Adjuvant Radiotherapy in Surgically Treated HPV-Positive Oropharyngeal Carcinoma with Adverse Pathological Features
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Variable Definitions
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
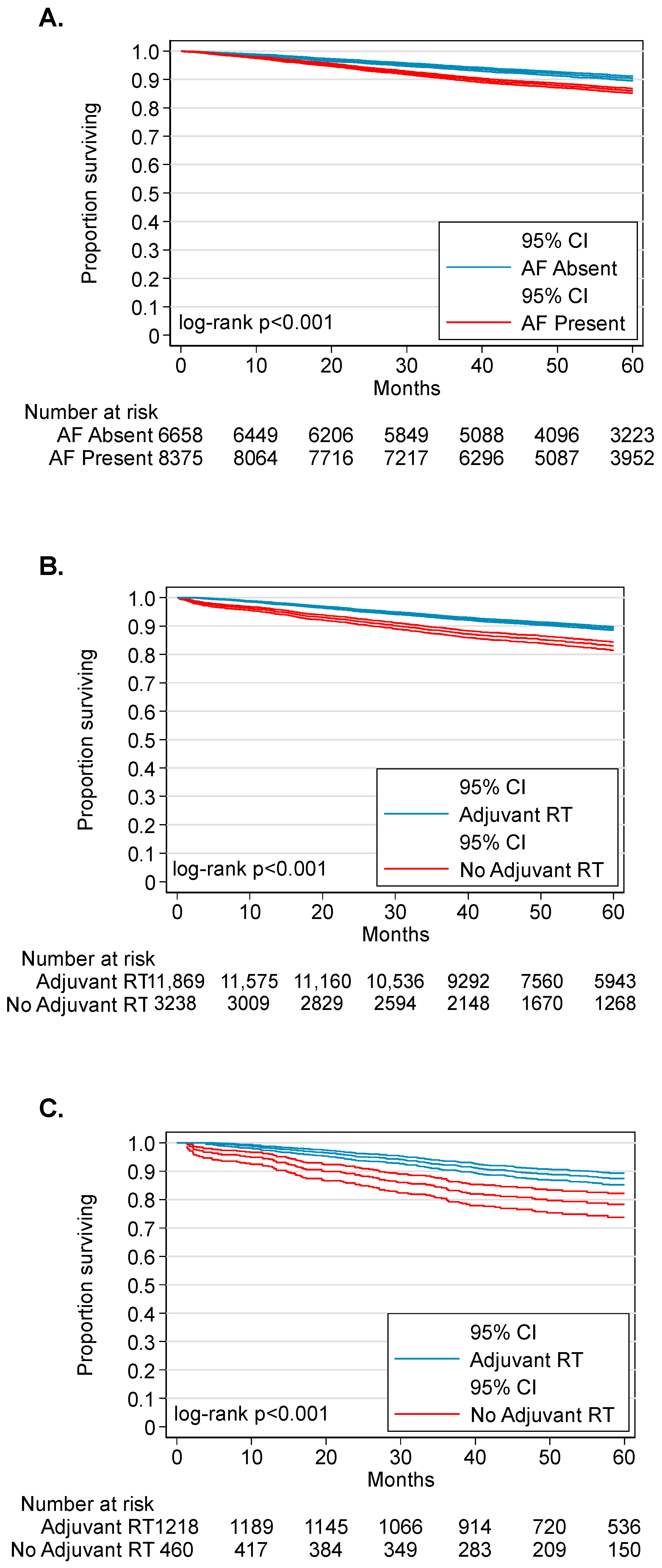
3.2. The Presence of Any Individual Adverse Feature Is Independently Associated with Survival in HPV-OPC
3.3. Adjuvant Radiotherapy Is Associated with Improved Survival in Patients with AF-HPV-OPC
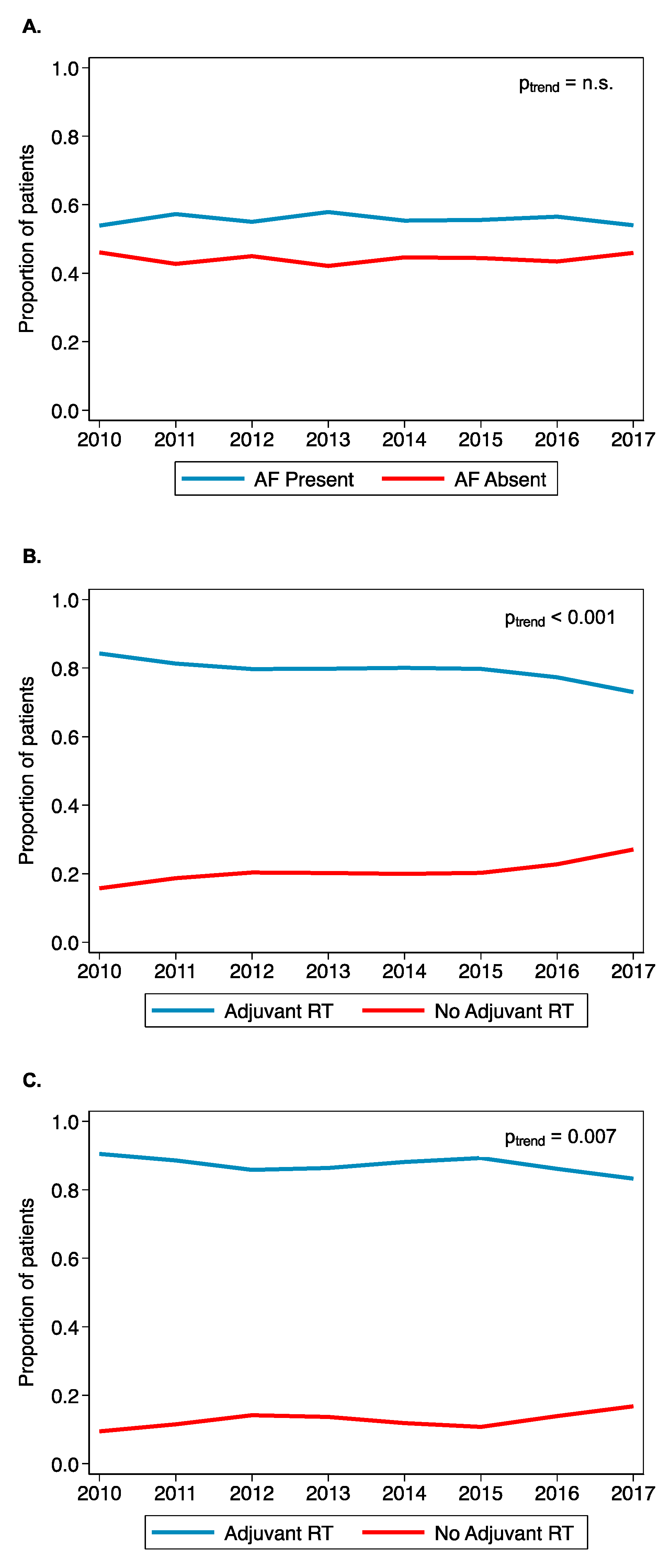
3.4. Trends of AF-HPV-OPC Treated with Adjuvant Radiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of human papillomavirus–positive head and neck squamous cell carcinoma. J. Clin. Oncol. 2015, 33, 3235. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef] [PubMed]
- Faraji, F.; Rettig, E.M.; Tsai, H.L.; El Asmar, M.; Fung, N.; Eisele, D.W.; Fakhry, C. The prevalence of human papillomavirus in oropharyngeal cancer is increasing regardless of sex or race, and the influence of sex and race on survival is modified by human papillomavirus tumor status. Cancer 2019, 125, 761–769. [Google Scholar] [CrossRef]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Cracchiolo, J.R.; Baxi, S.S.; Morris, L.G.; Ganly, I.; Patel, S.G.; Cohen, M.A.; Roman, B.R. Increase in primary surgical treatment of T1 and T2 oropharyngeal squamous cell carcinoma and rates of adverse pathologic features: National Cancer Data Base. Cancer 2016, 122, 1523–1532. [Google Scholar] [CrossRef]
- Moore, E.J.; Olsen, S.M.; Laborde, R.R.; García, J.J.; Walsh, F.J.; Price, D.L.; Janus, J.R.; Kasperbauer, J.L.; Olsen, K.D. Long-Term Functional and Oncologic Results of Transoral Robotic Surgery for Oropharyngeal Squamous Cell Carcinoma. Mayo Clin. Proc. 2012, 87, 219–225. [Google Scholar] [CrossRef]
- Ferris, R.L.; Flamand, Y.; Weinstein, G.S.; Li, S.; Quon, H.; Mehra, R.; Garcia, J.J.; Chung, C.H.; Gillison, M.L.; Duvvuri, U.; et al. Phase II Randomized Trial of Transoral Surgery and Low-Dose Intensity Modulated Radiation Therapy in Resectable p16+ Locally Advanced Oropharynx Cancer: An ECOG-ACRIN Cancer Research Group Trial (E3311). J. Clin. Oncol. 2022, 40, 138–149. [Google Scholar] [CrossRef]
