Therapeutic Benefit of Systematic Lymphadenectomy in Node-Negative Uterine-Confined Endometrioid Endometrial Carcinoma: Omission of Adjuvant Therapy
Abstract
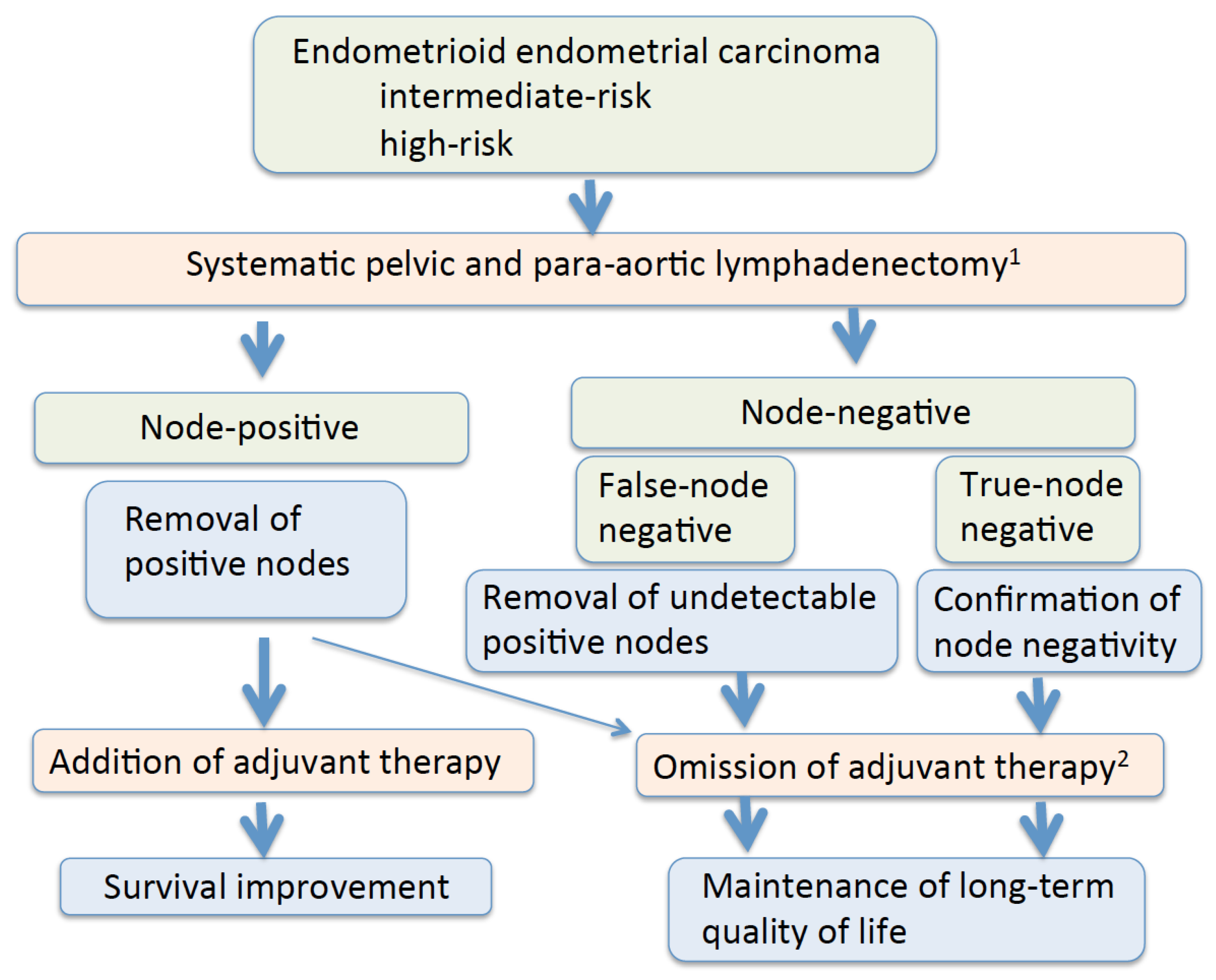
Simple Summary
Abstract
1. Introduction
2. What Is the True Incidence of Lymph Node Metastasis?
3. Role of Adjuvant Therapy in Endometrial Cancer
4. Sentinel Node-Negative Patients: Can Adjuvant Therapy Be Omitted?
5. Benefit of Systematic Lymphadenectomy in Node-Negative Patients: Omission of Adjuvant Therapy
6. Patients Treated with Minimally Invasive Surgery: Can Adjuvant Therapy Be Omitted?
7. Preoperative Prediction of Lymph Node Metastasis and Surgical Therapy
8. Concluding Remarks and Future Direction
Funding
Acknowledgments
Conflicts of Interest
References
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef]
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef]
- Benedetti Panici, P.; Basile, S.; Maneschi, F.; Lissoni, A.A.; Signorelli, M.; Scambia, G.; Angioli, R.; Tateo, S.; Mangili, G.; Katsaros, D.; et al. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. J. Natl. Cancer Inst. 2008, 100, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Kitchener, H.; Swart, A.M.C.; Qian, Q.; Amos, C.; Parmar, M.K.B. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2009, 373, 125–136. [Google Scholar] [PubMed]
- Creutzberg, C.L.; van Putten, W.L.J.; Koper, P.C.M.; Lybeert, M.L.M.; Jobsen, J.J.; Warlam-Rodenhuis, C.C.; De Winter, K.A.; Lutgens, L.C.; van den Bergh, A.C.; van de Steen-Banasik, E.; et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: Multicentre random trial. Lancet 2000, 355, 1404–1411. [Google Scholar] [CrossRef]
- Keys, H.M.; Roberts, J.A.; Brunetto, V.L.; Zaino, R.J.; Spirtos, N.M.; Bloss, J.D.; Pearlman, A.; Maiman, M.A.; Bell, J.G. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2004, 92, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Onsrud, M.; Cvancarova, M.; Hellebust, T.P.; Trope, C.G.; Kristensen, G.B.; Lindemann, K. Long-term outcomes after pelvic radiation for early-stage endometrial cancer. J. Clin. Oncol. 2013, 31, 3951–3956. [Google Scholar] [CrossRef] [PubMed]
- Maggi, R.; Lissoni, A.; Spina, F.; Melpignano, M.; Zola, P.; Favalli, G.; Colombo, A.; Fossati, R. Adjuvant chemotherapy vs. radiotherapy in high-risk endometrial carcinoma: Results of a randomised trial. Br. J. Cancer 2006, 95, 266–271. [Google Scholar] [CrossRef]
- Susumu, N.; Sagae, S.; Udagawa, Y.; Niwa, K.; Kuramoto, H.; Satoh, S.; Kudo, R. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: A Japanese Gynecologic Oncology Group study. Gynecol. Oncol. 2008, 108, 226–233. [Google Scholar] [CrossRef]
- Ebina, Y.; Katabuchi, H.; Mikami, M.; Nagase, S.; Yaegashi, N.; Udagawa, Y.; Kato, H.; Kubushiro, K.; Takamatsu, K.; Ino, K.; et al. Japan Society of Gynecologic Oncology guidelines 2013 for the treatment of uterine body neoplasms. Int. J. Clin. Oncol. 2016, 21, 419–434. [Google Scholar] [CrossRef]
- Colombo, N.; Preti, E.; Landoni, F.; Carinelli, S.; Colombo, A.; Marini, C.; Sessa, C. ESMO Guidelines Working Group (2013) Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. S6), vi33–vi38. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Corn, B.W.; Lanciano, R.M.; Greven, K.M.; Noumoff, J.; Schultz, D.; Hanks, G.E.; Fowble, B.L. Impact of improved irradiation technique, age, and lymph node sampling on the severe complication rate of surgically staged endometrial cancer patients: A multivariate analysis. J. Clin. Oncol. 1994, 12, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Nout, R.A.; Putter, H.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.H.W.; Van Der Steen-Banasik, E.M.; Mens, J.W.M.; Slot, A.; Kroese, M.C.S.; Nijman, H.W.; et al. Five-year quality of life of endometrial cancer patients treated in the randomized Post Operative Radiation Therapy in Endometrial Cancer (PORTEC-2) trial and comparison with norm data. Eur. J. Cancer 2012, 48, 1638–1648. [Google Scholar] [CrossRef]
- van de Poll-Franse, L.V.; Pijnenborg, J.M.A.; Boll, D.; Vos, M.C.; van den Berg, H.; Lybeert, M.L.M.; de Winter, K.; Kruitwagen, R.F.P.M. Health related quality of life and symptoms after pelvic lymphadenectomy or radiotherapy vs. no adjuvant regional treatment in early-stage endometrial carcinoma: A large population-based study. Gynecol. Oncol. 2012, 127, 153–160. [Google Scholar] [CrossRef]
- Pellizzon, A.C.A.; Fogarolli, R.C.; Miziara, M.; Baraldi, H.; Soares, C.R. Morbidity of adjuvant high-dose-rate brachytherapy for low to intermediate risk endometrial adenocarcinoma completely resected. Int. J. Cancer 2001, 96, 105–108. [Google Scholar] [CrossRef]
- Harkenrider, M.M.; Block, A.M.; Siddiqui, Z.A.; Small, W., Jr. The role of vaginal cuff brachytherapy in endometrial cancer. Gynecol. Oncol. 2015, 136, 365–372. [Google Scholar] [CrossRef]
- Cella, D.; Huang, H.; Homesley, H.D.; Montag, A.; Salani, R.; De Geest, K.; Lee, R.; Spirtos, N.M. Patient-reported peripheral neuropathy of doxorubicin and cisplatin with and without paclitaxel in the treatment of advanced endometrial cancer: Results from GOG 184. Gynecol. Oncol. 2010, 119, 538–542. [Google Scholar] [CrossRef]
- Forsse, D.; Barbero, M.L.; Werner, H.M.J.; Woie, K.; Nordskar, N.; Nielsen, E.B.; Engh, M.E.; Vistad, I.; Rege, A.; Sævik-Lode, M.; et al. Longitudinal effects of adjuvant chemotherapy and lymph node staging on patient-reported outcomes in endometrial cancer survivors: A prospective cohort study. Am. J. Obstet. Gynecol. 2022, 226, 90.e1–90.e20. [Google Scholar] [CrossRef]
- Creasman, W.T.; Morrow, C.P.; Bundy, B.N.; Homesley, H.D.; Graham, J.E.; Heller, P.B. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer 1987, 60, 2035–2041. [Google Scholar] [CrossRef]
- AlHilli, M.M.; Podratz, K.C.; Dowdy, S.C.; Bakkum-Gamez, J.N.; Weaver, A.L.; McGree, M.E.; Keeney, G.L.; Cliby, W.A.; Mariani, A. Risk-scoring system for the individualized prediction of lymphatic dissemination in patients with endometrioid endometrial cancer. Gynecol. Oncol. 2013, 131, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Mariani, A.; Keeney, G.L.; Aletti, G.; Webb, M.J.; Haddock, M.G.; Podratz, K.C. Endometrial carcinoma: Paraaortic dissemination. Gynecol. Oncol. 2004, 92, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Mariani, A.; Dowdy, S.C.; Cliby, W.A.; Gostout, B.S.; Jones, M.B.; Wilson, T.O.; Podratz, K.C. Prospective assessment of lymphatic dissemination in endometrial cancer: A paradigm shift in surgical staging. Gynecol. Oncol. 2008, 109, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Todo, Y.; Suzuki, Y.; Azuma, M.; Hatanaka, Y.; Konno, Y.; Watari, H.; Kato, H.; Matsuno, Y.; Yamashiro, K.; Sakuragi, N. Ultrastaging of para-aortic lymph nodes in stage IIIC1 endometrial cancer: A preliminary report. Gynecol. Oncol. 2012, 127, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Odagiri, T.; Watari, H.; Kato, T.; Mitamura, T.; Hosaka, M.; Sudo, S.; Takeda, M.; Kobayashi, N.; Dong, P.; Todo, Y.; et al. Distribution of lymph node metastasis sites in endometrial cancer undergoing systematic pelvic and para-aortic lymphadenectomy: A proposal of optimal lymphadenectomy for future clinical trials. Ann. Surg. Oncol. 2014, 21, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, I.; Matsuura, T.; Mitani, T.; Otsuka, K.; Kanamoto, Y. Open surgery including lymphadenectomy without adjuvant therapy for uterine-confined intermediate- and high-risk endometrioid endometrial carcinoma. Curr. Oncol. 2022, 29, 298. [Google Scholar] [CrossRef]
