protein interaction in the bone tissue involved in lung, breast, and prostate metastatic tumors. The most prominent DEGs were the upregulated JUN and downregulated PCNA. JUN regulates gene expression of the RANK–RANKL system which is indirectly responsible for bone resorption via osteoclast differentiation. The lack of PCNA is hypothesized to inhibit the maturation of immunological cells, as well as the E2-dependent DNA repair process, leading to an unchecked differentiation process of the surrounding tissue. The other hub genes presented in the aforementioned studies such as HRAS and RHOC also play a crucial role in activating osteoclast cells [141].
Further study is required to better understand the genetic basis of metastatic changes preferring a certain type of tissue.
5. Bone-Targeted Agents
Bone-targeted agents (BTAs) are the most popular drugs used for patients suffering from spine metastases. BTAs can decrease the incidence of skeletal-related events (SREs) including pathological fractures, pain, and hypercalcemia, which are common problems in these patients [142,143,144,145]. In light of extending the overall survival of oncological patients with bone metastases due to more and more effective anticancer treatment, the role of BTAs increases significantly [146]. BTAs include two main classes of agents—bisphosphonates and RANKL inhibitor denosumab.
5.1. Bisphosphonates
The first BTAs approved by the FDA for the treatment of bone metastases were bisphosphonates. Apart from the treatment of bone metastases, bisphosphonates are commonly applied in the therapy of osteoporosis and Paget’s disease [147]. Currently, they are also investigated as a potential treatment for otosclerosis, osteoarthritis of the knee, inflammatory rheumatic diseases, and many others diseases [148,149,150]
Clodronate and etidronate are non-nitrogen-containing bisphosphonates and represent the first generation of these drugs. The second generation is known as aminobisphosphonates and includes pamidronate and ibandronate [145]. Zoledronic acid (ZA) represents the third-generation agents, also known chemically as aminobisphosphonates, and exhibits the highest affinity for bone and antiresorptive strength [12]. ZA was regarded as a standard agent for SREs prevention in patients with bone metastases for almost a decade [151,152]. Bisphosphonates are characterized by the ability to permanently bind to hydroxyapatite molecules, which, on the one hand, provide their high affinity to the bone in vivo; however, on the other hand, they may for many years remain sequestrated in patients’ bones [153,154].
Bisphosphonates achieve the antitumor effect by three mechanisms of action—anti-resorptive effect, immunomodulation, and direct interaction with tumor cells [12]. The antiresorptive activity of the first-generation bisphosphonates results from the formation of cytotoxic metabolites through incorporation into nonhydrolyzable ATP analogs, which leads to osteoclast apoptosis [155]. Aminobisphosphonates owe their antiresorptive properties to the inhibition of farnesyl pyrophosphate synthase (FDPS) and through their ability to inhibit the dissolution of hydroxyapatite crystals [155,156]. FDPS suppression leads to the accumulation of intermediate compounds of the mevalonate (cholesterol biosynthesis) pathway in osteoclasts such as dimethylallyl diphosphate (DMAPP) and isopentenyl diphosphate (IPP) [153]. Overproduction of DMAPP and IPP results in the formation of ATP analogs ApppD and toxic ApppI, respectively. Accumulating ApppI inhibits ATP-dependent protein kinases (which probably impairs the function of the EGF receptor) and causes osteoclast apoptosis through mitochondrial ANT blocking [157]. Moreover, inhibition of FDPS leads to blocking prenylation of small GTPase proteins, such as Rho, Rac, and Cdc42, crucial signaling proteins in osteoclasts, which play the main role in cytoskeletal organization, intracellular vesicles trafficking, membrane ruffling, and apoptosis [158]. These mechanisms impair osteoclast function and survival, which leads to cell apoptosis and decreased bone resorption [159]. Regarding immunomodulatory activity, aminobisphosphonates might induce apoptosis of macrophages related to osteoclasts and activate γδT cells, a subset of T cells characterized by antitumor properties [12,160]. However, activation of γδT cells is responsible for the acute-phase reaction, which can occur in 20% of the patients treated with aminobisphosphonates [161]. The third mechanism of action results from direct interactions with tumor cells, which include negative influence on proliferation, migration, invasion, and induction of apoptosis [12,162].
5.2. Denosumab
Denosumab is a fully human monoclonal IgG2 antibody that binds and inhibits the receptor activator of nuclear factor kappa B ligand (RANKL) acting like osteoprotegerin (OPG). That interaction results in the blockage of the RANK/RANKL axis responsible for the maturation of osteoclast precursors [163]. It leads to inhibiting the activation of osteoclasts and a decrease in bone resorption. Moreover, the RANK/RANKL pathway generates regulatory T cells, increases the production of cytokines, and induces chemoresistance in vitro [164,165]. Additionally, overexpression of the RANK/RANKL axis is commonly found in many tumors, including prostate, breast, cervix, endometrium, thyroid, esophagus, and stomach tumors [165]. Therefore, the use of anti-RANKL agents such as denosumab may potentially sensitize resistant tumors to immunotherapy, suggesting its possible antitumor effect [164].
In contrast to bisphosphonates, denosumab does not accumulate in bone tissue and its effect can be reversible after termination of the treatment [166]. Similarly to bisphosphonates, denosumab can also be used in the treatment of osteoporosis, but in lower doses and at shorter time intervals [167].
5.3. Treatment Effectiveness of BTAs
Many studies and meta-analyses showed that BTAs are effective in decreasing the incidence of SREs, delaying the time incidence of the first SREs and enhancing the patients’ quality of life [168,169].
The majority of studies demonstrated a significant superiority of denosumab over ZA in delaying the time to the first onset of SREs and the development of multiple SREs [170,171,172,173]. Moreover, denosumab more effectively reduces strong analgesic use and better improves the HRQoL rate than bisphosphonates [170,173].
However, their influence on the overall survival or disease-free survival has not been observed [170,171,174]. Interestingly, some retrospective studies on non-small-cell lung cancer (NSCLC) have shown that denosumab may improve the OS [175,176] due to the direct blocking of RANKL in NSCLC tumors, in which RANK and RANKL expression was identified. However, in a randomized open-label phase III trial evaluating the addition of denosumab to the standard first-line treatment in advanced NSCLC (SPLENDOUR trial), improvement of the OS after adding denosumab to standard first-line platinum-based doublet chemotherapy was not observed [177]. Furthermore, in patients with early-stage breast cancer treated with neoadjuvant therapy, RANKL inhibition did not improve disease-related outcomes (D-CARE trial) [178]. On the other hand, in the ABCSG-18 trial, adding denosumab to adjuvant aromatase inhibitor treatment increased disease-free survival in nonmetastatic breast cancer patients [179]. Pantano et al. suggested that the conflicting outcomes of the D-CARE and ABCSG-18 trials result from high heterogeneity of breast tumor cells, which can affect treatment effectiveness [180]. They also for the first time demonstrated the expression of RANK on the circulating tumor cells of breast cancer, which may identify a subset of patients sensitive to denosumab treatment. However, the direct in vivo antitumor activity of denosumab still remains under discussion.
Regarding adverse effects, bisphosphonates and denosumab present similar overall rates [170]. However, in the case of certain complications, such as pyrexia, acute-phase reactions, and renal impairment, denosumab displays a significantly lower occurrence in comparison to ZA [151,170,173]. On the other hand, osteonecrosis of the jaw is more commonly observed in patients treated with denosumab [181,182]. Furthermore, due to the stronger antiresorptive properties of denosumab compared with bisphosphonates, hypocalcemia is a more frequent adverse effect during treatment with denosumab [173,183].
Despite the common use of BTAs in clinical practice, the optimal duration of treatment has not been established. According to the ASCO guidelines and the NCCN Clinical Practice Guidelines, treatment with BTAs ends up when the patient has a substantial decline in his general performance status or in case of severe toxicity [184,185]. The optimal dosing interval of BTAs also still remains undetermined. Initially, studies suggested that BTAs should be administered intravenously every 3–4 weeks [186,187]. However, less intensive treatment (every 12 weeks) was shown to be noninferior in comparison to the standard schedule [188,189,190]. The currently conducted REaCT-HOLD BMA randomized study evaluates the noninferiority of 24-week BTA schedule compared with a 12-week schedule (NCT04549207). Changing a 4-week schedule to a 12-week one, and even a 24-week treatment schedule may be beneficial for patients due to a decrease in BTA-related adverse effects, especially renal impairment. Moreover, it may be more cost-effective for healthcare systems [191]. Moreover, in a retrospective cohort study, Alzahrani et al. observed that the greatest risk for SREs was during the first year of BTA treatment compared with the second and third years [191]. Those findings emphasize the appropriateness of increasing dosing intervals and reducing the time of BTA treatment.
Among bisphosphonates, the FDA and the EMA approved pamidronate disodium (90 mg intravenously every 3–4 weeks) and zoledronic acid (4 mg intravenously every 3–4 weeks) for bone metastases from breast cancer and multiple myeloma in conjunction with standard neoplastic therapy. ZA has also been approved for use in patients with bone metastases from other solid tumors (in the case of prostate cancer, only in the castrate-resistant type) [192]. Additionally, the EMA approved oral bisphosphonate ibandronate for bone metastases in patients with breast cancer [193]. Denosumab, at a dose of 120 mg subcutaneously every 4 weeks, has been approved by the FDA and the EMA for the prevention of SREs in adults with bone metastases from solid tumors, excluding bone metastases from multiple myeloma (FDA) [193].
5.4. Complications of BTA Usage
With the increasingly common long-term use of BTAs, the prevalence of side effects is also increasing. The most frequent complications induced by BTAs include impaired wound healing, osteonecrosis of the jaw, hypocalcemia, and atypical femoral fractures [185,194,195,196]. Moreover, acute-phase reactions and renal impairment have been observed in patients with prolonged use of bisphosphonates, especially ZA [197]. Steller et al. investigated the levels of growth factors in patients after antiresorptive treatment and observed a significant decrease in the EGF and TGF-β1 concentrations in the patients treated with ZA and lower levels of TGF-β1 in the patients on anti-RANKL therapy. These observations may explain worse wound healing in these patients [194]. In the case of denosumab treatment, the use of platelet-rich fibrin may be beneficial to overcome this problem.
Prevalence of osteonecrosis of the jaw is only 1.03% among the patients treated with intravenous bisphosphonates and 3.64% in the case of high-dose denosumab treatment [198]. Therefore, it is not a frequent but severe complication. The pathophysiological mechanism underlying osteonecrosis of the jaw is not yet identified. The proposed theories include impaired bone remodeling, decreased angiogenesis, and the role of inflammatory or infectious factors [199]. Furthermore, the risk of osteonecrosis development is directly proportional to the duration of antiresorptive treatment and the dosage used [200]. The current management algorithm varies from providing good oral care and eliminating risk factors such as periodontal disease or tobacco smoking [200,201,202] to intravenous antibiotics and radical oral surgery in advanced cases [203,204]. However, prophylactic and treatment guidelines have not been established; thus, treatment of this complication may be challenging.
To prevent another complication, hypocalcemia, supplementation of vitamin D and calcium is crucial, especially in the case of denosumab, which is the strongest antiresorptive agent among BTAs [192,204].
6. Bone-Targeted Radioisotopes
Targeted radioisotope therapy is currently applied for patients with diffuse metastases both for palliative therapy and to improve survival. In contrast to bisphosphonates and denosumab, radiopharmaceuticals target the osteoblastic parts of osteosclerotic metastases. In the past, β-emitters such as samarium-153 (153Sa), strontium-89 (89Sr), and phosphorus-32 (32P) were used for pain treatment [205,206,207]. However, their use was associated with hematologic toxicity due to deep penetration into the bone tissue. Radium-223 (223Ra), an α-emitter, showed a lower toxicity than β-emitting radiopharmaceuticals due to a less penetrating character of α radiation (about 80 μm) [208].
Ra-223, as a calcium mimetic, can be deposited by activated osteoblasts near metastatic cells due to its ability of binding to hydroxyapatite in newly formed bone [209]. High-energy radiation delivered by Ra-223 to adjacent cancer cells leads to their destruction, sparing healthy tissues from irradiation at the same time. In the ALSYMPCA trial, six doses of Ra-223 (50 kBq per kg) administered intravenously every 4 weeks for patients with castrate-resistant prostate cancer and symptomatic bone metastases improved the median overall survival (14.9 vs. 11.3 months; HR, 0.70; 95% CI, 0.58–0.83; p < 0.001), reduced SREs, and prolonged the time to the first SRE compared with a placebo [210]. Ongoing clinical trials focus on combining Ra-223 with chemotherapy and immunotherapy (NCT03230734, NCT03996473, NCT04071223). However, the clinical trials with Ra-223 combined with chemotherapy and immunotherapy conducted to date, such as ERA-223, showed unsatisfactory results [211].
Based on the results of the ALSYMPCA trial, treatment of castrate-resistant prostate cancer and symptomatic bone metastases without visceral metastases with Ra-223 got approval from the FDA and the first-category recommendation by the NCCN [212], whereas 89Sr and 153Sa have been approved by the FDA for control of the pain from bone metastases [213].
Treatment with Ra-223 is regarded as well-tolerated and safe; however, thrombocytopenia and aplastic anemia have been observed in some cases [214,215].
7. Emerging Targeted Therapies
As bone-targeted agents such as bisphosphonates and denosumab only delay or prevent SREs in patients with bone metastases, exerting insignificant and disputable influence on the overall survival, it is necessary to search for new molecular agents. With the development of knowledge about bone metabolism and physiopathology of bone metastases during the last few decades, many potential agents have emerged in preclinical studies as potential inhibitors of specific biomarkers essential for metastatic progression.
Everolimus, a mammalian target of rapamycin kinase (mTOR) inhibitor, suppresses metastatic progression in the bone through inhibition of tumor-induced osteoclastogenesis. Moreover, everolimus in combination with standard therapies has been approved for the treatment of HER2-negative breast cancer and hormone receptor-positive advanced breast cancer [216,217].