- Swisher-McClure, S.; Lukens, J.N.; Aggarwal, C.; Ahn, P.; Basu, D.; Bauml, J.M.; Brody, R.; Chalian, A.; Cohen, R.B.; Fotouhi-Ghiam, A. A phase 2 trial of alternative volumes of oropharyngeal irradiation for de-intensification (AVOID): Omission of the resected primary tumor bed after transoral robotic surgery for human papilloma virus–related squamous cell carcinoma of the oropharynx. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Owadally, W.; Hurt, C.; Timmins, H.; Parsons, E.; Townsend, S.; Patterson, J.; Hutcheson, K.; Powell, N.; Beasley, M.; Palaniappan, N.; et al. PATHOS: A phase II/III trial of risk-stratified, reduced intensity adjuvant treatment in patients undergoing transoral surgery for Human papillomavirus (HPV) positive oropharyngeal cancer. BMC Cancer 2015, 15, 602. [Google Scholar] [CrossRef]
- Bryne, M.; Koppang, H.S.; Lilleng, R.; Kjærheim, Å. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J. Pathol. 1992, 166, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Albergotti, W.G.; Schwarzbach, H.L.; Abberbock, S.; Ferris, R.L.; Johnson, J.T.; Duvvuri, U.; Kim, S. Defining the Prevalence and Prognostic Value of Perineural Invasion and Angiolymphatic Invasion in Human Papillomavirus–Positive Oropharyngeal Carcinoma. JAMA Otolaryngol.–Head Neck Surg. 2017, 143, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Bauer, E.; Mazul, A.; Chernock, R.; Rich, J.; Jackson, R.S.; Paniello, R.; Pipkorn, P.; Oppelt, P.; Gay, H.; Daly, M. Extranodal extension is a strong prognosticator in HPV-positive oropharyngeal squamous cell carcinoma. Laryngoscope 2020, 130, 939–945. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Park, H.S.; Kelly, J.R.; Stahl, J.M.; Yarbrough, W.G.; Burtness, B.A.; Contessa, J.N.; Decker, R.H.; Koshy, M.; Husain, Z.A. The prognostic value of extranodal extension in human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer 2017, 123, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Dundar, Y.; Hotaling, J.; Raza, S.N.; Lin, H.S. Development and Assessment of a Novel Composite Pathologic Risk Stratification for Surgically Resected Human Papillomavirus–Associated Oropharyngeal Cancer. JAMA Otolaryngol.–Head Neck Surg. 2019, 145, 1105–1114. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Guidelines. Head and Neck Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 3 March 2022).