- Fujimoto, T.; Nanjo, H.; Nakamura, A.; Yokoyama, Y.; Takano, T.; Shoji, T.; Nakahara, K.; Yamada, H.; Mizunuma, H.; Yaegashi, N.; et al. Para-aortic lymphadenectomy may improve disease-related survival in patients with multipositive pelvic lymph node stage IIIc endometrial cancer. Gynecol. Oncol. 2007, 107, 253–259. [Google Scholar] [CrossRef]
- Karube, Y.; Fujimoto, T.; Yakahashi, O.; Nanjyo, H.; Mizunuma, H.; Yaegashi, N.; Sugiyama, T.; Kurachi, H.; Sato, A.; Tanaka, T. Histopathologic prognostic factors predicting para-aortic lymph node metastasis in patients with endometrioid uterine cancer. Gynecol. Oncol. 2010, 118, 151–154. [Google Scholar] [CrossRef]
- Fotopoulou, C.; El-Balat, A.; du Bois, A.; Sehouli, J.; Harter, P.; Muallem, M.Z.; Krätschell, R.W.; Traut, A.; Heitz, F. Systematic pelvic and paraaortic lymphadenectomy in early high-risk or advanced endometrial cancer. Arch. Gynecol. Obstet. 2015, 292, 1321–1327. [Google Scholar] [CrossRef]
- Otsuka, I.; Kubota, T.; Aso, T. Lymphadenectomy and adjuvant therapy in endometrial carcinoma: Role of adjuvant chemotherapy. Br. J. Cancer 2002, 87, 377–380. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Gomez, J.D.; Alektiar, K.M.; Soslow, R.A.; Hensley, M.L.; Leitao, M.M., Jr.; Gardner, G.J.; Sonda, Y.; Chi, D.S.; Barakat, R.R. The incidence of isolated paraaortic nodal metastasis in surgically staged endometrial cancer patients with negative pelvic lymph nodes. Gynecol. Oncol. 2009, 115, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Chiang, A.J.; Yu, K.J.; Chao, K.C.; Teng, N.N.H. The incidence of isolated para-aortic nodal metastasis in completely staged endometrial cancer patients. Gynecol. Oncol. 2011, 121, 122–125. [Google Scholar] [CrossRef]
- Kumar, S.; Podratz, K.C.; Bakkum-Gamez, J.N.; Dowdy, S.C.; Weaver, A.L.; McGree, M.E.; Cliby, W.A.; Keeney, G.L.; Thomas, G.; Mariani, A. Prospective assessment of the prevalence of pelvic, paraaortic and high paraaortic lymph node metastasis in endometrial cancer. Gynecol. Oncol. 2014, 132, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Multinu, F.; Casarin, J.; Cappuccio, S.; Keeney, G.L.; Glaser, G.E.; Cliby, W.A.; Weaver, A.L.; McGree, M.E.; Angioni, S.; Faa, G.; et al. Ultrastaging of negative pelvic lymph nodes to decrease the true prevalence of isolated paraaortic dissemination in endometrial cancer. Gynecol. Oncol. 2019, 154, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Piedimonte, S.; Richer, L.; Souhami, L.; Arseneau, J.; Fu, L.; Gilbert, L.; Alfieri, J.; Jardon, K.; Zeng, X.Z. Clinical significance of isolated tumor cells and micrometastasis in low-grade, stage Iendometrial cancer. J. Surg. Oncol. 2018, 118, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Mariani, A.; Paolini, B.; Ditto, A.; Raspagliesi, F. Low-volume disease in endometrial cancer: The role of micrometastasis and isolated tumor cells. Gynecol. Oncol. 2019, 153, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Blake, P.; Swart, A.M.; Orton, J.; Kitchener, H.; Whelan, T.; Lukka, H.; Eisenhauer, E.; Bacon, M.; Tu, D.; Parmar, M.K.B.; et al. The ASTEC/EN.5 writing committee on behalf of the ASTEC/EN.5 Study Group. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): Pooled trial results, systematic review, and meta-analysis. Lancet 2009, 373, 137–146. [Google Scholar]
- Nout, R.A.; van de Poll-Francse, L.V.; Lybeert, M.L.M.; Warlam-Rodenhuis, C.C.; Jobsen, J.J.; Mens, J.W.M.; Lutgens, L.C.H.W.; Pras, B.; van Putten, W.L.J.; Creutzberg, C.L. Long-term outcome and quality of life of patients with endometrial carcinoma treated with or without pelvic radiotherapy in the Post Operative Radiation Therapy in Endometrial Carcinoma 1 (PORTEC-1) trial. J. Clin. Oncol. 2011, 29, 1692–1700. [Google Scholar] [CrossRef]
- Onstad, M.; Ducie, J.; Fellman, B.M.; Abu-Rustum, N.R.; Leitao, M.; Mariani, A.; Multinu, F.; Lu, K.H.; Soliman, P. Adjuvant therapy for grade 3, deeply invasive endometrioid adenocarcinoma of the uterus. Int. J. Gynecol. Cancer 2020, 30, 485–490. [Google Scholar] [CrossRef]
- Creutzberg, C.L.; van Putten, W.L.J.; Koper, P.C.; Lybeert, M.L.M.; Jobsen, J.J.; Wárlám-Rodenhius, C.C.; De Winter, K.A.L.; Lutgens, L.C.H.W.; van den Bergh, A.C.M.; van den Steen-Banasik, E.; et al. Survival after relapse in patients with endometrial cancer: Results from a randomized trial. Gynecol. Oncol. 2003, 89, 201–209. [Google Scholar] [CrossRef]
- Petignat, P.; Jolicoeur, M.; Alobaid, A.; Drouin, P.; Gauthier, P.; Provencher, D.; Donath, D.; Nguyen, T.V. Salvage treatment with high-dose-rate brachytherapy for isolated vaginal endometrial cancer recurrence. Gynecol. Oncol. 2006, 101, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, I.; Uno, M.; Wakabayashi, A.; Kameda, S.; Udagawa, H.; Kubota, T. Predictive factors for prolonged survival in recurrent endometrial carcinoma: Implications for follow-up protocol. Gynecol. Oncol. 2010, 119, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Watanabe, M.; Amikura, T.; Obata, H.; Sekine, M.; Yahata, T.; Fujita, K.; Tanaka, K. Adjuvant chemotherapy as treatment of high-risk stage I and II endometrial cancer. Gynecol. Oncol. 2004, 94, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Multinu, F.; Garzon, S.; Weaver, A.L.; McGree, M.E.; Sartori, E.; Landoni, F.; Zola, P.; Dinoi, G.; Aletti, G.; Block, M.S.; et al. Adjuvant Chemotherapy in Early Stage Endometrioid Endometrial Cancer with >50% Myometrial Invasion and Negative Lymph Nodes. Int. J. Gynecol. Cancer 2021, 31, 537–544. [Google Scholar] [CrossRef]
- Randall, M.E.; Filiaci, V.; McMeekin, D.S.; von Gruenigen, V.; Huang, H.; Yashar, C.M.; Mannel, R.S.; Kim, J.W.; Salani, R.; DiSilvestro, P.A.; et al. Phase III Trial: Adjuvant Pelvic Radiation Therapy Versus Vaginal Brachytherapy Plus Paclitaxel/Carboplatin in High-Intermediate and High-Risk Early Stage Endometrial Cancer. J. Clin. Oncol. 2019, 37, 1810–1818. [Google Scholar] [CrossRef]
- Fujimoto, T.; Nanjyo, H.; Fukuda, J.; Nakamura, A.; Mizunuma, H.; Yaegashi, N.; Sugiyama, T.; Kurachi, H.; Sato, A.; Tanaka, T. Endometrioid uterine cancer: Histopathological risk factors of local and distant recurrence. Gynecol. Oncol. 2009, 112, 342–347. [Google Scholar] [CrossRef]
- Bakkum-Gamez, J.N.; Mariani, A.; Dowdy, S.C.; Weaver, A.L.; McGree, M.E.; Martin, J.R.; Keeney, G.L.; Jatoi, A.; Gostout, B.S.; Podratz, K.C. Efficacy of contemporary chemotherapy in stage IIIC endometrial cancer: A histologic dichotomy. Gynecol. Oncol. 2014, 132, 578–584. [Google Scholar] [CrossRef]
- Cragun, J.M.; Havrilesky, L.J.; Calingaert, B.; Synan, I.; Secord, A.A.; Soper, J.T.; Clarke-Pearson, D.L.; Berchuck, A. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J. Clin. Oncol. 2005, 23, 3668–3675. [Google Scholar] [CrossRef]
- Chan, J.K.; Cheung, M.K.; Huh, W.K.; Osann, K.; Husain, A.; Teng, N.N.; Kapp, D.S. Therapeutic role of lymph node resection in endometrioid corpus cancer. A study of 12,333 patients. Cancer 2006, 107, 1823–1830. [Google Scholar] [CrossRef]
- Jeong, N.H.; Lee, J.M.; Lee, J.K.; Kim, M.K.; Kim, Y.J.; Cho, C.H.; Kim, S.M.; Park, S.Y.; Park, C.Y.; Kim, K.T. Role of systematic lymphadenectomy and adjuvant radiation in early-stage endometrioid uterine cancer. Ann. Surg. Oncol. 2010, 17, 2951–2957. [Google Scholar] [CrossRef]