TGF-β induces molecular pathways, which promote tumor growth in the advanced stage of cancer progression [218]. Suppression of this biomarker reduced bone metastases in multiple preclinical studies on breast and prostate cancer [219]. Moreover, the TGF-β2 signaling pathway is involved in the formation of metastatic niches [220]. Therefore, TGF-β should also be considered a target of novel potential therapies. Recent clinical studies evaluated TGF-β inhibitors such as M7824, fresolimumab, and galunisertib [221,222,223]. The results of these studies regarding effectiveness are promising. Additionally, each of them was well-tolerated.
The Src kinase has been identified as the key factor for the development of bone metastases through the PTHrP-mediated effect on osteoclasts. Its overexpression in breast and prostate tumors is related to shortened survival and a greater risk of metastasis [224]. Dasatinib is an oral tyrosine kinase inhibitor with multitargeted activity against the Src family kinases (SFKs), platelet-derived growth factor receptor (PDGFR), BCR-ABL, and mast/stem cell growth factor receptor (c-KIT) [224,225]. Dasatinib inhibits osteoclasts activation and cancer metastasis to the bone. Moreover, this agent may reduce bone pain. Other potential anti-src agents evaluated in clinical trials include saracatinib and bosutinib [226,227].
Endothelin A antagonists, such as atrasentan and zibotentan demonstrated a promising potential for bone metastases-targeted therapy in preclinical studies [228,229]. However, a placebo-controlled phase III trial showed the ineffectiveness of atrasentan in delaying the progression of bone metastatic lesions in castrate-resistant prostate cancer [230]. Interestingly, a preclinical study on zibotentan demonstrated that the effectiveness of that endothelin A inhibitor considerably depends on androgen ablation [231].
Glycoprotein Dikkopf-1 (DKK-1), an endogenous WNT pathway antagonist, enhances osteoclastic activity through inhibition of osteoblastic differentiation and increasing levels of RANKL [232]. Studies showed that DKK-1 was overexpressed in prostate and breast cancers and in multiple myeloma bone lesions [233]. In a preclinical study in a murine model of breast cancer, elevated DKK-1 promoted osteolytic metastases and increased the number of osteoclasts [234]. In a phase IB multicenter dose determination clinical study, a humanized monoclonal antibody targeting DKK1, BHQ880 in combination with ZA and anti-myeloma treatment increased bone strength and density [235]. However, the prevention of SREs was not achieved.
Anti-sclerostin inhibitors, such as romosozumab, have been recently approved for the therapy of severe postmenopausal osteoporosis [236]. However, its antimetastatic properties have not been investigated. In a preclinical study, BPS804, a monoclonal antibody targeting sclerostin, enhanced bone density and strength and decreased the number of metastatic bone lesions [237].
CXCR4, chemokine, and also one of the CXCL12 receptors, play an important role in the modulation of colonization of the bone by metastatic cells. The CXCR4 inhibitors investigated in studies include plerixafor and pentixafor [238]. Interestingly, it has been suggested that plerixafor can reverse homing of the tumor cells disseminated to the bone marrow and return them to the bloodstream [239]. The homing of cancer cells to the bone may also be potentially suppressed by E-selectin antagonists such as uproleselan (GMI-1271), according to a preclinical study [240].
The agents for the therapy of bone metastases currently investigated in preclinical and clinical studies are summarized in Table 2.

Table 2.
The agents for the therapy of bone metastases currently investigated in preclinical and clinical studies.
8. Radiotherapy
Radiotherapy (RT) is another vital tool in spinal metastasis treatment for both neoadjuvant therapies following surgery and focal control of metastatic diseases. Due to continuous development of radiation oncology, nearly 50% of patients diagnosed with cancer undergo RT eventually in the course of treatment [247,248]. It is also utilized as a palliative method implemented in order to alleviate pain symptoms, improve quality of life, and attenuate the possibility of disease-accompanying pathologic fractures. RT might also be adopted with the aim to provide spinal cord decompression [249].
8.1. Types of Radiotherapy Modalities
The most common modalities utilized in spinal metastasis treatment are ERBT (external beam radiotherapy) and SRBT (stereotactic body radiotherapy) [250,251]. ERBT precisely delivers high-energy x-rays to the targeted tumor tissue using a computer-mediated and radiologic image-adjusted linear accelerator [252]. SRBT distributes a high irradiation dose to the target extracranial tumor tissue in one or few highly ablative fractions with the use of image-guided technology [253]. Nonetheless, SBRT is considered more precise and allows sparing of spinal cord tissue [254]. Table 3 presents a classification of the available radiotherapeutic methods (Table 3).

Table 3.
Classification of the available RT modalities [3,25]
8.2. Mechanism of Action
Regardless of the type of modality, the mechanism of action of ionizing irradiation is based on the influence of an electromagnetic wave or radiation of particles elicited on cancer cells [255]. As mentioned previously, cell’s energy absorption leads to reactive oxygen species (ROS) formation as well as destruction of various intracellular molecules, including DNA strands [256,257]. Although both cancer and normal cells are capable of reparation, a cancer cell’s ability to restore damaged DNA is far less effective [17]. Furthermore, the higher proliferation rate of cancer cells over the normal ones provides a higher significance to irradiation [247]. Ultimately, inefficient reparation processes lead to the arrest of cancer cell cycle, senescence, and necrosis [258,259].
The beneficial effect of ionizing radiation on the underlying cellular mechanisms of irradiated bone cells is not fully understood. Nevertheless, human and animal studies provide evidence that irradiation catalyzes depletion of osteoclast-activating factor (OAF) formation and damage of OCs (osteoclasts) within the tumor mass. Thus, inhibition of bone resorption and promotion of new bone tissue formation consequentially reduce the occurrence of pathologic fractures.
Cancer-induced bone pain (CIBP) is caused by the alteration of biological equilibrium within the bone microenvironment by secretion of chemical and pain mediators by tumor cells. Nociception-associated proinflammatory interleukins (IL-1β, IL-6, IL-11), tumor necrosis factor (TNF-α), chemokines, e.g., monocyte chemoattractant protein (MCP-1), macrophage inflammatory protein (MIP-1α), CXCR4, as well as matrix metalloproteinases and anti-inflammatory cytokine-transforming growth factor (TGF-β) contribute to tumor growth and metastasis [8,260,261]. Tumor cells then release cytokines that aim to further stimulate osteoclasts and, in consequence, bone resorption. Novel literature delivers evidence that radiation treatment not only acts on OCs and OBs, but also changes the bone microenvironment, including by reduction of inflammatory cells and chemical pain mediators which, in turn, results in cancer-induced pain relief [262,263,264] (Figure 4).
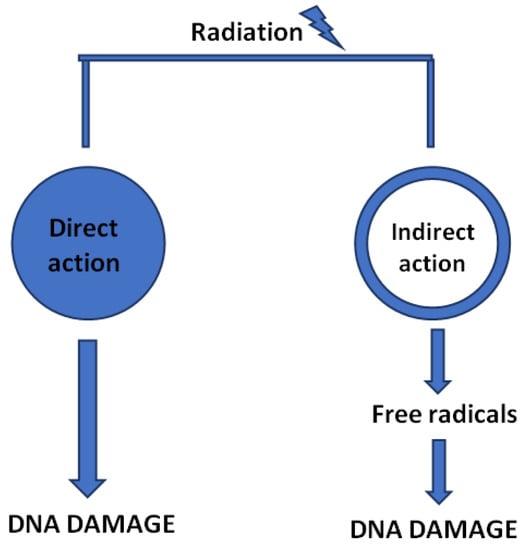
Figure 4.
Direct and indirect influence of ionizing radiation leading to the damage of DNA strands.
8.3. ESTRO-ACROP Guidelines
Skeletal-related events (SRE) that include pathologic fractures, pain, or spinal cord compression aggravate quality of life of affected patients and demand immediate medical intervention. In order to alleviate the aforementioned symptoms, The European Society for Radiation and Oncology/Advisory Committee on Radiation Oncology Practice (ESTRO-ACROP) formulated guidelines that aim to assist clinicians in accurately diagnosing and managing spinal metastases with special attention directed to external beam radiation treatment. These guidelines were printed in two issues following clinical dichotomization into complicated and uncomplicated metastases that require altered treatment. Complicated metastases (one third of the cases) comprise cases that involve spinal fractures, focal neurological deficits, or an associated soft tissue mass that cause spinal cord or cauda equina compression [265,266,267].
8.3.1. Uncomplicated Spinal Metastases
The guidelines recommend bone scintigraphy followed by CT, PET/CT, or MRI for diagnosis of symptomatic bone metastases. MRI should also be performed in the case of neural or soft tissues infiltration or compression. However, in the event of a metastatic tumor of an unknown origin or where molecular profiling improves the available treatment options, core biopsy is particularly advocated.
According to the authors, conventional radiotherapy with a single fraction of 8 Gy ought to be used in case of pain symptoms that are refractory to pharmacological treatment whereas a single-fraction-hemibody or wide-field irradiation should be performed in disseminated pain triggered by polymetastatic disease. Sustained post-RT pain symptoms are advised to be recurrently irradiated with an unmodified dose. The guidelines also address the issue of respective techniques implied in palliative pain treatment; however, due to lack of high-level evidence regarding superiority of one over another to date, each approach should be considered on the individual basis. However, the authors recommend 3D conformal image-guided radiotherapy for uncomplicated bone metastases and clinical target volume (CTV)-based RT in the event of soft tissue mass involved [266].
8.3.2. Complicated Spinal Metastases
The guidelines recommend an altered approach in complicated spinal metastases. On the assumption of spinal cord compression, whole spinal column MRI (or when contrast-enhanced CT is contraindicated) should be undertaken within 24 h post-diagnosis. When the tumor origin site is yet undiscovered, further diagnosis should include appropriate laboratory tests as well as histopathologic examination through definitive surgery or image-guided biopsy.
Following diagnosis, dexamethasone (10–16 mg IV) should be administered and followed by tapering doses of oral dexamethasone over the course of 10–14 days. No evidence supports administration of higher steroid doses. Furthermore, high steroid doses are equivalent to the increased risk of gastric ulcers. Therefore, proton pump inhibitors are recommended, especially when NSAIDS are used concomitantly.
Patients with relatively long life expectancy (over 3 months) with identified spinal instability (evaluated with the Spinal Instability Neoplastic Score (SINS) (Table 4)), paraplegia lasting less than two days, and with a single spinal metastatic site should undergo urgent surgery (decompression with/without stabilization) and subsequent neoadjuvant irradiation. However, the patients who fail to meet these criteria should be immediately referred to RT and receive a single fraction of 8–10 Gy. Although in some circumstances reradiation is necessary, it ought not to be repeated sooner than 6 months after, with the overall biological effective dose (BED) that does not exceed 100–135.5 Gy. In order to treat neuropathic pain, apart from adequate pharmacologic treatment and neurostimulation, a single dose of 8 Gy for conventional RT is advocated.

Table 4.
Spinal Instability Neoplastic Score (SINS).
Pathologic fractures should be treated through both surgery as well as postoperative RT, obviating cases of poor prognosis and low-performance status, where RT is suggested to suffice. Currently, compression fractures are considered to be favorable candidates for balloon kyphoplasty or percutaneous vertebroplasty. Regardless of that, a single dose of 8 Gy or five or ten fractions of 20–30 Gy are advised to be used in the event of pathologic fracture prophylaxis or when recalcification is predetermined. Equivalent RT doses are also used for bone-overgrowing tumor masses [267].
9. Immunotherapy
Immunotherapy revolutionized the standards of cancer treatment and brought new hopes for improving the quality of life and overall survival in oncological patients [268]. The use of immune checkpoint inhibitors (ICIs) represents the most successful type of immunotherapy for metastasis treatment [269]. The most common agents include CTLA-4 inhibitor ipilimumab and PD-1 inhibitors pembrolizumab and nivolumab [270]. However, these drugs showed sufficient efficacy for a minority of patients, and a great number of them developed resistance against the CTLA-4 and PD-1 inhibitors [271]. Thus, the current studies focus on blocking other signaling molecules influencing metastatic processes such as TIM3, LAG3, VISTA, TIGIT, or NKG2A [272,273,274,275,276]. Inhibition of the abovementioned immune checkpoints expressed by T cells increased their immune activity against tumor cells. Therefore, stimulating receptors which enhance the antitumor effect of T cells such as OX40, ICOS, CD40L, or CD27 leads to a similar effect [277,278,279,280]. The currently studied immunotherapeutic approaches to metastasis also involves adoptive cell therapy (ACT), application of CAR-T cells, and inhibiting protumor immune cells in the metastatic tumor’s microenvironment [271,281,282]. However, the bone microenvironment exhibits specific immune characteristics that distinguish bone from other metastatic sites [283,284]. Compared with other organs, bone is characterized by a decreased number of effective cytotoxic cells, a greater number of suppressive immune cells, and the presence of immune cells interactions with osteoclasts and osteoblasts [283]. Therefore, this may affect therapeutic outcomes after use of popular immune checkpoint inhibitors for bone metastases, which has been shown by some studies [285]. Moreover, due to that phenomenon, the presence of bone metastases may impair the efficacy of immunotherapy used for primary tumor treatment, e.g., of NSCLC, where the bone microenvironment provides a “shelter” for disseminated tumor cells [283,284,286,287]. However, emerging studies investigating interactions between the bone microenvironment and metastatic cells reveal further essential molecules and raise hope for overcoming immunotherapy resistance. Indeed, combinatorial therapy composed of anti-CTLA4 agent ipilimumab and TGF-β inhibitor suppressed the expansion of bone metastatic lesions [285]. In the recent studies, a growing body of evidence is observed for the increased clinical efficacy of ICIs after concomitant use of bisphosphonates or denosumab [165,288,289]. Moreover, combination immunotherapy with radiotherapy has also proven to be beneficial [290]. The currently ongoing clinical trials evaluate efficacy of the ICIs’ combination with denosumab, Ra-223, radiotherapy or chemotherapy (NCT03669523, NCT03996473, NCT05502315, NCT05378334, NCT03795207). However, the above data regarding clinical efficacy of ICIs for bone metastasis remain scarce and are represented mainly by small prospective and retrospective studies. Thus, conducting prospective randomized controlled studies is necessary to confirm these results.