- Wahle, B.; Zevallos, J. Transoral Robotic Surgery and De-escalation of Cancer Treatment. Otolaryngol. Clin. N. Am. 2020, 53, 981–994. [Google Scholar] [CrossRef]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef]
- Nichols, D.S.; Zhao, J.; Boyce, B.J.; Amdur, R.; Mendenhall, W.M.; Danan, D.; Hitchcock, K.; Ning, K.; Keyes, K.; Lee, J.H.; et al. HPV/p16-positive oropharyngeal cancer treated with transoral robotic surgery: The roles of margins, extra-nodal extension and adjuvant treatment. Am. J. Otolaryngol. 2021, 42, 102793. [Google Scholar] [CrossRef]
- Han, M.; Stanford-Moore, G.B.; Larson, A.R.; Schoppy, D.W.; Cognetti, D.M.; Joshi, A.S.; Houlton, J.J.; Ryan, W.R. Predictors of Mortality in HPV-Associated Oropharynx Carcinoma Treated With Surgery Alone. Laryngoscope 2020, 130, E423–E435. [Google Scholar] [CrossRef]
- Molony, P.; Kharytaniuk, N.; Boyle, S.; Woods, R.S.R.; O’Leary, G.; Werner, R.; Heffron, C.; Feeley, L.; Sheahan, P. Impact of positive margins on outcomes of oropharyngeal squamous cell carcinoma according to p16 status. Head Neck 2017, 39, 1680–1688. [Google Scholar] [CrossRef]
- Kaczmar, J.M.; Tan, K.S.; Heitjan, D.F.; Lin, A.; Ahn, P.H.; Newman, J.G.; Rassekh, C.H.; Chailan, A.A.; O’Malley Jr, B.W.; Cohen, R.B.; et al. HPV-related oropharyngeal cancer: Risk factors for treatment failure in patients managed with primary transoral robotic surgery. Head Neck 2016, 38, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Anderson, W.F.; Gillison, M.L. Incidence trends for human papillomavirus-related and-unrelated oral squamous cell carcinomas in the United States. J. Clin. Oncol. 2008, 26, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Rettig, E.M.; Zaidi, M.; Faraji, F.; Eisele, D.W.; El Asmar, M.; Fung, N. D’Souza, G.; Fakhry, C. Oropharyngeal cancer is no longer a disease of younger patients and the prognostic advantage of Human Papillomavirus is attenuated among older patients: Analysis of the National Cancer Database. Oral Oncol. 2018, 83, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Malm, I.J.; Fan, C.J.; Yin, L.X.; Li, D.X.; Koch, W.M.; Gourin, C.G.; Pitman, K.T.; Richmon, J.D.; Westra, W.H.; Kang, H.; et al. Evaluation of proposed staging systems for human papillomavirus-related oropharyngeal squamous cell carcinoma. Cancer 2017, 123, 1768–1777. [Google Scholar] [CrossRef]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef]