- Todo, Y.; Kato, H.; Minobe, S.; Okamoto, K.; Suzuki, Y.; Sudo, S.; Takeda, M.; Watari, H.; Kaneuchi, M.; Sakuragi, N. Initial failure site according to primary treatment with or without para-aortic lymphadenectomy in endometrial cancer. Gynecol. Oncol. 2011, 121, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Leitao, M.M., Jr.; Zhou, Q.C.; Gomez-Hidalgo, N.R.; Iasonos, A.; Baser, R.; Mezzancello, M.; Chang, K.; Ward, J.; Chi, D.S.; Roche, K.L.; et al. Patient-reported outcomes after surgery for endometrial carcinoma: Prevalence of lower-extremity lymphedema after sentinel lymph node mapping versus lymphadenectomy. Gynecol. Oncol. 2020, 156, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Dioun, S.; Chen, L.; Melamed, A.; Gockley, A.; St Clair, C.M.; Hou, J.Y.; Khoury-Collado, F.; Hershman, D.L.; Wright, J.D. Uptake and outcomes of sentinel lymph node mapping in women undergoing minimally invasive surgery for endometrial cancer. BJOG 2022, 129, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Frimer, M.; Khoury-Collado, F.; Murray, M.P.; Barakat, R.R.; Abu-Rustum, N.R. Micrometastases of endometrial cancer to sentinel lymph nodes: Is it an artifact of uterine manipulation? Gynecol. Oncol. 2010, 119, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Nottegar, A.; Veronese, N.; Senthil, M.; Roumen, R.M.; Stubbs, B.; Choi, A.H.; Verheuvel, N.C.; Solmi, M.; Pea, A.; Capelli, P.; et al. Extra-nodal extension of sentinel lymph node metastasis is a marker of poor prognosis in breast cancer patients: A systematic review and an exploratory meta-analysis. Eur. J. Surg. Oncol. 2016, 42, 919–925. [Google Scholar] [CrossRef]
- Faries, M.B.; Thompson, J.F.; Cochran, A.J.; Andtbacka, R.H.; Mozzillo, N.; Zager, J.S.; Jahkola, T.; Bowles, T.L.; Testori, A.; Beitsch, P.D.; et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef]
- Khoury-Collado, F.; Murray, M.P.; Hensley, M.L.; Sonoda, Y.; Alektiar, K.M.; Levine, D.A.; Chi, D.S.; Barakat, R.R.; Abu-Rustum, N.R. Sentinel lymph node mapping for endometrial cancer improves the detection of metastatic disease to regional lymph nodes. Gynecol. Oncol. 2011, 122, 251–254. [Google Scholar] [CrossRef]
- Ballester, M.; Naoura, I.; Chéreau, E.; Seror, J.; Bats, A.S.; Bricou, A.; Daraï, E. Sentinel node biopsy upstages patients with presumed low- and intermediate-risk endometrial cancer: Results of a multicenter study. Ann. Surg. Oncol. 2013, 20, 407–412. [Google Scholar] [CrossRef]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef]
- Bodurtha Smith, A.J.; Fader, A.N.; Tanner, E.J. Sentinel lymph node assessment in endometrial cancer: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 459–476. [Google Scholar] [CrossRef]
- Holloway, R.W.; Gupta, S.; Stavitzski, N.M.; Zhu, X.; Takimoto, E.L.; Gubbi, A.; Bigsby, G.E.; Brudie, L.A.; Kendrick, J.E.; Ahmad, S. Sentinel lymph node mapping with staging lymphadenectomy for patients with endometrial cancer increases the detection of metastasis. Gynecol. Oncol. 2016, 141, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.W.; Levenback, C.; Tornos, C.; Morris, M.; Wharton, J.T.; Gershenson, D.M. Intraabdominal lymphatic mapping to direct selective pelvic and paraaortic lymphadenectomy in women with high-risk endometrial cancer: Results of a pilot study. Gynecol. Oncol. 1996, 62, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Coleman, R.C.; Soliman, P.T.; Ramirez, P.T.; Levenback, C.F. A case for caution in the pursuit of the sentinel node in women with endometrial carcinoma. Gynecol. Oncol. 2014, 132, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Ditto, A.; Casarin, J.; Pinelli, C.; Perrone, A.M.; Scollo, P.; Martinelli, F.; Bogani, G.; Maggiore, U.L.R.; Signorelli, M.; Chiappa, V.; et al. Hysteroscopic versus cervical injection for sentinel node detection in endometrial cancer: A multicenter prospective randomised controlled trial from the Multicenter Italian Trials in Ovarian cancer (MITO) study group. Eur. J. Cancer 2020, 140, 1–10. [Google Scholar] [CrossRef]
- Persson, J.; Salehi, S.; Bollino, M.; Lonnerfors, C.; Falconer, H.; Geppert, B. Pelvic Sentinel lymph node detection in High-Risk Endometrial Cancer (SHREC-trial)--the final step towards a paradigm shift in surgical staging. Eur. J. Cancer 2019, 116, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Perrone, A.M.; Casadio, P.; Formelli, G.; Levorato, M.; Ghi, T.; Costa, S.; Meriggiola, M.C.; Pelusi, G. Cervical and hysteroscopic injection for identification of sentinel lymph node in endometrial cancer. Gynecol. Oncol. 2008, 111, 62–67. [Google Scholar] [CrossRef]
- Kataoka, F.; Susumu, N.; Yamagami, W.; Kuwahata, M.; Takigawa, A.; Nomura, H.; Takeuchi, H.; Nakahara, T.; Kameyama, K.; Aoki, D. The importance of para-aortic lymph nodes in sentinel lymph node mapping for endometrial cancer by using hysteroscopic radio-isotope tracer injection combined with subserosal dye injection: Prospective study. Gynecol. Oncol. 2016, 140, 400–404. [Google Scholar] [CrossRef]
- Martinelli, F.; Ditto, A.; Signorelli, M.; Bogani, G.; Chiappa, V.; Lorusso, D.; Scaffa, C.; Recalcati, D.; Perotto, S.; Haeusler, E.; et al. Sentinel node mapping in endometrial cancer following Hysteroscopic injection of tracers: A single center evaluation over 200 cases. Gynecol. Oncol. 2017, 146, 525–530. [Google Scholar] [CrossRef]
- Angeles, M.A.; Migliorelli, F.; Vidal-Sicart, S.; Saco, A.; Ordi, J.; Ros, C.; Fuste, P.; Munmany, M.; Escura, S.; Carreras, N.; et al. Paraaortic sentinel lymph node detection in intermediate and high-risk endometrial cancer by transvaginal ultrasound-guided myometrial injection of radiotracer (TUMIR). J. Gynecol. Oncol. 2021, 32, e52. [Google Scholar] [CrossRef]
- Altın, D.; Taşkın, S.; Ortac, F.; Tokgozoğlu, N.; Vatansever, D.; Güler, A.H.; Güng, M.; Taşci, T.; Beşe, T.; Turan, H.; et al. Diagnostic accuracy of sentinel node biopsy in non-endometrioid, high-grade and/or deep myoinvasive endometrial cancer: A Turkish gynecologic oncology group study (TRSGO-SLN-006). Gynecol. Oncol. 2022, 164, 492–497. [Google Scholar] [CrossRef]
- Niikura, H.; Okamura, C.; Utsunomiya, H.; Yoshinaga, K.; Akahira, J.; Ito, K.; Yaegashi, N. Sentinel lymph node detection in patients with endometrial cancer. Gynecol. Oncol. 2004, 92, 669–674. [Google Scholar] [CrossRef]
- Pristauz, G.; Bader, A.A.; Regitnig, P.; Haas, J.; Winter, R.; Tamussino, K. How accurate is frozen section histology of pelvic lymph nodes in patients with endometrial cancer? Gynecol. Oncol. 2009, 115, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ballester, M.; Dubernard, G.; Lecuru, F.; Heitz, D.; Mathevet, P.; Marret, H.; Querleu, D.; Golfier, F.; Leblanc, E.; Rouzier, R.; et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: A prospective multicentre study (SENTI-ENDO). Lancet Oncol. 2011, 12, 469–476. [Google Scholar] [CrossRef]
- Kim, C.H.; Khoury-Collado, F.; Barber, E.L.; Soslow, R.A.; Makker, V.; Leitao, M.M., Jr.; Sonoda, Y.; Alektiar, K.M.; Barakat, R.R.; Abu-Rustum, N. Sentinel lymph node mapping with pathologic ultrastaging: A valuable tool for assessing nodal metastasis in low-grade endometrial cancer with superficial myoinvasion. Gynecol. Oncol. 2013, 131, 714–719. [Google Scholar] [CrossRef]
- Baiocchi, G.; Mantoan, H.; Goncalves, B.T.; Faloppa, C.C.; Kumagai, L.Y.; Badiglian-Filho, L.; da Costa, A.A.B.A.; De Brot, L. Size of Sentinel Node Metastasis Predicts Non-sentinel Node Involvement in Endometrial Cancer. Ann. Surg. Oncol. 2020, 27, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Casarin, J.; Maggiore, U.L.R.; Ditto, A.; Pinelli, C.; Dell’acqua, A.; Lopez, S.; Chiappa, V.; Brusadelli, C.; Guerrisi, R.; et al. Survival outcomes in endometrial cancer patients having lymphadenectomy, sentinel node mapping followed by lymphadectomy and sentinel node mapping alone: Long-term results of a propensity-matched analysis. Gynecol. Oncol. 2020, 158, 77–83. [Google Scholar] [CrossRef]