10. Conclusions
Tumors often metastasize to the bone, therefore, an important task for contemporary medicine is to understand the processes that lead to such a state and find different therapeutic options. Thanks to continuous research, there is more and more detailed knowledge about cancer and metastasis, but these transformations are extremely complicated, e.g., due to the complexity of reactions, the variety of places where they occur, or the participation of both tumor cells and host cells in these transitions. The right target points in tumor metastasis mechanisms that will help us make a proper diagnosis as well as find the right treatment are still being researched. Nowadays, there are therapies for cancer metastasis to spine available that give patients a chance of starting treatment. Nevertheless, they are often burdened with numerous side effects and are not always as effective as we would expect. Their application still gives little chance of recovery from metastatic disease to the vertebral column. The more we learn about the molecular aspects of cancer metastasis, for example, about the EMT, osteolytic and osteoblastic mechanisms of metastasis, the easier it will be to look for treatment methods that will allow us to precisely kill tumor cells with good effectiveness and without side effects for the entire body. It is also important to create therapies that will allow us to affect the neoplasm at various stages in as many grip points as possible and give a chance for treatment event to patients with very advanced neoplastic disease, which is undoubtedly tumor metastasis to the spinal column.
Author Contributions
Conceptualization, J.L. (Jakub Litak); methodology J.L. (Jakub Litak) and M.S.; software, W.C.; validation, J.L. (Jakub Litak), P.K. and W.C.; formal analysis, W.C.; investigation, J.L. (Jakub Litak); resources, Z.H. and J.L. (Joanna Litak); data curation, L.S.; writing—original draft preparation, M.S., W.C., B.P. and L.S.; writing—review and editing, M.S., W.C., B.P. and L.S.; visualization, J.L. (Jakub Litak).; supervision, P.K.; project administration P.K. and J.R.; funding acquisition, J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by account sources of Medical University of Lublin
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kurisunkal, V.; Gulia, A.; Gupta, S. Principles of Management of Spine Metastasis. Indian J. Orthop. 2020, 54, 181–193. [Google Scholar] [CrossRef]
- Igoumenou, V.G.; Mavrogenis, A.F.; Angelini, A.; Baracco, R.; Benzakour, A.; Benzakour, T.; Bork, M.; Vazifehdan, F.; Nena, U.; Ruggieri, P.; et al. Complications of spine surgery for metastasis. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kido, A.; Tanaka, Y.; Facchini, G.; Peta, G.; Rossi, G.; Mavrogenis, A.F. Current Overview of Treatment for Metastatic Bone Disease. Curr. Oncol. 2021, 28, 3347–3372. [Google Scholar] [CrossRef]
- Algra, P.R.; Heimans, J.J.; Valk, J.; Nauta, J.J.; Lachniet, M.; van Kooten, B. Do metastases in vertebrae begin in the body or the pedicles? Imaging study in 45 patients. AJR Am. J. Roentgenol. 1992, 158, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.; Núñez, S.V.; Gonçalves, A.; Abreu, C.; Costa, L. Bone Health in Metastatic Cancer. Semin. Oncol. Nurs. 2022, 38, 151278. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Zheng, S.; Yang, A.; Zhang, X.; Zou, Y.; Tang, H.; Xie, X. Breast cancer subtypes and the risk of distant metastasis at initial diagnosis: A population-based study. Cancer Manag. Res. 2018, 10, 5329–5338. [Google Scholar] [CrossRef] [PubMed]
- Constans, J.P.; de Divitiis, E.; Donzelli, R.; Spaziante, R.; Meder, J.F.; Haye, C. Spinal metastases with neurological manifestations. Review of 600 cases. J. Neurosurg. 1983, 59, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Croucher, P.I.; Padhani, A.R.; Clézardin, P.; Chow, E.; Fallon, M.; Guise, T.; Colangeli, S.; Capanna, R.; Costa, L.; et al. Bone metastases. Nat. Rev. Dis. Primers 2020, 6, 83. [Google Scholar] [CrossRef]
- Ziu, E.; Viswanathan, V.K.; Mesfin, F.B. Spinal Metastasis. StatPearls. Published online March 3. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441950/ (accessed on 6 August 2022).
- Maccauro, G.; Spinelli, M.S.; Mauro, S.; Perisano, C.; Graci, C.; Rosa, M.A. Physiopathology of Spine Metastasis. Int. J. Surg.Oncol. 2011, 2011, 107969. [Google Scholar] [CrossRef]
- Papachristou, D.J.; Basdra, E.K.; Papavassiliou, A.G. Bone metastases: Molecular mechanisms and novel therapeutic interventions. Med. Res. Rev. 2012, 32, 611–636. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Bozec, A.; Rauner, M.; Jakob, F.; Perner, S.; Pantel, K. Novel approaches to target the microenvironment of bone metastasis. Nat. Rev. Clin. Oncol. 2021, 18, 488–505. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Asemi, Z.; Yousefi, B. Potential anticancer properties and mechanisms of thymoquinone in osteosarcoma and bone metastasis. Cell. Mol. Biol. Lett. 2022, 27, 1–28. [Google Scholar] [CrossRef]
- Sarkar, S.; Horn, G.; Moulton, K.; Oza, A.; Byler, S.; Kokolus, S.; Longacre, M. Cancer Development, Progression, and Therapy: An Epigenetic Overview. Int. J. Mol. Sci. 2013, 14, 21087–21113. [Google Scholar] [CrossRef]
- Biologii, P.; Tom, K. Przejście epitelialno-mezenchymalne w procesach nowotworzenia [Epithelial to mesenchymal transition in carcinogenesis]. Postępy Biologii Komórki 2018, 45, 223–236. [Google Scholar]
- Yin, W.; Wang, J.; Jiang, L.; James Kang, Y. Cancer and stem cells. Exp. Biol. Med. 2021, 246, 1791–1801. [Google Scholar] [CrossRef]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Welch, D.R.; Hurst, D.R. Defining the Hallmarks of Metastasis. Cancer Res. 2019, 79, 3011–3027. [Google Scholar] [CrossRef]
- Leong, S.P.; Naxerova, K.; Keller, L.; Pantel, K.; Witte, M. Molecular mechanisms of cancer metastasis via the lymphatic versus the blood vessels. Clin. Exp. Metastasis 2021, 39, 159–179. [Google Scholar] [CrossRef]
- Font-Clos, F.; Zapperi, S.; la Porta, C.A.M. Blood Flow Contributions to Cancer Metastasis. iScience 2020, 23, 101073. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.C.; Rathore, A.; Younas, H.; Gilkes, D.; Polotsky, V.Y. Hypoxia-Inducible Factors and Cancer. Curr. Sleep Med. Rep. 2017, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. Biomed. Res. Int. 2015, 2015, 549412. [Google Scholar] [CrossRef] [PubMed]
- Dudas, J.; Ladanyi, A.; Ingruber, J.; Steinbichler, T.B.; Riechelmann, H. Epithelial to Mesenchymal Transition: A Mechanism that Fuels Cancer Radio/Chemoresistance. Cells 2020, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Stone, R.C.; Pastar, I.; Ojeh, N.; Chen, V.; Liu, S.; Garzon, K.I.; Tomic-Canic, M. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016, 365, 495–506. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef]
- Norgard, R.J.; Stanger, B.Z. Isolation and Identification of EMT Subtypes. Methods Mol. Biol. 2021, 2179, 315–326. [Google Scholar] [CrossRef]
- Aiello, N.M.; Maddipati, R.; Norgard, R.J.; Balli, D.; Li, J.; Yuan, S.; Yamazoe, T.; Black, T.; Sahmoud, A.; Furth, E.E.; et al. EMT Subtype Influences Epithelial Plasticity and Mode of Cell Migration. Dev. Cell 2018, 45, 681–695.e4. [Google Scholar] [CrossRef]
- Liao, T.T.; Yang, M.H. Revisiting epithelial-mesenchymal transition in cancer metastasis: The connection between epithelial plasticity and stemness. Mol. Oncol. 2017, 11, 792–804. [Google Scholar] [CrossRef]
- Li, Q.; Hutchins, A.; Chen, Y.; Li, S.; Shan, Y.; Liao, B.; Zheng, D.; Shi, X.; Li, Y.; Chan, W.Y.; et al. A sequential EMT-MET mechanism drives the differentiation of human embryonic stem cells towards hepatocytes. Nat. Commun. 2017, 8, 15166. [Google Scholar] [CrossRef]
- Ban, J.; Fock, V.; Aryee, D.N.T.; Kovar, H. Mechanisms, Diagnosis and Treatment of Bone Metastases. Cells 2021, 10, 2944. [Google Scholar] [CrossRef]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef]
- Basu, S.; Cheriyamundath, S.; Ben-Ze’ev, A. Cell–cell adhesion: Linking Wnt/β-catenin signaling with partial EMT and stemness traits in tumorigenesis. F1000Research 2018, 7, 1488. [Google Scholar] [CrossRef]
- Zheng, M.; Jiang, Y.-P.; Chen, W.; Li, K.-D.; Liu, X.; Gao, S.-Y.; Feng, H.; Wang, S.-S.; Jiang, J.; Ma, X.-R.; et al. Snail and Slug collaborate on EMT and tumor metastasis through miR-101-mediated EZH2 axis in oral tongue squamous cell carcinoma. Oncotarget 2015, 6, 6794. [Google Scholar] [CrossRef]
- Li, J.; Riedt, T.; Goossens, S.; García, C.C.; Szczepanski, S.; Brandes, M.; Pieters, T.; Dobrosch, L.; Gütgemann, I.; Farla, N.; et al. The EMT transcription factor Zeb2 controls adult murine hematopoietic differentiation by regulating cytokine signaling. Blood 2017, 129, 460–472. [Google Scholar] [CrossRef]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Battaglia, R.A.; Delic, S.; Herrmann, H.; Snider, N.T. Vimentin on the move: New developments in cell migration. F1000Research 2018, 7, 1796. [Google Scholar] [CrossRef]
- Huang, X.; Xiang, L.; Wang, B.; Hu, J.; Liu, C.; Ren, A.; Du, K.; Ye, G.; Liang, Y.; Tang, Y.; et al. CMTM6 promotes migration, invasion, and EMT by interacting with and stabilizing vimentin in hepatocellular carcinoma cells. J. Transl. Med. 2021, 19, 120. [Google Scholar] [CrossRef]
- Huang, H.; Wright, S.; Zhang, J.; Brekken, R.A. Getting a grip on adhesion: Cadherin switching and collagen signaling. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2019, 1866, 118472. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol. Hematol. 2019, 137, 57–83. [Google Scholar] [CrossRef]
- Newland, R.; Chan, C.; Chapuis, P.; Keshava, A.; Rickard, M.; Stewart, P.; Suen, M.; Lee, K.; Dent, O. Relative effects of direct spread, lymph node metastasis and venous invasion in relation to blood borne distant metastasis present at the time of resection of colorectal cancer. Pathology 2020, 52, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Amelot, A.; Terrier, L.-M.; Cristini, J.; LeNail, L.-R.; Buffenoir, K.; Pascal-Moussellard, H.; Bonaccorsi, R.; Mathon, B. Approaching spinal metastases spread profile. Surg. Oncol. 2019, 31, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Tan, W.L.B.; Wei, W.; Vellayappan, B.A. An overview of the tumors affecting the spine—Inside to out. Neurooncol.Pract. 2020, 7 (Suppl. 1), i10. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Z.; Shen, J.F.; Zhou, Y.; Chen, X.Y.; Liu, J.M.; Liu, Z.L. Clinical characteristics and risk factors for developing bone metastases in patients with breast cancer. Sci. Rep. 2017, 7, 11325. [Google Scholar] [CrossRef] [PubMed]
- Klusa, D.; Lohaus, F.; Furesi, G.; Rauner, M.; Benešová, M.; Krause, M.; Kurth, I.; Peitzsch, C. Metastatic Spread in Prostate Cancer Patients Influencing Radiotherapy Response. Front. Oncol. 2020, 10, 627379. [Google Scholar] [CrossRef]