- Austin, P.C. A comparison of 12 algorithms for matching on the propensity score. Stat. Med. 2014, 33, 1057–1069. [Google Scholar] [CrossRef]
- Fakhry, C.; Gillison, M.L. Clinical implications of human papillomavirus in head and neck cancers. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 2606. [Google Scholar] [CrossRef]
- Bernier, J.; Cooper, J.S.; Pajak, T.F.; van Glabbeke, M.; Bourhis, J.; Forastiere, A.; Ozsahin, E.M.; Jacobs, J.R.; Jassem, J.; Ang, K.K.; et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005, 27, 843–850. [Google Scholar]
- Cooper, J.S.; Zhang, Q.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Long-term follow-up of the RTOG 9501/intergroup phase III trial: Postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 1198–1205. [Google Scholar] [CrossRef]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefèbvre, J.L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef]
- Iyer, N.G.; Dogan, S.; Palmer, F.; Rahmati, R.; Nixon, I.J.; Lee, N.; Patel, S.G.; Shah, J.P.; Ganly, I. Detailed analysis of clinicopathologic factors demonstrate distinct difference in outcome and prognostic factors between surgically treated HPV-positive and negative oropharyngeal cancer. Ann. Surg. Oncol. 2015, 22, 4411–4421. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Kallogjeri, D.; Gay, H.; Thorstad, W.L.; Lewis, J.S.; Chernock, R.; Nussenbaum, B.; Haughey, B.H. High metastatic node number, not extracapsular spread or N-classification is a node-related prognosticator in transorally-resected, neck-dissected p16-positive oropharynx cancer. Oral Oncol. 2015, 51, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Kang, M.S.; Koh, Y.W.; Choi, E.C.; Kim, S.H. Does p16+ Predict a Favorable Prognosis for Oropharyngeal Cancer? Risk Factors for Treatment Failure for Patients Who Underwent Surgery-Based Therapy. Ann. Surg. Oncol. 2019, 26, 547–554. [Google Scholar] [CrossRef]
- Yom, S.S.; Torres-Saavedra, P.; Caudell, J.J.; Waldron, J.N.; Gillison, M.L.; Xia, P.; Truong, M.T.; Kong, C.; Jordon, R.; Subramaniam, R.M.; et al. Reduced-Dose Radiation Therapy for HPV-Associated Oropharyngeal Carcinoma (NRG Oncology HN002). J. Clin. Oncol. 2021, 39, 956–965. [Google Scholar] [CrossRef]