- Daraï, E.; Dubernard, G.; Bats, A.S.; Heitz, D.; Mathevet, P.; Marret, H.; Querleu, D.; Glfoier, F.; Leblanc, E.; Rouzier, R.; et al. Sentinel node biopsy for the management of early stage endometrial cancer: Long-term results of the SENTI-ENDO study. Gynecol. Oncol. 2015, 136, 54–59. [Google Scholar] [CrossRef]
- Cusimano, M.C.; Vicus, D.; Pulman, K.; Maganti, M.; Bernardini, M.Q.; Bouchard-Fortier, G.; Laframboise, S.; May, T.; Hogen, L.F.; Covens, A.L.; et al. Assessment of Sentinel Lymph Node Biopsy vs Lymphadenectomy for Intermediate- and High-Grade Endometrial Cancer Staging. JAMA Surg. 2021, 156, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Di, S.D.; Lu, W.; He, Q.Z.; Li, Y.R.; Li, B.L.; Wang, Z.J.; Yan, Q.; Wan, X.P. A Prospective Study of Sentinel Lymph Node Mapping for Endometrial Cancer: Is It Effective in High-Risk Subtypes? Oncologist 2019, 24, e1381. [Google Scholar] [CrossRef]
- Torrent, A.; Amengual, J.; Sampol, C.M.; Ruiz, M.; Rioja, J.; Matheu, G.; Roca, P.; Cordoba, O. Sentinel Lymph Node Biopsy in Endometrial Cancer: Dual Injection, Dual Tracer—A Multidisciplinary Exhaustive Approach to Nodal Staging. Cancers 2022, 14, 929. [Google Scholar] [CrossRef]
- Barlin, J.N.; Khoury-Collado, F.; Kim, C.H.; Leitao, M.M., Jr.; Chi, D.S.; Sonoda, Y.; Alektiar, K.; DeLair, D.F.; Barakat, R.R.; Abu-Rustum, N.R. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: Beyond removal of blue nodes. Gynecol. Oncol. 2012, 125, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Buda, A.; Di Martino, G.; Restaino, S.; De Ponti, E.; Monterossi, G.; Giuliani, D.; Ercoli, A.; Dell’Orto, F.; Dinoi, G.; Grassi, T.; et al. The impact on survival of two different staging strategies in apparent early stage endometrial cancer comparing sentinel lymph nodes mapping algorithm and selective lymphadenectomy: An Italian retrospective analysis of two reference centers. Gynecol. Oncol. 2017, 147, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Buda, A.; Gasparri, M.L.; Puppo, A.; Mereu, L.; De Ponti, E.; Di Martino, G.; Novelli, A.; Tateo, S.; Muller, M.; Landoni, F.; et al. Lymph node evaluation in high-risk early stage endometrial cancer: A multi-institutional retrospective analysis comparing the sentinel lymph node (SLN) algorithm and SLN with selective lymphadenectomy. Lymph node evaluation in high-risk early stage endometrial cancer: A multi-institutional retrospective analysis comparing the sentinel lymph node (SLN) algorithm and SLN with selective lymphadenectomy. Gynecol. Oncol. 2018, 150, 261–266. [Google Scholar] [PubMed]
- Schlappe, B.A.; Weaver, A.L.; Ducie, J.A.; Eriksson, A.G.Z.; Dowdy, S.C.; Cliby, W.A.; Glaser, G.E.; Soslow, R.A.; Alektiar, K.M.; Makker, V.; et al. Multicenter study comparing oncologic outcomes between two nodal assessment methods in patients with deeply invasive endometrioid endometrial carcinoma: A sentinel lymph node algorithm versus a comprehensive pelvic and paraaortic lymphadenectomy. Gynecol. Oncol. 2018, 151, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Salman, L.; Cusimano, M.C.; Marchocki, Z.; Ferguson, S.E. Sentinel Lymph Node Mapping in High-Grade Endometrial Cancer. Curr. Oncol. 2022, 29, 96. [Google Scholar] [CrossRef]
- Takahashi, A.; Matsuura, M.; Matoda, M.; Nomura, H.; Okamoto, S.; Kanao, H.; Kondo, E.; Omatsu, K.; Kato, K.; Utsugi, K.; et al. Clinicopathological Features of Early and Late Recurrence of Endometrial Carcinoma After Surgical Resection. Int. J. Gynecol. Cancer 2017, 27, 967–972. [Google Scholar] [CrossRef]
- Podratz, K.C.; Mariani, A.; Webb, M.J. Staging and therapeutic value of lymphadenectomy in endometrial cancer. Gynecol. Oncol. 1998, 70, 163–164. [Google Scholar] [CrossRef]
- Chan, J.K.; Kapp, D.S. Role of complete lymphadenectomy in endometrioid uterine cancer. Lancet Oncol. 2007, 8, 831–841. [Google Scholar] [CrossRef]
- Dowdy, S.C.; Borah, B.J.; Bakkum-Gamez, J.N.; Weaver, A.L.; McGree, M.E.; Haas, L.R.; Keeney, G.L.; Mariani, A.; Podratz, K.C. Prospective assessment of survival, morbidity, and cost associated with lymphadenectomy in low-risk endometrial cancer. Gynecol. Oncol. 2012, 127, 5–10. [Google Scholar] [CrossRef]
- Creasman, W.T. ASTEC lymphadenectomy and radiation therapy studies: Are conclusions valid? Gynecol. Oncol. 2010, 116, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Naumann, R.W. The role of lymphadenectomy in endometrial cancer: Was the ASTEC trial doomed by design and are we destined to repeat that mistake? Gynecol. Oncol. 2012, 126, 5–11. [Google Scholar] [CrossRef]
- Huang, C.Y.; Tang, Y.H.; Chiang, Y.C.; Wang, K.L.; Fu, H.C.; Ke, Y.M.; Lau, H.Y.; Hsu, K.F.; Wu, C.H.; Cheng, W.F. Impact of management on the prognosis of pure uterine papillary serous cancer—A Taiwanese Gynecologic Oncology Group (TGOG) study. Gynecol. Oncol. 2014, 133, 221–228. [Google Scholar] [CrossRef]
- Leath, C.A., III; Numnum, T.M.; Kendrick, J.E.; Frederick, P.J.; Rocconi, R.P.; Conner, M.G.; Straughn, J.M., Jr. Patterns of Failure for Conservatively Managed Surgical Stage I Uterine Carcinosarcoma. Implications for Adjuvant Therapy. Int. J. Gynecol. Cancer 2009, 19, 888–891. [Google Scholar] [CrossRef]
- Jeppesen, M.M.; Jensen, P.T.; Hansen, D.G.; Iachina, M.; Mogensen, O. The nature of early-stage endometrial cancer recurrence—national cohort study. Eur. J. Cancer 2016, 69, 51–60. [Google Scholar] [CrossRef]
- Mariani, A.; Webb, M.J.; Galli, L.; Podratz, K.C. Potential therapeutic role of para-aortic lymphadenectomy in node-positive endometrial cancer. Gynecol. Oncol. 2000, 76, 348–356. [Google Scholar] [CrossRef]
- Alektiar, K.M.; Venkatraman, E.; Chi, D.S.; Barakat, R.R. Intravaginal brachytherapy alone for intermediate-risk endometrial cancer. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 111–117. [Google Scholar] [CrossRef]
- Todo, Y.; Kato, H.; Kaneuchi, M.; Watari, H.; Takeda, M.; Sakuragi, N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): A retrospective cohort analysis. Lancet 2010, 375, 1165–1172. [Google Scholar] [CrossRef]
- Huang, C.Y.; Ho, C.M.; Chen, Y.L.; You, S.L.; Chen, C.A.; Cheng, W.F. Impact of lymphadenectomy in uterine endometrioid carcinoma. Eur. J. Surg. Oncol. 2013, 39, 350–357. [Google Scholar] [CrossRef]
- Eggemann, H.; Ignatov, T.; Kaiser, K.; Burger, E.; Dan Costa, S.; Ignatov, A. Survival advantage of lymphadenectomy in endometrial cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 1051–1060. [Google Scholar] [CrossRef]
- Nugent, E.K.; Bishop, E.A.; Mathews, C.A.; Moxley, K.M.; Tenney, M.; Mannel, R.S.; Walker, J.L.; Moore, K.N.; Landrum, L.M.; McMeekin, D.S. Do uterine risk factors or lymph node metastasis more significantly affect recurrence in patients with endometrioid adenocarcinoma? Gynecol. Oncol. 2012, 125, 94–98. [Google Scholar] [CrossRef]
- Ouldamer, L.; Bendifallah, S.; Body, G.; Canlorbe, G.; Touboul, C.; Graesslin, O.; Raimond, E.; Collinet, P.; Coutant, C.; Lavoué, V.; et al. Call for Surgical Nodal Staging in Women with ESMO/ESGO/ESTRO High-Intermediate Risk Endometrial Cancer: A Multicentre Cohort Analysis from the FRANCOGYN Study Group. Ann. Surg. Oncol. 2017, 24, 1660–1666. [Google Scholar] [CrossRef]
- Morrow, C.P.; Bundy, B.N.; Kurman, R.J.; Creasman, W.T.; Heller, P.; Homesley, H.D.; Graham, J.E. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: A Gynecologic Oncology Group Study. Gynecol. Oncol. 1991, 40, 55–65. [Google Scholar] [CrossRef]