- Schmied, L.; Höglund, P.; Meinke, S. Platelet-Mediated Protection of Cancer Cells from Immune Surveillance—Possible Implications for Cancer Immunotherapy. Front. Immunol. 2021, 12, 527. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Ding, Y.; Zhuang, R. Platelet-mediated tumor metastasis mechanism and the role of cell adhesion molecules. Crit. Rev. Oncol. Hematol. 2021, 167, 103502. [Google Scholar] [CrossRef]
- Anvari, S.; Osei, E.; Maftoon, N. Interactions of platelets with circulating tumor cells contribute to cancer metastasis. Sci. Rep. 2021, 11, 15477. [Google Scholar] [CrossRef]
- Akhtar, M.; Haider, A.; Rashid, S.; Al-Nabet, A.D.M.H. Paget’s “Seed and Soil” Theory of Cancer Metastasis: An Idea Whose Time has Come. Adv. Anat. Pathol. 2019, 26, 69–74. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Jiang, X.; Qian, C.; Liu, Z.; Luo, D. Factors involved in cancer metastasis: A better understanding to “seed and soil” hypothesis. Mol. Cancer 2017, 16, 176. [Google Scholar] [CrossRef]
- Pienta, K.J.; Robertson, B.A.; Coffey, D.S.; Taichman, R.S. The Cancer Diaspora: Metastasis beyond the seed and soil hypothesis. Clin. Cancer Res. 2013, 19, 5849–5855. [Google Scholar] [CrossRef]
- Chernysheva, O.; Markina, I.; Demidov, L.; Kupryshina, N.; Chulkova, S.; Palladina, A.; Antipova, A.; Tupitsyn, N. Bone Marrow Involvement in Melanoma. Potentials for Detection of Disseminated Tumor Cells and Characterization of Their Subsets by Flow Cytometry. Cells 2019, 8, 627. [Google Scholar] [CrossRef]
- Høilund-Carlsen, P.F.; Hess, S.; Werner, T.J.; Alavi, A. Cancer metastasizes to the bone marrow and not to the bone: Time for a paradigm shift! Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 893–897. [Google Scholar] [CrossRef]
- Xiang, L.; Gilkes, D.M. The Contribution of the Immune System in Bone Metastasis Pathogenesis. Int. J. Mol. Sci. 2019, 20, 999. [Google Scholar] [CrossRef]
- Li, H.; Wu, M.; Zhao, X. Role of chemokine systems in cancer and inflammatory diseases. MedComm 2022, 3, e147. [Google Scholar] [CrossRef]
- Fathi, E.; Farahzadi, R.; Valipour, B.; Sanaat, Z. Cytokines secreted from bone marrow derived mesenchymal stem cells promote apoptosis and change cell cycle distribution of K562 cell line as clinical agent in cell transplantation. PLoS ONE 2019, 14, e0215678. [Google Scholar] [CrossRef]
- Chen, P.; Wu, B.; Ji, L.; Zhan, Y. Cytokine Consistency Between Bone Marrow and Peripheral Blood in Patients with Philadelphia-Negative Myeloproliferative Neoplasms. Front. Med. 2021, 8, 598182. [Google Scholar] [CrossRef]
- Florentin, J.; O’Neil, S.P.; Ohayon, L.L.; Uddin, A.; Vasamsetti, S.B.; Arunkumar, A.; Ghosh, S.; Boatz, J.C.; Sui, J.; Kliment, C.R.; et al. VEGF Receptor 1 Promotes Hypoxia-Induced Hematopoietic Progenitor Proliferation and Differentiation. Front. Immunol. 2022, 13, 882484. [Google Scholar] [CrossRef]
- Ganta, V.C.; Choi, M.; Kutateladze, A.; Annex, B.H. VEGF 165 b Modulates Endothelial VEGFR1-STAT3 Signaling Pathway and Angiogenesis in Human and Experimental Peripheral Arterial Disease. Circ. Res. 2017, 120, 282–295. [Google Scholar] [CrossRef]
- Massena, S.; Christoffersson, G.; Vågesjö, E.; Seignez, C.; Gustafsson, K.; Binet, F.; Hidalgo, C.H.; Giraud, A.; Lomei, J.; Weström, S.; et al. Identification and characterization of VEGF-A–responsive neutrophils expressing CD49d, VEGFR1, and CXCR4 in mice and humans. Blood 2015, 126, 2016–2026. [Google Scholar] [CrossRef]
- Marçola, M.; Rodrigues, C.E. Endothelial progenitor cells in tumor angiogenesis: Another brick in the wall. Stem Cells Int. 2015, 2015, 832649. [Google Scholar] [CrossRef]
- Sanmartin, M.C.; Borzone, F.R.; Giorello, M.B.; Pacienza, N.; Yannarelli, G.; Chasseing, N.A. Bone marrow/bone pre-metastatic niche for breast cancer cells colonization: The role of mesenchymal stromal cells. Crit. Rev. Oncol. Hematol. 2021, 164, 103416. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef]
- Jelgersma, C.; Vajkoczy, P. How to Target Spinal Metastasis in Experimental Research: An Overview of Currently Used Experimental Mouse Models and Future Prospects. Int. J. Mol. Sci. 2021, 22, 5420. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Esposito, M.; Kang, Y. Bone metastasis and the metastatic niche. J. Mol. Med. 2015, 93, 1203. [Google Scholar] [CrossRef]
- Dong, Q.; Liu, X.; Cheng, K.; Sheng, J.; Kong, J.; Liu, T. Pre-metastatic Niche Formation in Different Organs Induced by Tumor Extracellular Vesicles. Front. Cell Dev. Biol. 2021, 9, 2643. [Google Scholar] [CrossRef]
- Marques, C.S.; Santos, A.R.; Gameiro, A.; Correia, J.; Ferreira, F. CXCR4 and its ligand CXCL12 display opposite expression profiles in feline mammary metastatic disease, with the exception of HER2-overexpressing tumors. BMC Cancer 2018, 18, 741. [Google Scholar] [CrossRef]
- Shi, Y.; Riese, D.J.; Shen, J. The Role of the CXCL12/CXCR4/CXCR7 Chemokine Axis in Cancer. Front. Pharmacol. 2020, 11, 1969. [Google Scholar] [CrossRef]
- Zielińska, K.A.; Katanaev, V.L. The Signaling Duo CXCL12 and CXCR4: Chemokine Fuel for Breast Cancer Tumorigenesis. Cancers 2020, 12, 3071. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Mao, X.; Fan, C.; Liu, C.; Guo, A.; Guan, S.; Jin, Q.; Li, B.; Yao, F.; Jin, F. CXCL12-CXCR4 axis promotes the natural selection of breast cancer cell metastasis. Tumour Biol. 2014, 35, 7765–7773. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhao, H.; Chen, H.; Yao, Q. CXCR4 in breast cancer: Oncogenic role and therapeutic targeting. Drug Des. Devel. Ther. 2015, 9, 4953–4964. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.L.; Ferando, B.; Eapen, A.S.; Yu, J.C.; Joy, A.R. Cancer Secretome May Influence BSP and DSP Expression in Human Salivary Gland Cells. J. Histochem. Cytochem. 2017, 65, 139–151. [Google Scholar] [CrossRef]
- Zabkiewicz, C.; Resaul, J.; Hargest, R.; Jiang, W.G.; Ye, L. Bone morphogenetic proteins, breast cancer, and bone metastases: Striking the right balance. Endocr. Relat. Cancer 2017, 24, R349–R366. [Google Scholar] [CrossRef]
- Rustamov, V.; Keller, F.; Klicks, J.; Hafner, M.; Rudolf, R. Bone sialoprotein shows enhanced expression in early, high- proliferation stages of three-dimensional spheroid cell cultures of breast cancer cell line MDA-MB-231. Front. Oncol. 2019, 9, 36. [Google Scholar] [CrossRef]
- Chen, F.; Han, Y.; Kang, Y. Bone marrow niches in the regulation of bone metastasis. Br. J. Cancer 2021, 124, 1912–1920. [Google Scholar] [CrossRef]
- Ming, J.; Cronin, S.J.F.; Penninger, J.M. Targeting the RANKL/RANK/OPG Axis for Cancer Therapy. Front. Oncol. 2020, 10, 1283. [Google Scholar] [CrossRef]
- Sisay, M.; Mengistu, G.; Edessa, D. The RANK/RANKL/OPG system in tumorigenesis and metastasis of cancer stem cell: Potential targets for anticancer therapy. OncoTargets Ther. 2017, 10, 3801–3810. [Google Scholar] [CrossRef]
- Litak, J.; Czyzewski, W.; Szymoniuk, M.; Pastuszak, B.; Litak, J.; Litak, G.; Grochowski, C.; Rahnama-Hezavah, M.; Kamieniak, P. Hydroxyapatite Use in Spine Surgery—Molecular and Clinical Aspect. Materials 2022, 15, 2906. [Google Scholar] [CrossRef]
- de Groot, A.F.; Appelman-Dijkstra, N.M.; van der Burg, S.H.; Kroep, J.R. The anti-tumor effect of RANKL inhibition in malignant solid tumors—A systematic review. Cancer Treat. Rev. 2018, 62, 18–28. [Google Scholar] [CrossRef]
- Sobacchi, C.; Menale, C.; Villa, A. The RANKL-RANK Axis: A Bone to Thymus Round Trip. Front. Immunol. 2019, 10, 629. [Google Scholar] [CrossRef]
- Li, B.; Wang, P.; Jiao, J.; Wei, H.; Xu, W.; Zhou, P. Roles of the RANKL–RANK Axis in Immunity—Implications for Pathogenesis and Treatment of Bone Metastasis. Front. Immunol. 2022, 13, 922. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, Y.; Hwang, H.G.; Sung, S.H.; Lee, M.; Son, Y.J. Betulin Suppresses Osteoclast Formation via Down-Regulating NFATc1. J. Clin. Med. 2018, 7, 154. [Google Scholar] [CrossRef]
- Wang, L.; Chen, K.; He, J.; Kenny, J.; Yuan, Y.; Chen, J.; Liu, Q.; Tan, R.; Zhao, J.; Xu, J. Cytochalasin Z11 inhibits RANKL-induced osteoclastogenesis via suppressing NFATc1 activation. RSC Adv. 2019, 9, 38438–38446. [Google Scholar] [CrossRef]
- Casimiro, S.; Vilhais, G.; Gomes, I.; Costa, L. The Roadmap of RANKL/RANK Pathway in Cancer. Cells 2021, 10, 1978. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Huang, Z.; Chen, X.; Zhang, B. The roles of osteoprotegerin in cancer, far beyond a bone player. Cell Death Discov. 2022, 8, 252. [Google Scholar] [CrossRef]
- Marley, K.; Bracha, S.; Seguin, B. Osteoprotegerin activates osteosarcoma cells that co-express RANK and RANKL. Exp. CellRes. 2015, 338, 32–38. [Google Scholar] [CrossRef]
- Tresguerres, F.G.F.; Torres, J.; López-Quiles, J.; Hernández, G.; Vega, J.A.; Tresguerres, I.F. The osteocyte: A multifunctional cell within the bone. Ann. Anat. 2020, 227, 151422. [Google Scholar] [CrossRef]
- Davis, H.M.; Pacheco-Costa, R.; Atkinson, E.G.; Brun, L.R.; Gortazar, A.R.; Harris, J.; Hiasa, M.; Bolarinwa, S.A.; Yoneda, T.; Ivan, M.; et al. Disruption of the Cx43/miR21 pathway leads to osteocyte apoptosis and increased osteoclastogenesis with aging. Aging Cell 2017, 16, 551–563. [Google Scholar] [CrossRef]
- Ottewell, P.D. The role of osteoblasts in bone metastasis. J. Bone Oncol. 2016, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Floranović, M.P.; Veličković, L.J. Effect of CXCL12 and Its Receptors on Unpredictable Renal Cell Carcinoma. Clin. Genitourin. Cancer 2020, 18, e337–e342. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, T. Bone metastasis: Interaction between cancer cells and bone microenvironment. J. Oral. Biosci. 2019, 61, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Kolb, A.D.; Shupp, A.B.; Mukhopadhyay, D.; Marini, F.C.; Bussard, K.M. Osteoblasts are “educated” by crosstalk with metastatic breast cancer cells in the bone tumor microenvironment. Breast Cancer Res. 2019, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Suhovskih, A.V.; Kashuba, V.I.; Klein, G.; Grigorieva, E.V. Prostate cancer cells specifically reorganize epithelial cell-fibroblast communication through proteoglycan and junction pathways. Cell Adhes. Migr. 2017, 11, 39. [Google Scholar] [CrossRef][Green Version]
- Yu, G.; Shen, P.; Lee, Y.-C.; Pan, J.; Song, J.H.; Pan, T.; Lin, S.-C.; Liang, X.; Wang, G.; Panaretakis, T.; et al. Multiple pathways coordinating reprogramming of endothelial cells into osteoblasts by BMP4. iScience. 2021, 24, 102388. [Google Scholar] [CrossRef]
- Ungefroren, H. Autocrine TGF-β in Cancer: Review of the Literature and Caveats in Experimental Analysis. Int. J. Mol. Sci. 2021, 22, 977. [Google Scholar] [CrossRef]
- Wei, X.F.; Chen, Q.L.; Fu, Y.; Zhang, Q.K. Wnt and BMP signaling pathways co-operatively induce the differentiation of multiple myeloma mesenchymal stem cells into osteoblasts by upregulating EMX2. J. Cell. Biochem. 2019, 120, 6515–6527. [Google Scholar] [CrossRef]
- Role of the Wnt/β-Catenin Signaling Pathway in Regulating the Phenotypic Transformation of Aortic Valvular Myofibroblasts to Osteoblast-like Cells. Available online: http://caod.oriprobe.com/articles/48652138/Role_of_the_Wnt_%CE%B2_catenin_signaling_pathway_in_regulating_the_phenotyp.htm (accessed on 6 August 2022).