- Euscher, E.D.; Bassett, R.; Malpica, A. Lymph node counts in endometrial cancer: Expectations versus reality. Am. J. Surg. Pathol. 2011, 35, 913–918. [Google Scholar] [CrossRef]
- Fanning, J.; Nanavati, P.J.; Hilgers, R. Surgical staging and high dose rate brachytherapy for endometrial cancer: Limiting external radiotherapy to node-positive tumors. Obstet. Gynecol. 1996, 87, 1041–1044. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Iasonos, A.; Zhou, Q.; Oke, E.; Soslow, R.A.; Alektiar, K.M.; Chi, D.S.; Barakat, R.R. Is there a therapeutic impact to regional lymphadenectomy in the surgical treatment of endometrial carcinoma? Am. J. Obstet. Gynecol. 2008, 198, 457.e1–457.e6. [Google Scholar] [CrossRef]
- Huang, M.; Chadha, M.; Musa, F.; Friedmann, P.; Kolev, V.; Holcomb, K. Lymph nodes: Is total number or station number a better predictor of lymph node metastasis in endometrial cancer? Gynecol. Oncol. 2010, 119, 295–298. [Google Scholar] [CrossRef]
- Kilgore, L.C.; Partridge, E.E.; Alvarez, R.D.; Austin, J.M.; Shingleton, H.M.; Noojin, F.; Conner, W. Adenocarcinoma of the endometrium: Survival comparison of patients with and without pelvic node sampling. Gynecol. Oncol. 1995, 56, 29–33. [Google Scholar] [CrossRef]
- Sosa, J.A.; Diener-West, M.; Gusev, Y.; Choti, M.A.; Lange, J.R.; Dooley, W.C.; Zeiger, M.A. Association between extent of axillary lymph node dissection and survival in patients with stage I breast cancer. Ann. Surg. Oncol. 1998, 5, 140–149. [Google Scholar] [CrossRef]
- Weir, L.; Speers, C.; D’Yachkova, Y.; Olivotto, I.A. Prognostic significance of the number of axillary lymph nodes removed in patients with node-negative breast cancer. J. Clin. Oncol. 2002, 20, 1793–1799. [Google Scholar] [CrossRef]
- Gajra, A.; Newman, N.; Gamble, G.P.; Kohman, L.J.; Graziano, S.L. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J. Clin. Oncol. 2003, 21, 1029–1034. [Google Scholar] [CrossRef]
- Shah, M.; Lewin, S.N.; Deutsch, I.; Burke, W.M.; Sun, X.; Herzog, T.J.; Wright, J.D. Therapeutic role of lymphadenectomy for cervical cancer. Cancer 2011, 117, 310–317. [Google Scholar] [CrossRef]
- Chen, S.S. Operative treatment in stage I endometrial carcinoma with deep myometrial invasion and/or grade 3 tumor surgically limited to the corpus uteri. No recurrence with only primary surgery. Cancer 1989, 63, 1843–1845. [Google Scholar] [CrossRef]
- Ayhan, A.; Taskiran, C.; Celik, C.; Guney, I.; Yuce, K.; Ozyar, E.; Atahan, L.; Kucukali, T. Is there a survival benefit to adjuvant radiotherapy in high-risk surgical stage I endometrial cancer? Gynecol. Oncol. 2002, 86, 259–263. [Google Scholar] [CrossRef]
- Straughn, J.M.; Huh, W.K.; Orr, J.W.; Kelly, F.J.; Roland, P.Y.; Gold, M.A.; Powell, M.; Mutch, D.G.; Partridge, E.E.; Kilgore, L.C.; et al. Stage IC adenocarcinoma of the endometrium: Survival comparisons of surgically staged patients with and without adjuvant radiotherapy. Gynecol. Oncol. 2003, 89, 295–300. [Google Scholar] [CrossRef]
- Orr, J.W., Jr.; Holimon, J.L.; Orr, P.F. Stage I corpus cancer: Is teletherapy necessary? Am. J. Obstet. Gynecol. 1997, 176, 777–789. [Google Scholar] [CrossRef]
- Berclaz, G.; Hänggi, W.; Kratzer-Berger, A.; Altermatt, H.J.; Greiner, R.H.; Dreher, E. Lymphadenectomy in high risk endometrial carcinoma stage I and II: No more morbidity and no need for external pelvic radiation. Int. J. Gynecol. Cancer 1999, 9, 322–328. [Google Scholar] [CrossRef]
- Horowitz, N.S.; Peters, W.A., III; Smith, M.R.; Drescher, C.W.; Artwood, M.; Mate, T.P. Adjuvant high dose rate vaginal brachytherapy as treatment of stage I and II endometrial carcinoma. Obstet. Gynecol. 2002, 99, 235–240. [Google Scholar]
- Mariani, A.; Webb, M.J.; Keeney, G.L.; Calori, G.; Podratz, K.C. Hematogenous dissemination in corpus cancer. Gynecol. Oncol. 2001, 80, 233–238. [Google Scholar] [CrossRef]
- Otsuka, I.; Ono, I.; Akamatsu, H.; Sunamori, K.; Aso, T. Pulmonary metastasis from endometrial carcinoma. Int. J. Gynecol. Cancer 2002, 12, 208–213. [Google Scholar] [CrossRef]
- Matsuo, K.; Yabuno, A.; Hom, M.S.; Shida, M.; Kakuda, M.; Adachi, S.; Mandelbaum, R.S.; Ueda, Y.; Hasegawa, K.; Enomoto, T.; et al. Significance of abnormal peritoneal cytology on survival of women with stage I–II endometrioid endometrial cancer. Gynecol. Oncol. 2018, 149, 301–309. [Google Scholar] [CrossRef]
- Brown, A.P.; Gaffney, D.K.; Dodson, M.K.; Soisson, A.P.; Belnap, T.W.; Alleman, K.; Sause, W.T. Survival analysis of endometrial cancer patients with positive lymph nodes. Int. J. Gynecol. Cancer 2013, 23, 861–868. [Google Scholar] [CrossRef]
- Otsuka, I.; Kadooka, M.; Matsuura, T. Long-term survival of a patient with stage IIIC2 grade 3 endometrioid endometrial carcinoma treated with surgery alone. Gynecol. Oncol. Rep. 2021, 38, 100869. [Google Scholar] [CrossRef]
- Goebel, E.A.; St Laurent, J.D.; Nucci, M.R.; Feltmate, C.M. Retrospective detection of isolated tumor cells by immunohistochemistry in sentinel lymph node biopsy performed for endometrial carcinoma: Is there clinical significance? Int. J. Gynecol. Cancer 2020, 30, 291–298. [Google Scholar] [CrossRef]
- Backes, F.J.; Felixb, A.S.; Plante, M.; Grégoire, J.; Sullivan, S.A.; Rossi, E.C.; Tanner, E.J., III; Stewart, K.I.; Soliman, P.T.; Holloway, R.W.; et al. Sentinel lymph node (SLN) isolated tumor cells (ITCs) in otherwise stage I/II endometrioid endometrial cancer: To treat or not to treat? Gynecol. Oncol. 2021, 161, 347–352. [Google Scholar] [CrossRef]
- Ghoniem, K.; Larisha, A.M.; Dinoi, G.; Zhou, X.C.; Alhilli, M.; Wallace, S.; Wohlmuth, C.; Baiocchi, G.; Tokgozoglu, N.; Raspagliesi, F.; et al. Oncologic outcomes of endometrial cancer in patients with low-volume metastasis in the sentinel lymph nodes: An international multi-institutional study. Gynecol. Oncol. 2021, 162, 590–598. [Google Scholar] [CrossRef]
- Plante, M.; Stanleigh, J.; Renaud, M.-C.; Sebastianelli, A.; Grondin, K.; Gregoire, J. Isolated tumor cells identified by sentinel lymph node mapping in endometrial cancer: Does adjuvant treatment matter? Gynecol. Oncol. 2017, 146, 240–246. [Google Scholar] [CrossRef]
- Ignatov, A.; Ivros, S.; Bozukova, T.; Papathemelis, T.; Ortmann, O.; Eggemann, H. Systematic lymphadenectomy in early stage endometrial cancer. Arch. Gynecol. Obstet. 2020, 302, 231–239. [Google Scholar] [CrossRef]
- Papathemelis, T.; Hassas, D.; Gerken, M.; Klinkhammer-Schalke, M.; Scharl, A.; Lux, M.P.; Beckmann, M.W.; Scharl, S. Is there a benefit of lymphadenectomy for overall and recurrence-free survival in type I FIGO IB G1-2 endometrial carcinoma? A retrospective population-based cohort analysis. J. Cancer Res. Clin. Oncol. 2018, 144, 2019–2027. [Google Scholar] [CrossRef]
- Gayar, O.H.; Robbins, J.R.; Parikh, K.; Lu, M.; Buekers, T.; Munkarah, A.; Elshaikh, M.A. Hysterectomy for uterine adenocarcinoma in the elderly: Tumor characteristics, and long-term outcome. Gynecol. Oncol. 2011, 123, 71–75. [Google Scholar] [CrossRef]
- Hachisuga, K.; Ohishi, Y.; Tomonobe, H.; Yahata, H.; Kato, K.; Oda, Y. Endometrial endometrioid carcinoma, G1, is more aggressive in the elderly than in the young. Histopathology 2021, 79, 708–719. [Google Scholar] [CrossRef]
- Pawelec, G. Does patient age influence anti-cancer immunity? Semin. Immunopathol. 2019, 41, 125–131. [Google Scholar] [CrossRef]
- Walker, J.L.; Piedmonte, M.R.; Spirtos, N.M.; Eisenkop, S.M.; Schalaerth, J.B.; Mannel, R.S.; Barakat, R.; Pearl, M.L.; Sharma, S.K. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J. Clin. Oncol. 2012, 30, 695–700. [Google Scholar] [CrossRef]
- Janda, M.V.; Gebski, L.C.; Davies, P.; Forder, A.; Brand, R.; Hogg, T.W.; Land, R.; Manolitsas, T.; Nascimento, M.; Neesham, D.; et al. Effect of total laparoscopic hysterectomy vs total abdominal hysterectomy on disease-free survival among women with stage I endometrial cancer: A randomized clinical trial. JAMA 2017, 317, 1224–1233. [Google Scholar] [CrossRef]
- Wright, J.D. Laparoscopic Hysterectomy for Endometrial Cancer. A Procedure 25 Years in the Making. JAMA 2017, 317, 1215–1216. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Z.; Wang, J. Survival of microsatellite-stable endometrioid endometrial cancer patients after minimally invasive surgery: An analysis of the Cancer Genome Atlas data. Gynecol. Oncol. 2020, 158, 92–98. [Google Scholar] [CrossRef]
- Wright, J.D.; Burke, W.M.; Tergas, A.I.; Hou, J.Y.; Huang, Y.; Hu, J.C.; Hillyer, G.C.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L. Comparative Effectiveness of Minimally Invasive Hysterectomy for Endometrial Cancer. J. Clin. Oncol. 2016, 34, 1087–1096. [Google Scholar] [CrossRef]
- Chu, C.S.; Randall, T.C.; Bandera, C.A.; Rubin, S.C. Vaginal Cuff Recurrence of Endometrial Cancer Treated by Laparoscopic-Assisted Vaginal Hysterectomy. Gynecol. Oncol. 2003, 88, 62–65. [Google Scholar] [CrossRef]
- Kim, S.I.; Park, D.C.; Lee, S.J.; Song, M.J.; Kim, C.J.; Lee, H.N.; Yoon, J.H. Survival rates of patients who undergo minimally invasive surgery for endometrial cancer with cervical involvement. Int. J. Med. Sci. 2021, 18, 2204–2208. [Google Scholar] [CrossRef]
- Philp, I.; Tannenbaum, S.; Haber, H.; Saini, A.; St Laurent, J.; James, K.; Feltmate, C.; Russo, A.; Growdon, W. Effect of surgical approach on risk of recurrence after vaginal brachytherapy in early-stage high-intermediate risk endometrial cancer. Gynecol. Oncol. 2021, 160, 389–395. [Google Scholar] [CrossRef]
- Feigenberg, T.; Cormier, B.; Gotlieb, W.H.; Jegatheeswaran, K.; Helpman, L.; Kim, S.R.; Lau, S.; May, T.; Saab, D.; Plante, M.; et al. Factors associated with an increased risk of recurrence in patients diagnosed with high-grade endometrial cancer undergoing minimally invasive surgery: A study of the society of gynecologic oncology of Canada (GOC) community of practice (CoP). Gynecol. Oncol. 2021, 162, 606–612. [Google Scholar] [CrossRef]
- Song, J.; Le, T.; Hopkins, L.; Fung-Kee-Fung, M.; Lupe, K.; Gaudet, M.; Rajiv Samant, C.E. A comparison of disease recurrence between robotic versus laparotomy approach in patients with intermediate-risk endometrial cancer. Int. J. Gynecol. Cancer 2020, 30, 160–166. [Google Scholar] [CrossRef]
- Argenta, P.A.; Mattson, J.; Rivard, C.L.; Luther, E.; Schefter, A.; Vogel, R.I. Robot-assisted versus laparoscopic minimally invasive surgery for the treatment of stage I endometrial cancer. Gynecol. Oncol. 2022, 165, 347–352. [Google Scholar] [CrossRef]
- Chang, E.J.; Jooya, N.D.; Ciesielski, K.M.; Shahzad, M.M.; Roman, L.D.; Matsuo, K. Intraoperative tumor spill during minimally invasive hysterectomy for endometrial cancer: A survey study on experience and practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 256–261. [Google Scholar] [CrossRef]
- Nasioudis, D.; Ko, E.M.; Cory, L.; Latif, N. Impact of surgical approach on prevalence of positive peritoneal cytology and lymph-vascular invasion in patients with early-stage endometrial carcinoma: A National Cancer Database study. Int. J. Gynecol. Cancer 2021, 31, 1001–1006. [Google Scholar] [CrossRef]
- Stolnicu, S.; Terinte, C.; Ioanid, N.; Silva, E. Presence of tumor cells in the vagina during surgical treatment could be the source of vaginal recurrence in patients with endometrial carcinoma—A pilot prospective study. Ann. Diagn. Pathol. 2020, 46, 151503. [Google Scholar] [CrossRef]
- Otsuka, I. Cutaneous metastasis after surgery, injury, lymphadenopathy, and peritonitis: Possible mechanisms. Int. J. Mol. Sci. 2019, 20, 3286. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Wolf, J.K.; Levenback, C. Laparoscopic port-site metastases: Etiology and prevention. Gynecol. Oncol. 2003, 91, 179–189. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lope, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Melamed, A.; Margul, D.J.; Chen, L.; Keating, N.L.; del Carmen, M.G.; Yang, J.; Seagle, B.L.L.; Alexander, A.; Barber, E.L.; Rice, L.W.; et al. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. N. Engl. J. Med. 2018, 379, 1905–1914. [Google Scholar] [CrossRef]
- Acar, O.; Esen, T.; Bavbek, S.; Pekerd, Ö.; Musaoglu, A. Port site and peritoneal metastases after robot-assisted radical prostatectomy. Int. J. Surg. Case Rep. 2014, 5, 131–134. [Google Scholar] [CrossRef]
- Jacquet, P.; Averbach, A.M.; Jacquet, N. Abdominal wall metastasis and peritoneal carcinomatosis after laparoscopic-assisted colectomy for colon cancer. Eur. J. Surg. Oncol. 1995, 21, 568–570. [Google Scholar] [CrossRef]
- Uccella, S.; Bonzini, M.; Malzoni, M.; Fanfani, F.; Palomba, S.; Aletti, G.; Corrado, G.; Ceccaroni, M.; Seracchioli, R.; Shakir, F.; et al. The effect of a uterine manipulator on the recurrence and mortality of endometrial cancer: A multi-centric study by the Italian Society of Gynecological Endoscopy. Am. J. Obstet. Gynecol. 2017, 216, 592.e1–592.e11. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Iserte, P.; Lago, V.; Tauste, C.; Diaz-Feijoo, B.; Gil-Moreno, A.; Oliver, R.; Coronado, P.; Salamanca, M.B.M.; Pantoja-Garrido, M.; Marcos-Sanmartin, J.; et al. Impact of uterine manipulator on oncological outcome in endometrial cancer surgery. Am. J. Obstet. Gynecol. 2021, 224, 65.e1–65.e11. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhao, J.M.; Luo, Z.C.; Zhang, P.; Chen, P.; Zhang, X.L.; Luo, S.; Yang, D.B.; Tan, J.; Zhou, Y.; et al. Diluted povidone-iodine inhibits tumor growth through apoptosis-induction and suppression of SOD activity. Oncol. Rep. 2012, 27, 383. [Google Scholar]
- Hah, J.H.; Roh, D.H.; Jung, Y.H.; Kim, K.H.; Sung, M.W. Selection of irrigation fluid to eradicate free cancer cells during head and neck cancer surgery. Head Neck 2012, 34, 546–550. [Google Scholar] [CrossRef]
- Bouwman, F.; Smits, A.; Lopes, A.; Das, N.; Pollard, A.; Massuger, L.; Bekkers, R.; Galaal, K. The impact of BMI on surgical complications and outcomes in endometrial cancer surgery—An institutional study and systematic review of the literature. Gynecol. Oncol. 2015, 139, 369–376. [Google Scholar] [CrossRef]
- Suidan, R.S.; He, W.; Sun, C.C.; Zhao, H.; Fleming, N.D.; Ramirez, P.T.; Soliman, P.T.; Westin, S.N.; Lu, K.H.; Giordano, S.H.; et al. Impact of body mass index and operative approach on surgical morbidity and costs in women with endometrial carcinoma and hyperplasia. Gynecol. Oncol. 2017, 145, 55–60. [Google Scholar] [CrossRef]
- Acharya, S.; Esthappan, J.; Badiyan, S.; DeWees, T.A.; Tanderup, K.; Schwarz, J.K.; Grigsby, P.W. Medically inoperable endometrial cancer in patients with a high body mass index (BMI): Patterns of failure after 3-D image-based high dose rate (HDR) brachytherapy. Radiother. Oncol. 2016, 118, 167–172. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Ko, E.M.; Walter, P.; Clark, L.; Jackson, A.; Franasiak, J.; Bolac, C.; Havrilesky, L.; Alvarez Secord, A.; Moore, D.T.; Gehrig, P.A.; et al. The complex triad of obesity, diabetes and race in Type I and II endometrial cancers: Prevalence and prognostic significance. Gynecol. Oncol. 2014, 133, 28–32. [Google Scholar] [CrossRef]