- Winter, E.M.; Appelman-Dijkstra, N.M. Parathyroid Hormone-Related Protein-Induced Hypercalcemia of Pregnancy Successfully Reversed by a Dopamine Agonist. J. Clin. Endocrinol. Metab. 2017, 102, 4417–4420. [Google Scholar] [CrossRef]
- Goltzman, D. Nonparathyroid Hypercalcemia. Front. Horm. Res. 2019, 51, 77–90. [Google Scholar] [CrossRef]
- Vičić, I.; Belev, B. The pathogenesis of bone metastasis in solid tumors: A review. Croat. Med. J. 2021, 62, 270. [Google Scholar] [CrossRef]
- Kamalakar, A.; Washam, C.L.; Akel, N.S.; Allen, B.J.; Williams, D.K.; Swain, F.L.; Leitzel, K.; Lipton, A.; Gaddy, D.; Suva, L.J. PTHrP(12-48) Modulates the Bone Marrow Microenvironment and Suppresses Human Osteoclast Differentiation and Lifespan. J. Bone Miner Res. 2017, 32, 1421–1431. [Google Scholar] [CrossRef]
- Liang, M.; Ma, Q.; Ding, N.; Luo, F.; Bai, Y.; Kang, F.; Gong, X.; Dong, R.; Dai, J.; Dai, Q.; et al. IL-11 is essential in promoting osteolysis in breast cancer bone metastasis via RANKL-independent activation of osteoclastogenesis. Cell Death Dis. 2019, 10, 353. [Google Scholar] [CrossRef]
- Reynaud, C.; Ferreras, L.; Di Mauro, P.; Kan, C.; Croset, M.; Bonnelye, E.; Pez, F.; Thomas, C.; Aimond, G.; Karnoub, A.E.; et al. Lysyl oxidase is a strong determinant of tumor cell colonization in bone. CancerRes. 2017, 77, 268–278. [Google Scholar] [CrossRef]
- le Pape, F.; Vargas, G.; Clézardin, P. The role of osteoclasts in breast cancer bone metastasis. J. Bone Oncol. 2016, 5, 93–95. [Google Scholar] [CrossRef]
- Brook, N.; Brook, E.; Dharmarajan, A.; Dass, C.R.; Chan, A. Breast cancer bone metastases: Pathogenesis and therapeutic targets. Int. J. Biochem. Cell Biol. 2018, 96, 63–78. [Google Scholar] [CrossRef]
- Zheng, X.; Kang, W.; Liu, H.; Guo, S. Inhibition effects of total flavonoids from Scutellaria barbata D. Don on human breast carcinoma bone metastasis via down-regulating PTHrP pathway. Int. J. Mol. Med. 2018, 41, 3137–3146. [Google Scholar] [CrossRef]
- Martin, T.J.; Johnson, R.W. Multiple actions of parathyroid hormone-related protein in breast cancer bone metastasis. Br. J.Pharmacol. 2021, 178, 1923–1935. [Google Scholar] [CrossRef]
- Cheng, J.N.; Frye, J.B.; Whitman, S.A.; Kunihiro, A.G.; Pandey, R.; Funk, J.L. A Role for TGFβ Signaling in Preclinical Osteolytic Estrogen Receptor-Positive Breast Cancer Bone Metastases Progression. Int. J. Mol. Sci. 2021, 22, 4463. [Google Scholar] [CrossRef]
- Wu, C.; Chen, M.; Sun, Z.; Ye, Y.; Han, X.; Qin, Y.; Liu, S. Wenshen Zhuanggu formula mitigates breast cancer bone metastasis through the signaling crosstalk among the Jagged1/Notch, TGF-β and IL-6 signaling pathways. J. Ethnopharmacol. 2019, 232, 145–154. [Google Scholar] [CrossRef]
- Meng, X.; Ark, A.V.; Lee, P.; Hostetter, G.; Bhowmick, N.A.; Matrisian, L.M.; Williams, B.O.; Miranti, C.K.; Li, X. Myeloid-specific TGF-β signaling in bone promotes basic-FGF and breast cancer bone metastasis. Oncogene 2016, 35, 2370–2378. [Google Scholar] [CrossRef]
- Das, D.; Hong, J. Prostaglandin E2 Receptor 4 (EP4): A Promising Therapeutic Target for the Treatment of Cancer and Inflammatory Diseases. Curr. Chem. Biol. 2020, 15, 50–68. [Google Scholar] [CrossRef]
- Johnston, K.A.; Lopez, K.M. Lysyl oxidase in cancer inhibition and metastasis. Cancer Lett. 2018, 417, 174–181. [Google Scholar] [CrossRef]
- Vaziri, N.; Shariati, L.; Javanmard, S.H. Leukemia inhibitory factor: A main controller of breast cancer. J. Biosci. 2020, 45, 143. [Google Scholar] [CrossRef]
- Wang, M.; Wang, M.; Wang, Z.; Yu, X.; Song, Y.; Wang, C.; Xu, Y.; Wei, F.; Zhao, Y.; Xu, Y. Long non-coding RNA-CTD-2108O9.1 represses breast cancer metastasis by influencing leukemia inhibitory factor receptor. Cancer Sci. 2018, 109, 1764–1774. [Google Scholar] [CrossRef]
- Hu, G.-F.; Wang, C.; Wu, G.; Zhang, C.; Zhu, W.; Chen, C.; Gu, Y.; Zhang, H.; Yang, Z. AZD3463, an IGF-1R inhibitor, suppresses breast cancer metastasis to bone via modulation of the PI3K-Akt pathway. Ann. Transl. Med. 2020, 8, 336. [Google Scholar] [CrossRef]
- Okamoto, K.; Takayanagi, H. Osteoimmunology. Cold Spring Harb. Perspect. Med. 2019, 9, a031245. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, Z. Immune Modulation of Metastatic Niche Formation in the Bone. Front. Immunol. 2021, 12, 765994. [Google Scholar] [CrossRef] [PubMed]
- Ponzetti, M.; Rucci, N. Updates on Osteoimmunology: What’s New on the Cross-Talk Between Bone and Immune System. Front.Endocrinol. 2019, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Oshi, M.; Tokumaru, Y.; Asaoka, M.; Yan, L.; Satyananda, V.; Matsuyama, R.; Matsuhashi, N.; Futamura, M.; Ishikawa, T.; Yoshida, K.; et al. M1 Macrophage and M1/M2 ratio defined by transcriptomic signatures resemble only part of their conventional clinical characteristics in breast cancer. Sci. Rep. 2020, 10, 16554. [Google Scholar] [CrossRef]
- Antonio, G.; Oronzo, B.; Vito, L.; Angela, C.; Antonel-La, A.; Roberto, C.; Giovanni, S.A.; Antonella, L. Immune system and bone microenvironment: Rationale for targeted cancer therapies. Oncotarget. 2020, 11, 480. [Google Scholar] [CrossRef][Green Version]
- Aielli, F.; Ponzetti, M.; Rucci, N. Bone Metastasis Pain, from the Bench to the Bedside. Int. J. Mol. Sci. 2019, 20, 280. [Google Scholar] [CrossRef]
- Akezaki, Y.; Nakata, E.; Kikuuchi, M.; Sugihara, S.; Katayama, Y.; Katayama, H.; Hamada, M.; Ozaki, T. Factors Affecting the Quality of Life of Patients with Painful Spinal Bone Metastases. Healthcare. 2021, 9, 1499. [Google Scholar] [CrossRef]
- Nowak, H.; Szwacka, D.M.; Pater, M.; Mrugalski, W.K.; Milczarek, M.G.; Staniszewska, M.; Jankowski, R.; Barciszewska, A.-M. Holistic Approach to the Diagnosis and Treatment of Patients with Tumor Metastases to the Spine. Cancers 2022, 14, 3480. [Google Scholar] [CrossRef]
- Hallinan, J.T.P.D.; Ge, S.; Zhu, L.; Zhang, W.; Lim, Y.T.; Thian, Y.L.; Jagmohan, P.; Kuah, T.; Lim, D.S.W.; Low, X.Z.; et al. Diagnostic Accuracy of CT for Metastatic Epidural Spinal Cord Compression. Cancers 2022, 14, 4231. [Google Scholar] [CrossRef]
- Kuah, T.; Vellayappan, B.A.; Makmur, A.; Nair, S.; Song, J.; Tan, J.H.; Kumar, N.; Quek, S.T.; Hallinan, J.T.P.D. State-of-the-Art Imaging Techniques in Metastatic Spinal Cord Compression. Cancers 2022, 14, 3289. [Google Scholar] [CrossRef]
- Lee, K.R. Interpretation of MR Imaging of Spinal Metastasis: Focus on the Understanding of Its Pathophysiology and the Next Step toward a Further Clinical Approach Using MRI Findings. Investig. Magn. Reson. Imaging. 2016, 20, 1–8. [Google Scholar] [CrossRef][Green Version]
- Stecco, A.; Trisoglio, A.; Soligo, E.; Berardo, S.I.; Sukhovei, L.I.; Carriero, A. Whole-Body MRI with Diffusion-Weighted Imaging in Bone Metastases: A Narrative Review. Diagnostics 2018, 8, 45. [Google Scholar] [CrossRef]
- Luining, W.I.; Meijer, D.; Dahele, M.R.; Vis, A.N.; Oprea-Lager, D.E. Nuclear Imaging for Bone Metastases in Prostate Cancer: The Emergence of Modern Techniques Using Novel Radiotracers. Diagnostics 2021, 11, 117. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, S.J. Bone scintigraphy in patients with pain. Korean J. Pain 2017, 30, 165. [Google Scholar] [CrossRef]
- Tanaka, M.; Sonawane, S.; Uotani, K.; Fujiwara, Y.; Sessumpun, K.; Yamauchi, T.; Sugihara, S. Percutaneous C-Arm Free O-Arm Navigated Biopsy for Spinal Pathologies: A Technical Note. Diagnostics 2021, 11, 636. [Google Scholar] [CrossRef]
- Filippiadis, D.; Mazioti, A.; Kelekis, A. Percutaneous, Imaging-Guided Biopsy of Bone Metastases. Diagnostics 2018, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, E.; Pazzaglia, L.; Cevolani, L.; Pratelli, L.; Pierini, M.; Quattrini, I.; Carretta, E.; Manara, M.C.; Pasello, M.; Frega, G.; et al. Bone Turnover Marker (BTM) Changes after Denosumab in Giant Cell Tumors of Bone (GCTB): A Phase II Trial Correlative Study. Cancers 2022, 14, 2863. [Google Scholar] [CrossRef] [PubMed]
- Pop, V.; Parvu, A.; Craciun, A.; Farcas, A.D.; Tomoaia, G.; Bojan, A. Modern markers for evaluating bone disease in multiple myeloma (Review). Exp. Ther. Med. 2021, 22, 1329. [Google Scholar] [CrossRef]
- Yates, L.R.; Knappskog, S.; Wedge, D.; Farmery, J.H.; Gonzalez, S.; Martincorena, I.; Alexandrov, L.B.; Van Loo, P.; Haugland, H.K.; Lilleng, P.K.; et al. Genomic Evolution of Breast Cancer Metastasis and Relapse. Cancer Cell 2017, 32, 169–184.e7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, R.; Baumbusch, L.O.; Tsavachidis, S.; Brewster, A.M.; Do, K.-A.; Sahin, A.; Hortobagyi, G.N.; Taube, J.H.; Mani, S.A.; et al. Genomic copy number imbalances associated with bone and non-bone metastasis of early-stage breast cancer. Breast Cancer Res. Treat. 2013, 143, 189–201. [Google Scholar] [CrossRef][Green Version]
- Parolia, A.; Cieslik, M.; Chu, S.-C.; Xiao, L.; Ouchi, T.; Zhang, Y.; Wang, X.; Vats, P.; Cao, X.; Pitchiaya, S.; et al. Distinct structural classes of activating FOXA1 alterations in advanced prostate cancer. Nature 2019, 571, 413–418. [Google Scholar] [CrossRef]
- Liu, W.; Liang, Y.; Bian, C.; Jiang, L.; Zheng, G.; Dong, J. Gene expression profile analysis of the bone microenvironment in patients with spinal metastases. Oncol. Lett. 2018, 15, 61–68. [Google Scholar] [CrossRef]
- Subramanian, C.R.; Talluri, S.; Mullangi, S.; Lekkala, M.R.; Moftakhar, B. Review of Bone Modifying Agents in Metastatic Breast Cancer. Cureus 2021, 13, e13332. [Google Scholar] [CrossRef]
- Shapiro, C.L. Bone-modifying Agents (BMAs) in Breast Cancer. Clin. Breast Cancer 2021, 21, e618–e630. [Google Scholar] [CrossRef]
- Lu, H.; Pundole, X.; Lee, H.C. The role of bone-modifying agents in myeloma bone disease. JBMR Plus 2021, 5, e10518. [Google Scholar] [CrossRef]
- Riffel, R.M.; Göbel, A.; Rachner, T.D. Bone Metastases: From Mechanisms to Treatment. Semin. Oncol. Nurs. 2022, 38, 151277. [Google Scholar] [CrossRef]
- Im, S.-A.; Lu, Y.-S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.-S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef]
- Kaboudin, B.; Daliri, P.; Faghih, S.; Esfandiari, H. Hydroxy- and Amino-Phosphonates and -Bisphosphonates: Synthetic Methods and Their Biological Applications. Front. Chem. 2022, 10, 890696. [Google Scholar] [CrossRef]
- Zimmerer, R.E.; Africa, R.E.; Westenhaver, Z.K.; McKinnon, B.J. Bisphosphonate therapy in otosclerosis: A scoping review. Laryngoscope Investig. Otolaryngol. 2022, 7, 242–249. [Google Scholar] [CrossRef]
- Horecka, A.; Hordyjewska, A.; Blicharski, T.; Kurzepa, J. Osteoarthritis of the knee—Biochemical aspect of applied therapies: A review. Bosn. J. Basic Med. Sci. 2022, 22, 488–498. [Google Scholar] [CrossRef]
- Peris, P.; Monegal, A.; Guañabens, N. Bisphosphonates in inflammatory rheumatic diseases. Bone 2021, 146, 115887. [Google Scholar] [CrossRef]
- Grávalos, C.; Rodríguez, C.; Sabino, A.; Seguí, M.; Virizuela, J.A.; Carmona, A.; Cassinello, J.; Isla, D.; Jara, C.; Martín, M. SEOM Clinical Guideline for bone metastases from solid tumours (2016). Clin. Transl. Oncol. 2016, 18, 1243–1253. [Google Scholar] [CrossRef]
- Terpos, E.; Kleber, M.; Engelhardt, M.; Zweegman, S.; Gay, F.; Kastritis, E.; van de Donk, N.W.; Bruno, B.; Sezer, O.; Broijl, A.; et al. European myeloma network guidelines for the management of multiple myeloma-related complications. Haematologica 2015, 100, 1254–1266. [Google Scholar]
- Rogers, M.J.; Mönkkönen, J.; Munoz, M.A. Molecular mechanisms of action of bisphosphonates and new insights into their effects outside the skeleton. Bone 2020, 139, 115493. [Google Scholar] [CrossRef] [PubMed]