- Papatla, K.; Huang, M.; Slomovitz, B. The obese endometrial cancer patient: How do we effectively improve morbidity and mortality in this patient population? Ann. Oncol. 2016, 27, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- Canlorbe, G.; Bendifallah, S.; Raimond, E.; Graesslin, O.; Hudry, D.; Coutant, C.; Touboul, C.; Bleu, G.; Collinet, P.; Darai, E.; et al. Severe Obesity Impacts Recurrence-Free Survival of Women with High-Risk Endometrial Cancer: Results of a French Multicenter Study. Ann. Surg. Oncol. 2015, 22, 2714–2721. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, H.; Watari, H.; Todo, Y.; Kato, T.; Konno, Y.; Hosaka, M.; Sakuragi, N. Lymphadenectomy can be omitted for low-risk endometrial cancer based on preoperative assessments. J. Gynecol. Oncol. 2014, 25, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Kato, H.; Katayama, K.; Nakanishi, K.; Kawano, A.; Iura, A.; Konnai, K.; Onose, R.; Hirahara, F.; Miyagi, E. A preoperative risk-scoring system to predict lymph node metastasis in endometrial cancer and stratify patients for lymphadenectomy. Gynecol. Oncol. 2016, 142, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Visser, N.C.M.; Reijnen, C.; Massuger, L.; Nagtegaal, I.D.; Bulten, J.; Pijnenborg, J.M.A. Accuracy of Endometrial Sampling in Endometrial Carcinoma: A Systematic Review and Meta-analysis. Obstet. Gynecol. 2017, 130, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.S.; Gebb, J.S.; Einstein, M.H.; Shahabi, S.; Novetsky, A.P.; Goldberg, G.L. Accuracy of preoperative endometrial sampling for the detection of high-grade endometrial tumors. Am. J. Obstet. Gynecol. 2007, 196, 243.e1–243.e5. [Google Scholar] [CrossRef]
- Iwai, K.; Fukuda, K.; Hachisuga, T.; Mori, M.; Uchiyama, M.; Iwasaka, T.; Sugimori, H. Prognostic significance of progesterone receptor immunohistochemistry for lymph node metastases in endometrial carcinoma. Gynecol. Oncol. 1999, 72, 351–359. [Google Scholar] [CrossRef]
- Trovik, J.; Wik, E.; Werner, H.M.J.; Krakstad, C.; Helland, H.; Vandenput, I.; Njolstad, T.S.; Stefansson, I.M.; Marcickiewicz, J.; Tingulstad, S.; et al. Hormone receptor loss in endometrial carcinoma curettage predicts lymph node metastasis and poor outcome in prospective multicentre trial. Eur. J. Cancer 2013, 49, 3431–3441. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar]
- Jamieson, A.; Huvila, J.; Thompson, E.F.; Leung, S.; Chiuc, D.; Lumc, A.; McConechy, M.; Grondin, K.; Aguirre-Hernandez, R.; Salvador, S.; et al. Variation in practice in endometrial cancer and potential for improved care and equity through molecular classification. Gynecol. Oncol. 2022, 165, 201–214. [Google Scholar] [CrossRef]
- Kolehmainen, A.M.; Pasanen, A.M.; Koivisto-Korander, R.L.; Bützow, R.C.; Loukovaara, M.J. Molecular characterization in the prediction of disease extent in endometrial carcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.; Thompson, E.F.; Huvila, J.; Leung, S.; Lumb, A.; Morin, C.; Ennour-Idrissi, K.; Sebastianelli, A.; Renaud, M.C.; Gregoire, J.; et al. Endometrial carcinoma molecular subtype correlates with the presence of lymph node metastases. Gynecol. Oncol. 2022, 165, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, C.M.; Tritchler, D.L.; Cohn, D.E.; Mutch, D.G.; Rush, C.M.; Lankes, H.A.; Creasman, W.T.; Miller, D.S.; Ramirez, N.C.; Geller, M.A.; et al. An NRG Oncology/GOG study of molecular classification for risk prediction in endometrioid endometrial cancer. Gynecol. Oncol. 2018, 148, 174–180. [Google Scholar] [CrossRef]
- Leon-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: Impact on prognosis and benefit from adjuvant therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef]
- Leon-Castillo, A.; Horeweg, N.; Peters, E.E.M.; Rutten, T.; ter Haar, N.; Smit, V.T.H.B.M.; Kroon, C.D.; Boennelycke, M.; Hogdall, E.; Hogdall, C.; et al. Prognostic relevance of the molecular classification in high-grade endometrial cancer for patients staged by lymphadenectomy and without adjuvant treatment. Gynecol. Oncol. 2022, 164, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Bosse, T.; Nout, R.A.; McAlpine, J.N.; McConechy, M.K.; Britton, H.; Hussein, Y.R.; Gonzalez, C.; Ganesan, R.; Steele, J.C.; Harrison, B.T.; et al. Molecular classification of grade 3 endometrioid endometrial cancers identifies distinct prognostic subgroups. Am. J. Surg. Pathol. 2018, 42, 561–568. [Google Scholar] [CrossRef]
- Schultheis, A.M.; Martelotto, A.M.; De Filippo, M.R.; Piscuglio, S.; Ng, C.K.Y.; Hussein, Y.R.; Reis-Filho, J.S.; Soslow, R.A.; Weigelt, B. TP53 Mutational Spectrum in Endometrioid and Serous Endometrial Cancers. Int. J. Gynecol. Pathol. 2016, 35, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Leon-Castillo, A.; Gilvazquez, E.; Nout, R.; Smit, V.T.H.B.M.; McAlpine, J.N.; McConechy, M.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; et al. Clinicopathological and molecular characterisation of ‘multiple-classifier’ endometrial carcinomas. J. Pathol. 2020, 250, 312–322. [Google Scholar] [CrossRef]
- Guntupalli, S.R.; Zighelboim, I.; Kizer, N.T.; Zhang, Q.; Powell, M.A.; Thaker, P.H.; Goodfellow, P.J.; Mutch, D.G. Lymphovascular space invasion is an independent risk factor for nodal disease and poor outcomes in endometrioid endometrial cancer. Gynecol. Oncol. 2012, 124, 31–35. [Google Scholar] [CrossRef]
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Jürgenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of PORTEC cohorts. Clin. Cancer. Res. 2006, 22, 4215–4224. [Google Scholar] [CrossRef]
- Billingsley, C.C.; Cohn, D.E.; Mutch, D.G.; Hade, E.M.; Goodfellow, P.J. Prognostic Significance of POLE Exonuclease Domain Mutations in High-Grade Endometrioid Endometrial Cancer on Survival and Recurrence. A Subanalysis. Int. J. Gynecol. Cancer 2016, 26, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, J.; Zhao, L.; Wang, Z.; Wang, J. Tumor Molecular Features Predict Endometrial Cancer Patients’ Survival after Open or Minimally Invasive Surgeries. Front. Oncol. 2021, 11, 634857. [Google Scholar] [CrossRef] [PubMed]
- Konno, Y.; Todo, Y.; Minobe, S.; Kato, H.; Okamoto, K.; Sudo, S.; Takeda, M.; Watar, I.H.; Kaneuchi, M.; Sakuragi, N. A Retrospective Analysis of Postoperative Complications with or Without Para-aortic Lymphadenectomy in Endometrial Cancer. Int. J. Gynecol. Cancer 2011, 21, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, F.; Uccella, S.; Cromi, A.; Bogani, G.; Robba, C.; Serati, M.; Bolis, P. Lymphoceles, lymphorrhea, and lymphedema after laparoscopic and open endometrial cancer staging. Ann. Surg. Oncol. 2012, 19, 259–267. [Google Scholar] [CrossRef]
- Menderes, G.; Azodi, M.; Schwartz, P.; Silasi, D.A. Comparison of Lymphedema Incidence Between 2 Lymphadenectomy Techniques in Patients With Uterine Cancer Undergoing Robotic Staging. Int. J. Gynecol. Cancer 2015, 25, 160–165. [Google Scholar] [CrossRef]
- Wedin, M.; Stalberg, K.; Marcickiewicz, J.; Ahlner, E.; Akesson, A.; Lindahl, G.; Kjolhede, P.; on behalf of the LASEC study group. Incidence of lymphedema in the lower limbs and lymphocyst formation within one year of surgery for endometrial cancer: A prospective longitudinal multicenter study. Gynecol. Oncol. 2020, 159, 201–208. [Google Scholar] [CrossRef]
- Tada, H.; Teramukai, S.; Fukushima, M.; Sasaki, H. Risk factors for lower limb lymphedema after lymph node dissection in patients with ovarian and uterine carcinoma. BMC Cancer 2009, 9, 47. [Google Scholar] [CrossRef]
- Mitra, D.; Catalano, P.J.; Cimbak, N.; Damato, A.L.; Muto, M.G.; Viswanathan, A.N. The risk of lymphedema after postoperative radiation therapy in endometrial cancer. J. Gynecol. Oncol. 2016, 27, e4. [Google Scholar] [CrossRef]