- Stopeck, A.T.; Fizazi, K.; Body, J.-J.; Brown, J.E.; Carducci, M.; Diel, I.; Fujiwara, Y.; Martín, M.; Paterson, A.; Tonkin, K.; et al. Safety of long-term denosumab therapy: Results from the open label extension phase of two phase 3 studies in patients with metastatic breast and prostate cancer. Support Care Cancer 2016, 24, 447–455. [Google Scholar] [CrossRef]
- Sousa, S.; Clézardin, P. Bone-Targeted Therapies in Cancer-Induced Bone Disease. Calcif Tissue Int. 2018, 102, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, H.; Russell, R.G.G.; Francis, M.D. Diphosphonates inhibit hydroxyapatite dissolution in vitro and bone resorption in tissue culture and in vivo. Science 1969, 165, 1262–1264. [Google Scholar] [CrossRef] [PubMed]
- Yuen, T.; Stachnik, A.; Iqbal, J.; Sgobba, M.; Gupta, Y.; Lu, P.; Colaianni, G.; Ji, Y.; Zhu, L.-L.; Kim, S.-M.; et al. Bisphosphonates inactivate human EGFRs to exert antitumor actions. Proc. Natl. Acad. Sci. USA 2014, 111, 17989–17994. [Google Scholar] [CrossRef]
- Itzstein, C.; Coxon, F.P.; Rogers, M.J. The regulation of osteoclast function and bone resorption by small GTPases. Small GTPases 2011, 2, 117–130. [Google Scholar] [CrossRef]
- Clézardin, P. Bisphosphonates’ antitumor activity: An unravelled side of a multifaceted drug class. Bone 2011, 48, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Silva-Santos, B.; Serre, K.; Norell, H. γδ T cells in cancer. Nat. Rev. Immunol. 2015, 15, 683–691. [Google Scholar] [CrossRef]
- Kunzmann, V.; Bauer, E.; Feurle, J.; Tony, F.W.H.P.; Wilhelm, M. Stimulation of γδ T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 2000, 96, 384–392. [Google Scholar] [CrossRef]
- D’Oronzo, S.; Coleman, R.; Brown, J.; Silvestris, F. Metastatic bone disease: Pathogenesis and therapeutic options: Up-date on bone metastasis management. J. Bone Oncol. 2018, 15. [Google Scholar] [CrossRef]
- Yee, A.J.; Raje, N.S. Denosumab for the treatment of bone disease in solid tumors and multiple myeloma. Future Oncol. 2018, 14, 195–203. [Google Scholar] [CrossRef]
- Peters, S.; Clézardin, P.; Márquez-Rodas, I.; Niepel, D.; Gedye, C. The RANK–RANKL axis: An opportunity for drug repurposing in cancer? Clin. Transl. Oncol. 2019, 21, 977–991. [Google Scholar] [CrossRef]
- van Dam, P.A.; van Dam, P.A.; Verhoeven, Y.; Verhoeven, Y.; Trinh, X.B.; Trinh, X.B.; Wouters, A.; Wouters, A.; Lardon, F.; Lardon, F.; et al. RANK/RANKL signaling inhibition may improve the effectiveness of checkpoint blockade in cancer treatment. Crit. Rev. Oncol. Hematol. 2019, 133, 85–91. [Google Scholar] [CrossRef]
- Tsourdi, E.; Langdahl, B.; Cohen-Solal, M.; Aubry-Rozier, B.; Eriksen, E.F.; Guañabens, N.; Obermayer-Pietsch, B.; Ralston, S.H.; Eastell, R.; Zillikens, M.C. Discontinuation of Denosumab therapy for osteoporosis: A systematic review position statement by, E.C.T.S. Bone 2017, 105, 11–17. [Google Scholar] [CrossRef]
- Kendler, D.L.; Cosman, F.; Stad, R.K.; Ferrari, S. Denosumab in the Treatment of Osteoporosis: 10 Years Later: A Narrative Review. Adv. Ther. 2022, 39, 58–74. [Google Scholar] [CrossRef]
- Jakob, T.; Tesfamariam, Y.M.; Macherey, S.; Kuhr, K. Bisphosphonates or RANK-ligand-inhibitors for men with prostate cancer and bone metastases: A network meta-analysis. Cochrane Database Syst. Rev. 2020, 12, CD013020. [Google Scholar] [CrossRef]
- Tesfamariam, Y.; Jakob, T.; Wöckel, A.; Adams, A.; Weigl, A.; Monsef, I.; Kuhr, K.; Skoetz, N. Adjuvant bisphosphonates or RANK-ligand inhibitors for patients with breast cancer and bone metastases: A systematic review and network meta-analysis. Crit. Rev. Oncol. Hematol. 2019, 137, 1–8. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, L.; Liu, X.; Wen, X.; Li, H.; Li, W. Meta-analysis of clinical trials to assess denosumab over zoledronic acid in bone metastasis. Int. J. Clin. Pharm. 2021, 43, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Chang, B.; Lin, F.; Xie, D.; Hu, Q.; Yu, G.; Du, S.; Li, X. Meta-analysis comparing denosumab and zoledronic acid for treatment of bone metastases in patients with advanced solid tumours. Eur. J. Cancer Care 2017, 26, e12541. [Google Scholar] [CrossRef]
- Lipton, A.; Fizazi, K.; Stopeck, A.; Henry, D.; Smith, M.; Shore, N.; Martin, M.; Vadhan-Raj, S.; Brown, J.; Richardson, G.; et al. Effect of denosumab versus zoledronic acid in preventing skeletal-related events in patients with bone metastases by baseline characteristics. Eur. J. Cancer 2016, 53, 75–83. [Google Scholar] [CrossRef]
- Chen, F.; Pu, F. Safety of Denosumab Versus Zoledronic Acid in Patients with Bone Metastases: A Meta-Analysis of Randomized Controlled Trials. Oncol. Res. Treat. 2016, 39, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.R.; Brungs, D.; Pavlakis, N. Osteoclast inhibitors to prevent bone metastases in men with high-risk, non-metastatic prostate cancer: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0191455. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, H.; Niho, S.; Kirita, K.; Umemura, S.; Matsumoto, S.; Yoh, K.; Goto, K. Impact of denosumab use on the survival of untreated non-squamous non-small cell lung cancer patients with bone metastases. J. Cancer Res. Clin. Oncol. 2017, 143, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.A.; Jones, R.; Elders, A.; Mulatero, C.; Royle, P.; Sharma, P.; Stewart, F.; Todd, R.; Mowatt, G. Denosumab for treatment of bone metastases secondary to solid tumours: Systematic review and network meta-analysis. Eur. J. Cancer 2013, 49, 416–430. [Google Scholar] [CrossRef]
- Peters, S.; Danson, S.; Hasan, B.; Dafni, U.; Reinmuth, N.; Majem, M.; Tournoy, K.G.; Mark, M.T.; Pless, M.; Cobo, M.; et al. A Randomized Open-Label Phase III Trial Evaluating the Addition of Denosumab to Standard First-Line Treatment in Advanced NSCLC: The European Thoracic Oncology Platform (ETOP) and European Organisation for Research and Treatment of Cancer (EORTC) SPLENDOUR Trial. J. Thorac. Oncol. 2020, 15, 1647–1656. [Google Scholar] [CrossRef]
- Coleman, R.; Finkelstein, D.M.; Barrios, C.; Martin, M.; Iwata, H.; Hegg, R.; Glaspy, J.; Periañez, A.M.; Tonkin, K.; Deleu, I.; et al. Adjuvant denosumab in early breast cancer (D-CARE): An international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 60–72. [Google Scholar] [CrossRef]
- Gnant, M.; Pfeiler, G.; Dubsky, P.C.; Hubalek, M.; Greil, R.; Jakesz, R.; Wette, V.; Balic, M.; Haslbauer, F.; Melbinger, E.; et al. Adjuvant denosumab in breast cancer (ABCSG-18): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015, 386, 433–443. [Google Scholar] [CrossRef]
- Pantano, F.; Rossi, E.; Iuliani, M.; Facchinetti, A.; Simonetti, S.; Ribelli, G.; Zoccoli, A.; Vincenzi, B.; Tonini, G.; Zamarchi, R.; et al. Dynamic changes of Receptor activator of nuclear factor-κB expression in Circulating Tumor Cells during Denosumab predict treatment effectiveness in Metastatic Breast Cancer. Sci. Rep. 2020, 10, 1288. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Angelini, A.; Vottis, C.; Pala, E.; Calabrò, T.; Papagelopoulos, P.J.; Ruggieri, P. Modern Palliative Treatments for Metastatic Bone Disease: Awareness of Advantages, Disadvantages, and Guidance. Clin. J. Pain 2016, 32, 337–350. [Google Scholar] [CrossRef]
- Higuchi, T.; Soga, Y.; Muro, M.; Kajizono, M.; Kitamura, Y.; Sendo, T.; Sasaki, A. Replacing zoledronic acid with denosumab is a risk factor for developing osteonecrosis of the jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 547–551. [Google Scholar] [CrossRef]
- Menshawy, A.; Mattar, O.; Abdulkarim, A.; Kasem, S.; Nasreldin, N.; Menshawy, E.; Mohammed, S.; Abdel-Maboud, M.; Gadelkarim, M.; El Ashal, G.G.; et al. Denosumab versus bisphosphonates in patients with advanced cancers-related bone metastasis: Systematic review and meta-analysis of randomized controlled trials. Support Care Cancer 2018, 26, 1029–1038. [Google Scholar] [CrossRef]
- Van Poznak, C.; Somerfield, M.R.; Barlow, W.E.; Biermann, J.S.; Bosserman, L.D.; Clemons, M.J.; Dhesy-Thind, S.K.; Dillmon, M.S.; Eisen, A.; Frank, E.S.; et al. Role of Bone-Modifying Agents in Metastatic Breast Cancer: An American Society of Clinical Oncology-Cancer Care Ontario Focused Guideline Update. J. Clin. Oncol. 2017, 35, 3978–3986. [Google Scholar] [CrossRef]
- Cianciolo, G.; Tondolo, F.; Barbuto, S.; Iacovella, F.; Zavatta, G.; Altieri, P.; Grandinetti, V.; Comai, G.; Cozzolino, M.; La Manna, G. Denosumab-Induced Hypocalcemia and Hyperparathyroidism in de novo Kidney Transplant Recipients. Am. J. Nephrol. 2021, 52, 611–619. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Van Poznak, C.; Harker, W.G.; Gradishar, W.J.; Chew, H.; Dakhil, S.R.; Haley, B.B.; Sauter, N.; Mohanlal, R.; Zheng, M.; et al. Continued Treatment Effect of Zoledronic Acid Dosing Every 12 vs 4 Weeks in Women with Breast Cancer Metastatic to Bone: The OPTIMIZE-2 Randomized Clinical Trial. JAMA Oncol. 2017, 3, 906–912. [Google Scholar] [CrossRef]
- Himelstein, A.L.; Foster, J.C.; Khatcheressian, J.L.; Roberts, J.D.; Seisler, D.K.; Novotny, P.J.; Qin, R.; Go, R.S.; Grubbs, S.S.; O’Connor, T.; et al. Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients with Bone Metastases: A Randomized Clinical Trial. JAMA 2017, 317, 48–58. [Google Scholar] [CrossRef]
- Templeton, A.; Stalder, L.; Sauvin, L.A.; Bernhard, J.; Brauchli, P.; Gillessen, S.; Hayoz, S.; Klingbiel, D.; Matter-Walstra, K.; Thürlimann, B.; et al. Prevention of Symptomatic Skeletal Events with Denosumab Administered Every 4 Weeks Versus Every 12 Weeks–A Non-Inferiority Phase III Trial: Sakk 96/12—Reduse. Ann. Oncol. 2014, 25, iv540. [Google Scholar] [CrossRef]
- Clemons, M.; Ong, M.; Stober, C.; Ernst, S.; Booth, C.; Canil, C.; Mates, M.; Robinson, A.; Blanchette, P.; Joy, A.A.; et al. A randomised trial of 4- versus 12-weekly administration of bone-targeted agents in patients with bone metastases from breast or castration-resistant prostate cancer. Eur. J. Cancer 2021, 142, 132–140. [Google Scholar] [CrossRef]
- Ibrahim, M.F.K.; Mazzarello, S.; Shorr, R.; Vandermeer, L.; Jacobs, C.; Hilton, J.; Hutton, B.; Clemons, M. Should de-escalation of bone-targeting agents be standard of care for patients with bone metastases from breast cancer? A systematic review and meta-analysis. Ann. Oncol. 2015, 26, 2205–2213. [Google Scholar] [CrossRef]
- Alzahrani, M.; Stober, C.; Liu, M.; Awan, A.; Ng, T.L.; Pond, G.; Alshamsan, B.; Vandermeer, L.; Clemons, M. Symptomatic skeletal-related events in patients receiving longer term bone-modifying agents for bone metastases from breast and castration resistant prostate cancers. Support Care Cancer 2022, 30, 3977–3984. [Google Scholar] [CrossRef]
- Coleman, R.; Body, J.J.; Aapro, M.; Hadji, P.; Herrstedt, J. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2014, 25 (Suppl. 3), 124–137. [Google Scholar] [CrossRef]
- Casimiro, S.; Ferreira, A.R.; Mansinho, A.; Alho, I.; Costa, L. Molecular Mechanisms of Bone Metastasis: Which Targets Came from the Bench to the Bedside? Int. J. Mol. Sci. 2016, 17, 1415. [Google Scholar] [CrossRef]
- Steller, D.; Simon, R.; von Bialy, R.; Pries, R.; Hakim, S.G. Impact of Zoledronic Acid and Denosumab Treatment on Growth Factor Concentration in Platelet Rich Fibrin of Patients with Osteolytic Bone Metastases. Anticancer Res. 2021, 41, 3917–3923. [Google Scholar] [CrossRef]
- Kizub, D.A.; Miao, J.; Schubert, M.M.; Paterson, A.H.G.; Clemons, M.; Dees, E.C.; Ingle, J.N.; Falkson, C.I.; Barlow, W.E.; Hortobagyi, G.N.; et al. Risk factors for bisphosphonate-associated osteonecrosis of the jaw in the prospective randomized trial of adjuvant bisphosphonates for early-stage breast cancer (SWOG 0307). Support Care Cancer 2021, 29, 2509–2517. [Google Scholar] [CrossRef] [PubMed]
- da Costa Golfieri, F.; Zanon, M.; Percio, P.P.V. Atypical femoral fractures due to the use of bisphosphonates: Epidemiologic study in a tertiary hospital. Acta Ortop. Bras. 2022, 30, e238821. [Google Scholar] [CrossRef]
- Brufsky, A.M.; Sereika, S.M.; Mathew, A.; Tomifumi, O.; Singh, V.; Rosenzweig, M. Long-term treatment with intravenous bisphosphonates in metastatic breast cancer: A retrospective study. Breast J. 2013, 19, 504–511. [Google Scholar] [CrossRef]
- Hallmer, F.; Andersson, G.; Götrick, B.; Warfvinge, G.; Anderud, J.; Bjørnland, T. Prevalence, initiating factor, and treatment outcome of medication-related osteonecrosis of the jaw-a 4-year prospective study. Oral Surg. Oral Med. Oral Pathol. OralRadiol. 2018, 126, 477–485. [Google Scholar] [CrossRef]
- Alsalih, A.; Dam, A.; Lindberg, P.; Truedsson, A. Medication-related osteonecrosis of the jaws initiated by zoledronic acid and potential pathophysiology. Dent. J. 2021, 9, 85. [Google Scholar] [CrossRef]
- McGowan, K.; McGowan, T.; Ivanovski, S. Risk factors for medication-related osteonecrosis of the jaws: A systematic review. Oral Dis. 2018, 24, 527–536. [Google Scholar] [CrossRef]
- Kawahara, M.; Kuroshima, S.; Sawase, T. Clinical considerations for medication-related osteonecrosis of the jaw: A comprehensive literature review. Int. J. Implant Dent. 2021, 7, 47. [Google Scholar] [CrossRef]
- Mücke, T.; Deppe, H.; Hein, J.; Wolff, K.-D.; Mitchell, D.A.; Kesting, M.R.; Retz, M.; Gschwend, J.E.; Thalgott, M. Prevention of bisphosphonate-related osteonecrosis of the jaws in patients with prostate cancer treated with zoledronic acid—A prospective study over 6 years. J. Cranio-Maxillofac. Surg. 2016, 44, 1689–1693. [Google Scholar] [CrossRef]
- Chien, H.-I.; Chen, L.-W.; Liu, W.-C.; Lin, C.-T.; Ho, Y.-Y.; Tsai, W.-H.; Yang, K.-C. Bisphosphonate-Related Osteonecrosis of the Jaw. Ann. Plast Surg. 2021, 86 (Suppl. 1), S78–S83. [Google Scholar] [CrossRef] [PubMed]
- Body, J.-J.; Bone, H.G.; de Boer, R.H.; Stopeck, A.; Van Poznak, C.; Damião, R.; Fizazi, K.; Henry, D.H.; Ibrahim, T.; Lipton, A.; et al. Hypocalcaemia in patients with metastatic bone disease treated with denosumab. Eur. J. Cancer 2015, 51, 1812–1821. [Google Scholar] [CrossRef]
- Silberstein, E.B.; Elgazzar, A.H.; Kapilivsky, A. Phosphorus-32 radiopharmaceuticals for the treatment of painful osseous metastases. Semin. Nucl. Med. 1992, 22, 17–27. [Google Scholar] [CrossRef]
- Smeland, S.; Erikstein, B.; Aas, M.; Skovlund, E.; Hess, S.L.; Fosså, S.D. Role of strontium-89 as adjuvant to palliative external beam radiotherapy is questionable: Results of a double-blind randomized study. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 1397–1404. [Google Scholar] [CrossRef]
- Sartor, O.; Reid, R.H.; Hoskin, P.J.; Quick, D.P.; Ell, P.J.; Coleman, R.E.; Kotler, J.A.; Freeman, L.M.; Olivier, P. Samarium-153-Lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology 2004, 63, 940–945. [Google Scholar] [CrossRef]
- Flux, G.D. Imaging and dosimetry for radium-223: The potential for personalized treatment. Br. J. Radiol. 2017, 90, 20160748. [Google Scholar] [CrossRef]
- Sartor, O.; Hoskin, P.; Bruland, Ø.S. Targeted radio-nuclide therapy of skeletal metastases. Cancer Treat. Rev. 2013, 39, 18–26. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Smith, M.; Parker, C.; Saad, F.; Miller, K.; Tombal, B.; Ng, Q.S.; Boegemann, M.; Matveev, V.; Piulats, J.M.; Zucca, L.E.; et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 408–419. [Google Scholar] [CrossRef]
- Smith, A.W.; Greenberger, B.A.; Den, R.B.; Stock, R.G. Radiopharmaceuticals for Bone Metastases. Semin. Radiat. Oncol. 2021, 31, 45–59. [Google Scholar] [CrossRef]
- Shiloh, R.; Krishnan, M. Radiation for Treatment of Painful Bone Metastases. Hematol. Oncol. Clin. N. Am. 2018, 32, 459–468. [Google Scholar] [CrossRef]
- Parker, C.C.; Coleman, R.E.; Sartor, O.; Vogelzang, N.J.; Bottomley, D.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; et al. Three-year Safety of Radium-223 Dichloride in Patients with Castration-resistant Prostate Cancer and Symptomatic Bone Metastases from Phase 3 Randomized Alpharadin in Symptomatic Prostate Cancer Trial. Eur. Urol. 2018, 73, 427–435. [Google Scholar] [CrossRef]
- Hoskin, P.; Sartor, O.; O’Sullivan, J.M.; Johannessen, D.C.; Helle, S.I.; Logue, J.; Bottomley, D.; Nilsson, S.; Vogelzang, N.J.; Fang, F.; et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: A prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014, 15, 1397–1406. [Google Scholar] [CrossRef]
- Irelli, A.; Sirufo, M.M.; Scipioni, T.; De Pietro, F.; Pancotti, A.; Ginaldi, L.; De Martinis, M. mTOR Links Tumor Immunity and Bone Metabolism: What are the Clinical Implications? Int. J. Mol. Sci. 2019, 20, 5841. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Andre, F.; Jiang, Z.; Shao, Z.; Mano, M.S.; Neciosup, S.P.; Tseng, L.-M.; Zhang, Q.; Shen, K.; Liu, D.; et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): A phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015, 16, 816–829. [Google Scholar] [CrossRef]
- Hansen, A.G.; Arnold, S.A.; Jiang, M.; Palmer, T.D.; Ketova, T.; Merkel, A.; Pickup, M.; Samaras, S.; Shyr, Y.; Moses, H.L.; et al. ALCAM/CD166 is a TGF-β-responsive marker and functional regulator of prostate cancer metastasis to bone. Cancer Res. 2014, 74, 1404–1415. [Google Scholar] [CrossRef]
- Juárez, P.; Fournier, P.G.; Mohammad, K.S.; McKenna, R.C.; Davis, H.W.; Peng, X.H.; Niewolna, M.; Mauviel, A.; Chirgwin, J.M.; Guise, T.A. Halofuginone inhibits TGF-β/BMP signaling and in combination with zoledronic acid enhances inhibition of breast cancer bone metastasis. Oncotarget 2017, 8, 86447. [Google Scholar] [CrossRef]
- Shiozawa, Y.; Pedersen, E.A.; Patel, L.R.; Ziegler, A.M.; Havens, A.M.; Jung, Y.; Wang, J.; Zalucha, S.; Loberg, R.D.; Pienta, K.J.; et al. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia 2010, 12, 116–127. [Google Scholar] [CrossRef]
- Melisi, D.; Garcia-Carbonero, R.; Macarulla, T.; Pezet, D.; Deplanque, G.; Fuchs, M.; Trojan, J.; Oettle, H.; Kozloff, M.; Cleverly, A.; et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br. J. Cancer 2018, 119, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Lee, P.; Adams, S.; Goldberg, J.D.; Li, X.; Xie, M.W.; Ratikan, J.A.; Felix, C.; Hwang, L.; Faull, K.F.; et al. Focal Irradiation and Systemic TGFβ Blockade in Metastatic Breast Cancer. Clin. CancerRes. 2018, 24, 2493–2504. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.; Heery, C.R.; Schlom, J.; Madan, R.A.; Cao, L.; Kang, Z.; Lamping, E.; Marté, J.L.; Donahue, R.N.; Grenga, I.; et al. Phase I Trial of M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGFβ, in Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Mitri, Z.; Nanda, R.; Blackwell, K.; Costelloe, C.M.; Hood, I.; Wei, C.; Brewster, A.M.; Ibrahim, N.K.; Koenig, K.B.; Hortobagyi, G.N.; et al. TBCRC-010: Phase I/II Study of Dasatinib in Combination with Zoledronic Acid for the Treatment of Breast Cancer Bone Metastasis. Clin. Cancer Res. 2016, 22, 5706–5712. [Google Scholar] [CrossRef]
- Yu, E.Y.; Duan, F.; Muzi, M.; Deng, X.; Chin, B.; Alumkal, J.J.; Taplin, M.-E.; Taub, J.M.; Herman, B.; Higano, C.S.; et al. Castration-resistant prostate cancer bone metastasis response measured by 18F-fluoride PET after treatment with dasatinib and correlation with progression-free survival: Results from American College of Radiology Imaging Network 6687. J. Nucl. Med. 2015, 56, 354–360. [Google Scholar] [CrossRef]
- Hu, A.N.; Chen, F.C.; Wang, K.T.; Wang, Z.Q.; Liang, Y.; Dong, J. CX3CL1 in the red bone marrow promotes renal cell carcinoma to metastasize to the spine by involving the Src-related pathway. Neoplasma 2022, 69, 670–679. [Google Scholar] [CrossRef]
- Danson, S.; Mulvey, M.R.; Turner, L.; Horsman, J.; Escott, K.; Coleman, R.E.; Ahmedzai, S.H.; Bennett, M.I.; Andrew, D. An exploratory randomized-controlled trial of the efficacy of the Src-kinase inhibitor saracatinib as a novel analgesic for cancer-induced bone pain. J. Bone Oncol. 2019, 19, 100261. [Google Scholar] [CrossRef]
- Drake, J.M.; Danke, J.R.; Henry, M.D. Bone-specific growth inhibition of prostate cancer metastasis by atrasentan. Cancer Biol. Ther. 2010, 9, 607–614. [Google Scholar] [CrossRef]
- Yin, J.J.; Mohammad, K.S.; Käkönen, S.M.; Harris, S.; Wu-Wong, J.R.; Wessale, J.L.; Padley, R.J.; Garrett, I.R.; Chirgwin, J.M.; Guise, T.A. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc. Natl. Acad. Sci. USA 2003, 100, 10954–10959. [Google Scholar] [CrossRef]
- Carducci, M.A.; Saad, F.; Abrahamsson, P.-A.; Dearnaley, D.P.; Schulman, C.C.; North, S.; Sleep, D.J.; Isaacson, J.D.; Nelson, J.B. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer 2007, 110, 1959–1966. [Google Scholar] [CrossRef]
- Moon, H.H.; Clines, K.L.; Cooks, M.A.; Cialek, C.A.; Esvelt, M.A.; Clines, G.A. Castration Determines the Efficacy of ETAR Blockade in a Mouse Model of Prostate Cancer Bone Metastasis. Endocrinology 2019, 160, 1786–1796. [Google Scholar] [CrossRef]
- Sethakorn, N.; Heninger, E.; Sánchez-De-Diego, C.; Ding, A.B.; Yada, R.C.; Kerr, S.C.; Kosoff, D.; Beebe, D.J.; Lang, J.M. Advancing Treatment of Bone Metastases through Novel Translational Approaches Targeting the Bone Microenvironment. Cancers 2022, 14, 757. [Google Scholar] [CrossRef]
- Clines, K.L.; Clines, G.A. DKK1 and Kremen Expression Predicts the Osteoblastic Response to Bone Metastasis. Transl. Oncol. 2018, 11, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Zhang, H.; Li, X.; Li, X.; Cong, M.; Peng, F.; Yu, J.; Zhang, X.; Yang, Q.; Hu, G. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat. Cell Biol. 2017, 19, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.P.; Beck, J.T.; Stewart, A.K.; Shah, J.; Kelly, K.R.; Isaacs, R.; Bilic, S.; Sen, S.; Munshi, N.C. A Phase IB multicentre dose-determination study of BHQ880 in combination with anti-myeloma therapy and zoledronic acid in patients with relapsed or refractory multiple myeloma and prior skeletal-related events. Br. J. Haematol. 2014, 167, 366–375. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- McDonald, M.M.; Reagan, M.R.; Youlten, S.E.; Mohanty, S.T.; Seckinger, A.; Terry, R.L.; Pettitt, J.A.; Simic, M.K.; Cheng, T.L.; Morse, A.; et al. Inhibiting the osteocyte-specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood 2017, 129, 3452–3464. [Google Scholar] [CrossRef]
- Devignes, C.-S.; Aslan, Y.; Brenot, A.; Devillers, A.; Schepers, K.; Fabre, S.; Chou, J.; Casbon, A.-J.; Werb, Z.; Provot, S. HIF signaling in osteoblast-lineage cells promotes systemic breast cancer growth and metastasis in mice. Proc. Natl. Acad. Sci. USA 2018, 115, E992–E1001. [Google Scholar] [CrossRef]
- Heckmann, D.; Maier, P.; Laufs, S.; Wenz, F.; Zeller, W.J.; Fruehauf, S.; Allgayer, H. CXCR4 Expression and Treatment with SDF-1α or Plerixafor Modulate Proliferation and Chemosensitivity of Colon Cancer Cells. Transl. Oncol. 2013, 6, 124. [Google Scholar] [CrossRef]
- Muz, B.; Abdelghafer, A.; Markovic, M.; Yavner, J.; Melam, A.; Salama, N.N.; Azab, A.K. Targeting E-selectin to Tackle Cancer Using Uproleselan. Cancers 2021, 13, 335. [Google Scholar] [CrossRef]
- Qiao, L.; Liang, Y.; Li, N.; Hu, X.; Luo, D.; Gu, J.; Lu, Y.; Zheng, Q. Endothelin-A receptor antagonists in prostate cancer treatment-a meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 3465. [Google Scholar]
- de Felice, M.; Lambert, D.; Holen, I.; Escott, K.J.; Andrew, D. Effects of Src-kinase inhibition in cancer-induced bone pain. Mol. Pain 2016, 12, 1744806916643725. [Google Scholar] [CrossRef]