- Kumar, S.; Shah, J.P.; Bryant, C.S.; Awonuga, A.O.; Imudia, A.N.; Ruterbusch, J.J.; Cote, M.L.; Ali-fehmi, R.; Morris, R.T.; Malone, J.M. Second neoplasms in survivors of endometrial cancer: Impact of radiation therapy. Gynecol. Oncol. 2009, 113, 233–239. [Google Scholar] [CrossRef]
- Todo, Y.; Yamazaki, H.; Takeshita, S.; Ohba, Y.; Sudo, S.; Minobe, S.; Okamoto, K.; Kato, H. Close relationship between removal of circumflex iliac nodes to distal external iliac nodes and postoperative lower-extremity lymphedema in uterine corpus malignant tumors. Gynecol. Oncol. 2015, 139, 160–164. [Google Scholar] [CrossRef]
- Milam, M.R.; Java, J.; Walker, J.L.; Metzinger, D.S.; Parker, L.P.; Coleman, R.L. Nodal metastasis risk in endometrioid endometrial cancer. Obstet. Gynecol. 2012, 119 2 Pt 1, 286–292. [Google Scholar] [CrossRef]
- AlHilli, M.M.; Podratz, K.C.; Dowdy, S.C.; Bakkum-Gamez, J.N.; Weaver, A.L.; McGree, M.E.; Kumar, S.; Keeney, G.L.; Cliby, W.A.; Mariani, A. Preoperative biopsy and intraoperative tumor diameter predict lymph node dissemination in endometrial cancer. Gynecol. Oncol. 2013, 128, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Jorge, S.; Hou, J.Y.; Tergas, A.I.; Burke, W.M.; Huang, Y.; Hu, J.C.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L.; Wright, J.D. Magnitude of risk for nodal metastasis associated with lymphvascular space invasion for endometrial cancer. Gynecol. Oncol. 2016, 140, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Stalberg, K.; Bjurberg, M.; Borgfeldt, C.; Carlson, J.; Dahm-Kähler, P.; Flöter-Rådestad, A.; Hellman, K.; Hjerpe, E.; Holmberg, E.; Kjølhede, P.; et al. Lymphovascular space invasion as a predictive factor for lymph node metastases and survival in endometrioid endometrial cancer—A Swedish Gynecologic Cancer Group (SweGCG) study. Acta Oncol. 2019, 58, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Matanes, E.; Eisenberg, N.; Lau, S.; Salvador, S.; Ferenczy, A.; Pelmus, M.; Gotlieb, W.H.; Kogan, L. Absence of prognostic value of lymphovascular space invasion in patients with endometrial cancer and negative sentinel lymph nodes. Gynecol. Oncol. 2021, 162, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Berman, M.L. Adjuvant radiotherapy following properly staged endometrial cancer: What role? Gynecol. Oncol. 2004, 92, 737–739. [Google Scholar] [CrossRef] [PubMed]
| Authors (Year) | Risk Group | Criteria | |
|---|---|---|---|
| JSGO | Ebina (2016) [10] | Low | Grade 1 or 2, Stage IA |
| Intermediate | Grade 1 or 2, Stage IB | ||
| Grade 3, Stage IA | |||
| Lymphovascular space invasion (LVSI) | |||
| High | Grade 3, Stage IB | ||
| Cervical stromal invasion | |||
| ESMO | Colombo (2013) [11] | Low | Grade 1 or 2, Stage IA |
| Intermediate | Grade 3, Stage IA | ||
| Grade 1 or 2, Stage IB | |||
| High | Grade 3, Stage IB | ||
| PORTEC-1 | Creutzberg (2000) [5] | Low | Grade 1, Stage IA |
| Intermediate | Grade 1, Stage IB | ||
| Grade 2, Stage IA and IB | |||
| Grade 3, Stage IA | |||
| High-intermediate | Age > 60 years + Grade 1 or 2 + Stage IB | ||
| Age >60 years + Grade 3 + Stage IA | |||
| GOG-99 | Keys (2004) [6] | Low | Grade 1 or 2, Stage IA |
| Low-intermediate | Age ≤ 50 years + ≤ 2 pathological risk factors | ||
| Age 50–69 years + ≤ 1 pathological risk factor | |||
| Age ≥ 70 years + no pathological risk factor | |||
| Risk factors (1) grade 2 or 3 histology, | |||
| (2) positive LVSI | |||
| (3) myometrial invasion to outer third | |||
| High-intermediate | Any age + 3 pathological risk factors | ||
| Age 50–69 years + ≥ 2 pathological risk factor | |||
| Age ≥ 70 years + ≥ 1 pathological risk factor | |||
| ESGO/ ESTRO/ESP | Coucin (2021) [12] | Low | Grade 1 or 2, Stage IA, LVSI negative or focal |
| Intermediate | Grade 1 or 2, Stage IB, LVSI negative or focal | ||
| Grade 3, Stage IA, LVSI negative or focal | |||
| High-intermediate | Stage I, substantial LVSI | ||
| Grade 3, Stage IB, regardless of LVSI status | |||
| Stage II |
| Author (Year) | No. of Patients | Stage, Grade; Histology | Lymphadenectomy (LA) | No. of Nodes Removed | 5-Year Survival Rate | Median Follow-Up |
|---|---|---|---|---|---|---|
| Chen (1989) [113] | 18 | IAG3, IB | Selective biopsy of pelvic and para-aortic lymph nodes | Not available | 100% (DFS) | 5–13 years |
| Ayhan (2002) [114] | 25 | IAG3, IB; endometrioid | Pelvic and para-aortic LA | 27, median | 92% (OS) | 96 months |
| Straughn (2003) [115] | 121 | IB; serous and clear cell were excluded | Pelvic and para-aortic LA | 20, mean | 92% (OS) | 41 months |
| Otsuka (2022) [26] | 77 | IAG3, IB, II; endometrioid | Pelvic LA in all patients and para-aortic LA in selected patients | 19 (pelvic) 8 (para-aortic), median | 97% (DSS) | 75 months |
| Author (Year) | No. of Patients | Lymphadenectomy | Number of Nodes Removed | Age | Tumor Type |
|---|---|---|---|---|---|
| Positive effects of PLA/PALA on survival | |||||
| Huang (2013) [99] | 961 | PLA, PALA | 18 (pelvic) 5 (para-aortic) | 53 y, median | Endometrioid |
| Todo (2010) [98] | 671 | PLA, PALA | 59 (pelvic) 23 (para-aortic) | 56 y, median | Other than low-risk (pT1A, G1-2) |
| Abu-Rustum (2008) [106] | 1035 | PLA, PALS (up to IMA) | 16 | 61 y, median | Endometrioid |
| Mariani (2000) [95] | 137 | PLA, PALA | 16 (pelvic) 6 (para-aortic) | 67 y, mean | Patients at high risk for para-aortic lymph node involvement * |
| Eggemann (2016) [100] | 1502 | PLA, PALA | 19 | 66 y (PLA and PALA) 68 y (PLA) 72 y (no LA), mean | Other than low-risk (pT1A, G1-2) |
| No effects of PLA/PALA on survival | |||||
| Papathemelis (2018) [129] | 299 | PLA, (PALA) | 26 | 69 y, median | pT1B G1-2, type I |
| Ignatov (2020) [128] | 2392 | PLA, PALA | 29 | 69 y (LA) 74 y (no LA), median | Endometrioid, intermediate-risk (pT1A G3, pT1B G1-2) high-risk (pT1B G3, pT2 Gany) |
| Author (Year) | No. of Patients | Lymphadenectomy (LA) | No. of Nodes Removed | Incidence of Lower Extremity Lymphedema | Incidence of Lymphocele |
|---|---|---|---|---|---|
| Menderes (2015) [185] | 238 | Robotically assisted PLA | 16 (mean) | 4.6 % | NA |
| Ghezzi (2012) [184] | 138 | Open PLA | 18 | 14.6% | 15.4% |
| Laparoscopic PLA | 13% | 1.4% | |||
| Konno (2011) [183] | 142 | Open PLA | 36 (median) | 28.3% | 9.4% |
| 138 | Open PLA + PALA | 87 (median) | 23.2% | 9.2% | |
| Wedin (2020) [186] | 116 | PLA or PLA + PALA | 25.4 (PLA), 38.4 (PLA + PALA) | 15.8% | 4.3% |
| 119 | no LA | 3.4% | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otsuka, I. Therapeutic Benefit of Systematic Lymphadenectomy in Node-Negative Uterine-Confined Endometrioid Endometrial Carcinoma: Omission of Adjuvant Therapy. Cancers 2022, 14, 4516. https://doi.org/10.3390/cancers14184516
Otsuka I. Therapeutic Benefit of Systematic Lymphadenectomy in Node-Negative Uterine-Confined Endometrioid Endometrial Carcinoma: Omission of Adjuvant Therapy. Cancers. 2022; 14(18):4516. https://doi.org/10.3390/cancers14184516
Chicago/Turabian StyleOtsuka, Isao. 2022. "Therapeutic Benefit of Systematic Lymphadenectomy in Node-Negative Uterine-Confined Endometrioid Endometrial Carcinoma: Omission of Adjuvant Therapy" Cancers 14, no. 18: 4516. https://doi.org/10.3390/cancers14184516
APA StyleOtsuka, I. (2022). Therapeutic Benefit of Systematic Lymphadenectomy in Node-Negative Uterine-Confined Endometrioid Endometrial Carcinoma: Omission of Adjuvant Therapy. Cancers, 14(18), 4516. https://doi.org/10.3390/cancers14184516