- Trivedi, T.; Pagnotti, G.M.; Guise, T.A.; Mohammad, K.S. The Role of TGF-β in Bone Metastases. Biomolecules 2021, 11, 1643. [Google Scholar] [CrossRef] [PubMed]
- Gkotzamanidou, M.; Dimopoulos, M.A.; Kastritis, E.; Christoulas, D.; Moulopoulos, L.A.; Terpos, E. Sclerostin: A possible target for the management of cancer-induced bone disease. Expert Opin. Ther. Targets 2012, 16, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; de Menna, M.; Groenewoud, A.; Thalmann, G.N.; Kruithof-de Julio, M.; Snaar-Jagalska, B.E. A NF-ĸB-Activin A signaling axis enhances prostate cancer metastasis. Oncogene 2019, 39, 1634–1651. [Google Scholar] [CrossRef] [PubMed]
- Straign, D.M.; Ihle, C.L.; Provera, M.D.; Owens, P. Targeting the BMP Pathway in Prostate Cancer Induced Bone Disease. Front. Endocrinol. 2021, 12, 1609. [Google Scholar] [CrossRef]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef]
- Haussmann, J.; Tamaskovics, B.; Bölke, E.; Djiepmo-Njanang, F.-J.; Kammers, K.; Corradini, S.; Hautmann, M.; Ghadjar, P.; Maas, K.; Schuler, P.J.; et al. Addition of chemotherapy to hyperfractionated radiotherapy in advanced head and neck cancer-a meta-analysis. Strahlenther. Onkol. 2019, 195, 1041–1049. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Sebastian, P.; Zaveri, G.R. Radiotherapy for spinal metastasis: A narrative review. Indian Spine J. 2022, 5, 185–192. [Google Scholar] [CrossRef]
- Huynh, M.A.; Roldan, C.; Nunes, P.; Kelly, A.; Taylor, A.; Richards, C.; Fareed, M.M.; Gorman, D.; Groff, M.; Ferrone, M.; et al. Characteristics of Patients and Treatment Recommendations from a Multidisciplinary Spinal Tumor Program. Palliat. Med. Rep. 2020, 1, 143. [Google Scholar] [CrossRef]
- Zhang, H.R.; Li, J.K.; Yang, X.G.; Qiao, R.Q.; Hu, Y.C. Conventional Radiotherapy and Stereotactic Radiosurgery in the Management of Metastatic Spine Disease. Technol. Cancer Res. Treat. 2020, 19, 1533033820945798. [Google Scholar] [CrossRef]
- Ito, K.; Ogawa, H.; Shimizuguchi, T.; Nihei, K.; Furuya, T.; Tanaka, H.; Karasawa, K. Stereotactic Body Radiotherapy for Spinal Metastases: Clinical Experience in 134 Cases from a Single Japanese Institution. Technol. Cancer Res. Treat. 2018, 17, 1533033818806472. [Google Scholar] [CrossRef]
- Guckenberger, M.; Andratschke, N.; Alheit, H.; Holy, R.; Moustakis, C.; Nestle, U.; Sauer, O. Definition of stereotactic body radiotherapy: Principles and practice for the treatment of stage I non-small cell lung cancer. Strahlentherapie Und Onkologie 2014, 190, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Osborn, V.W.; Lee, A.; Yamada, Y. Stereotactic Body Radiation Therapy for Spinal Malignancies. Technol. Cancer Res. Treat. 2018, 17, 1533033818802304. [Google Scholar] [CrossRef]
- Donaubauer, A.J.; Deloch, L.; Becker, I.; Fietkau, R.; Frey, B.; Gaipl, U.S. The Influence of Radiation on Bone and Bone Cells—Differential Effects on Osteoclasts and Osteoblasts. Int. J. Mol. Sci. 2020, 21, 6377. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, L.; Fu, J.; Shi, C.; Xu, J.; Zhu, Y. Efficient free radical generation against cancer cells by low-dose X-ray irradiation with a functional SPC delivery nanosystem. J. Mater. Chem. B 2016, 4, 5863–5872. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhuang, Y.; Li, R.; Liu, Y.; Mei, Z.; He, Z.; Zhou, F.; Zhou, Y. Effects of different doses of X-ray irradiation on cell apoptosis, cell cycle, DNA damage repair and glycolysis in HeLa cells. Oncol. Lett. 2019, 17, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.; Wang, J.; Zhai, J.; Ren, L.; Zhu, G. Radiation-Induced Osteocyte Senescence Alters Bone Marrow Mesenchymal Stem Cell Differentiation Potential via Paracrine Signaling. Int. J. Mol. Sci. 2021, 22, 9323. [Google Scholar] [CrossRef]
- Maier, P.; Hartmann, L.; Wenz, F.; Herskind, C. Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization. Int. J. Mol. Sci. 2016, 17, 102. [Google Scholar] [CrossRef]
- Habberstad, R.; Frøseth, T.C.S.; Aass, N.; Abramova, T.; Baas, T.; Mørkeset, S.T.; Caraceni, A.; Laird, B.; Boland, J.W.; Rossi, R.; et al. The Palliative Radiotherapy and Inflammation Study (PRAIS)—Protocol for a longitudinal observational multicenter study on patients with cancer induced bone pain. BMC Palliative Care 2018, 17, 110. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, Z.; Ma, S.; Liu, X. Radiotherapy and Cytokine Storm: Risk and Mechanism. Front. Oncol. 2021, 11, 1648. [Google Scholar] [CrossRef]
- Sierko, E.; Hempel, D.; Zuzda, K.; Wojtukiewicz, M.Z. Personalized Radiation Therapy in Cancer Pain Management. Cancers 2019, 11, 390. [Google Scholar] [CrossRef]
- Palata, O.; Podzimkova, N.H.; Nedvedova, E.; Umprecht, A.; Sadilkova, L.; Jelinkova, L.P.; Spisek, R.; Adkins, I. Radiotherapy in Combination with Cytokine Treatment. Front. Oncol. 2019, 9, 367. [Google Scholar] [CrossRef]
- Tiwana, M.S.; Barnes, M.; Yurkowski, E.; Roden, K.; Olson, R.A. Incidence and treatment patterns of complicated bone metastases in a population-based radiotherapy program. Radiother. Oncol. 2016, 118, 552–556. [Google Scholar] [CrossRef]
- van der Velden, J.; Willmann, J.; Spałek, M.; Oldenburger, E.; Brown, S.; Kazmierska, J.; Andratschke, N.; Menten, J.; van der Linden, Y.; Hoskin, P. ESTRO ACROP guidelines for external beam radiotherapy of patients with uncomplicated bone metastases. Radiother. Oncol. 2022, 173, 197–206. [Google Scholar] [CrossRef]
- Oldenburger, E.; Brown, S.; Willmann, J.; van der Velden, J.M.; Spałek, M.; van der Linden, Y.M.; Kazmierska, J.; Menten, J.; Andratschke, N.; Hoskin, P. ESTRO ACROP guidelines for external beam radiotherapy of patients with complicated bone metastases. Radiother. Oncol. 2022, 173, 240–253. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; del Rincon, S.V.; Papneja, N.; Miller, W.H. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27 (Suppl. 2), S87. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Liu, C.; Wang, Z.; Wu, W.; Zhang, N.; Zhang, L.; Hu, J.; Luo, P.; Zhang, J.; et al. Immune checkpoint modulators in cancer immunotherapy: Recent advances and emerging concepts. J. Hematol. Oncol. 2022, 15, 111. [Google Scholar] [CrossRef]
- Anwar, M.A.; El-Baba, C.; Elnaggar, M.; Elkholy, Y.O.; Mottawea, M.; Johar, D.; Al Shehabi, T.S.; Kobeissy, F.; Moussalem, C.; Massaad, E.; et al. Novel therapeutic strategies for spinal osteosarcomas. Semin. Cancer Biol. 2020, 64, 83–92. [Google Scholar] [CrossRef]
- Edwards, S.C.; Hoevenaar, W.H.M.; Coffelt, S.B. Emerging immunotherapies for metastasis. Br. J. Cancer 2021, 124, 37–48. [Google Scholar] [CrossRef]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Zhou, G.; Noordam, L.; Sprengers, D.; Doukas, M.; Boor, P.P.C.; Van Beek, A.A.; Erkens, R.; Mancham, S.; Grünhagen, D.; Menon, A.G.; et al. Blockade of LAG3 enhances responses of tumor-infiltrating T cells in mismatch repair-proficient liver metastases of colorectal cancer. Oncoimmunology 2018, 7, e1448332. [Google Scholar] [CrossRef] [PubMed]
- ElTanbouly, M.A.; Schaafsma, E.; Noelle, R.J.; Lines, J.L. VISTA: Coming of age as a multi-lineage immune checkpoint. Clin. Exp.Immunol. 2020, 200, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H.; et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Cluxton, C.D.; Spillane, C.; O’Toole, S.A.; Sheils, O.; Gardiner, C.M.; O’Leary, J.J. Suppression of Natural Killer cell NKG2D and CD226 anti-tumour cascades by platelet cloaked cancer cells: Implications for the metastatic cascade. PLoS ONE 2019, 14, e0211538. [Google Scholar] [CrossRef]
- Wang, R.; Gao, C.; Raymond, M.; Dito, G.; Kabbabe, D.; Shao, X.; Hilt, E.; Sun, Y.; Pak, I.; Gutierrez, M.; et al. An Integrative Approach to Inform Optimal Administration of OX40 Agonist Antibodies in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2019, 25, 6709–6720. [Google Scholar] [CrossRef]
- Solinas, C.; Gu-Trantien, C.; Willard-Gallo, K. The rationale behind targeting the ICOS-ICOS ligand costimulatory pathway in cancer immunotherapy. ESMO Open 2020, 5, e000544. [Google Scholar] [CrossRef]
- Medina-Echeverz, J.; Hinterberger, M.; Testori, M.; Geiger, M.; Giessel, R.; Bathke, B.; Kassub, R.; Gräbnitz, F.; Fiore, G.; Wennier, S.T.; et al. Synergistic cancer immunotherapy combines MVA-CD40L induced innate and adaptive immunity with tumor targeting antibodies. Nat. Commun. 2019, 10, 5041. [Google Scholar] [CrossRef]
- Sanborn, R.E.; Pishvaian, M.J.; Callahan, M.K.; Weise, A.; Sikic, B.I.; Rahma, O.; Cho, D.C.; Rizvi, N.A.; Sznol, M.; Lutzky, J.; et al. Safety, tolerability and efficacy of agonist anti-CD27 antibody (varlilumab) administered in combination with anti-PD-1 (nivolumab) in advanced solid tumors. J. Immunother. Cancer 2022, 10, e005147. [Google Scholar] [CrossRef]
- Borch, T.H.; Andersen, R.; Ellebaek, E.; Met, Ö.; Donia, M.; Marie Svane, I. Future role for adoptive T-cell therapy in checkpoint inhibitor-resistant metastatic melanoma. J. Immunother. Cancer 2020, 8, e000668. [Google Scholar] [CrossRef]
- Soltantoyeh, T.; Akbari, B.; Karimi, A.; Chalbatani, G.M.; Ghahri-Saremi, N.; Hadjati, J.; Hamblin, M.; Mirzaei, H. Chimeric Antigen Receptor (CAR) T Cell Therapy for Metastatic Melanoma: Challenges and Road Ahead. Cells 2021, 10, 1450. [Google Scholar] [CrossRef]
- Liu, C.; Wang, M.; Xu, C.; Li, B.; Chen, J.; Chen, J.; Wang, Z. Immune Checkpoint Inhibitor Therapy for Bone Metastases: Specific Microenvironment and Current Situation. J. Immunol. Res. 2021, 2021, 8970173. [Google Scholar] [CrossRef]
- Del Conte, A.; De Carlo, E.; Bertoli, E.; Stanzione, B.; Revelant, A.; Bertola, M.; Spina, M.; Bearz, A. Bone Metastasis and Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer (NSCLC): Microenvironment and Possible Clinical Implications. Int. J. Mol. Sci. 2022, 23, 6832. [Google Scholar] [CrossRef]
- Jiao, S.; Subudhi, S.K.; Aparicio, A.; Ge, Z.; Guan, B.; Miura, Y.; Sharma, P. Differences in Tumor Microenvironment Dictate T Helper Lineage Polarization and Response to Immune Checkpoint Therapy. Cell 2019, 179, 1177–1190.e13. [Google Scholar] [CrossRef]
- Landi, L.; D’Incà, F.; Gelibter, A.; Chiari, R.; Grossi, F.; Delmonte, A.; Passaro, A.; Signorelli, D.; Gelsomino, F.; Galetta, D.; et al. Bone metastases and immunotherapy in patients with advanced non-small-cell lung cancer. J. Immunother. Cancer 2019, 7, 316. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Chang, X.-S.; Zhou, R.; Chen, Y.-D.; Ma, H.-C.; Xiao, Z.-Z.; Qu, X.; Liu, Y.-H.; Liu, L.-R.; Li, Y.; et al. Bone metastasis attenuates efficacy of immune checkpoint inhibitors and displays “cold” immune characteristics in Non-small cell lung cancer. Lung Cancer 2022, 166, 189–196. [Google Scholar] [CrossRef]
- Bongiovanni, A.; Foca, F.; Menis, J.; Stucci, S.L.; Artioli, F.; Guadalupi, V.; Forcignanò, M.R.; Fantini, M.; Recine, F.; Mercatali, L.; et al. Immune Checkpoint Inhibitors with or Without Bone-Targeted Therapy in NSCLC Patients with Bone Metastases and Prognostic Significance of neutrophil-to-Lymphocyte Ratio. Front. Immunol. 2021, 12, 697298. [Google Scholar] [CrossRef]
- Li, Y.; Du, Y.; Sun, T.; Xue, H.; Jin, Z.; Tian, J. PD-1 blockade in combination with zoledronic acid to enhance the antitumor efficacy in the breast cancer mouse model. BMC Cancer 2018, 18, 669. [Google Scholar] [CrossRef]
- Qiang, H.; Lei, Y.; Shen, Y.; Li, J.; Zhong, H.; Zhong, R.; Zhang, X.; Chang, Q.; Lu, J.; Feng, H.; et al. Pembrolizumab monotherapy or combination therapy for bone metastases in advanced non-small cell lung cancer: A real-world retrospective study. Transl. Lung Cancer Res. 2022, 11, 87–99. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).