- Ma, D.J.; Price, K.A.; Moore, E.J.; Patel, S.H.; Hinni, M.L.; Garcia, J.J.; Graner, D.E.; Foster, N.R.; Ginos, B.; Neben-Wittich, M.; et al. Phase II Evaluation of Aggressive Dose De-Escalation for Adjuvant Chemoradiotherapy in Human Papillomavirus-Associated Oropharynx Squamous Cell Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 1909–1918. [Google Scholar] [CrossRef]
- Carey, R.M.; Shimunov, D.; Weinstein, G.S.; Cannady, S.B.; Lukens, J.N.; Lin, A.; Swisher-McClure, S.; Bauml, J.M.; Aggarwal, C.; Cohen, R.B.; et al. Increased rate of recurrence and high rate of salvage in patients with human papillomavirus–associated oropharyngeal squamous cell carcinoma with adverse features treated with primary surgery without recommended adjuvant therapy. Head Neck 2021, 43, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Graboyes, E.M.; Halbert, C.H.; Li, H.; Warren, G.W.; Alberg, A.J.; Calhoun, E.A.; Nussenbaum, B.; Marsh, C.H.; McCay, J.; Day, T.; et al. Barriers to the Delivery of Timely, Guideline-Adherent Adjuvant Therapy Among Patients With Head and Neck Cancer. JCO Oncol. Pract. 2020, 16, e1417–e1432. [Google Scholar] [CrossRef]
- Graboyes, E.M.; Garrett-Mayer, E.; Sharma, A.K.; Lentsch, E.J.; Day, T.A. Adherence to National Comprehensive Cancer Network guidelines for time to initiation of postoperative radiation therapy for patients with head and neck cancer. Cancer 2017, 123, 2651–2660. [Google Scholar] [CrossRef]
- Williamson, C.W.; Nelson, T.J.; Thompson, C.A.; Vitzthum, L.K.; Zakeri, K.; Riviere, P.J.; Bryant, A.K.; Sharabi, A.B.; Zou, J.; Mell, L.K. Bias Reduction through Analysis of Competing Events (BRACE) Correction to Address Cancer Treatment Selection Bias in Observational Data. Clin. Cancer Res. 2022, 28, 1832–1840. [Google Scholar] [CrossRef]
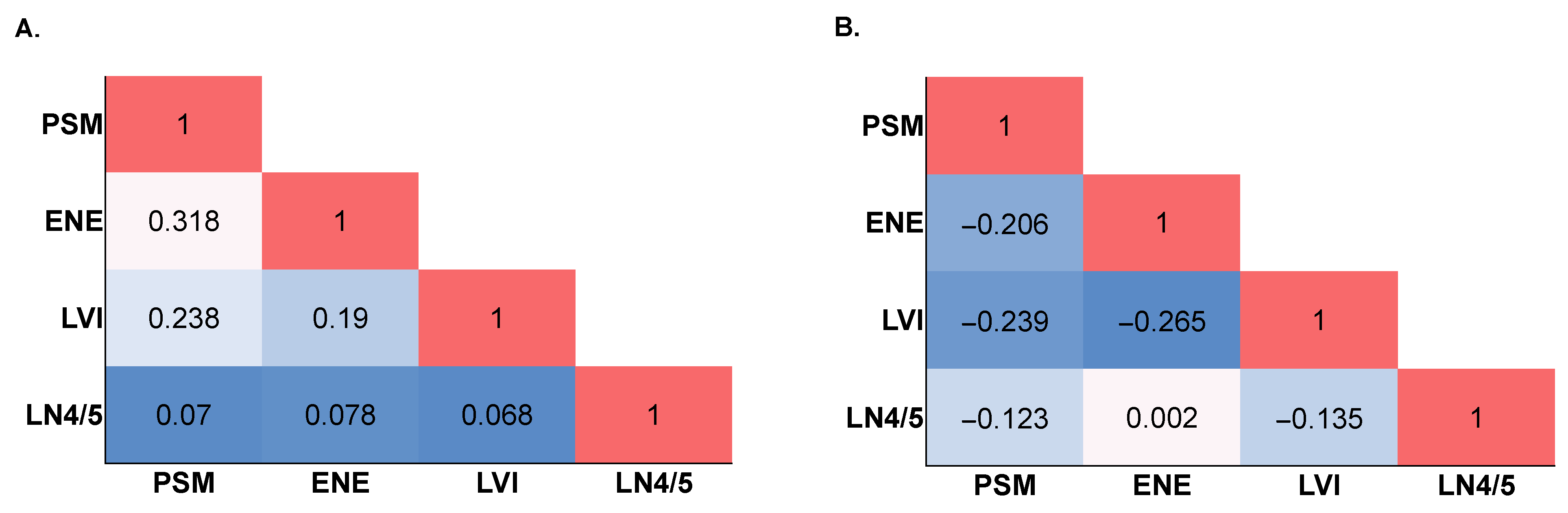
| Variable | Overall n = 15,036 | AF Negative n = 6661 | AF Positive n = 8375 | p-Value | |
|---|---|---|---|---|---|
| Age (mean, SD) | 58.3(9.4) | 58.3 (9.3) | 58.3 (9.4) | 0.858 | |
| Sex | Male | 12,564 | 5484 (82.3) | 7080 (84.5) | <0.001 |
| Race | White | 13,790 | 6067 (91.1) | 7723 (92.2) | 0.036 |
| Black | 525 | 240 (3.6) | 285 (3.4) | ||
| Hispanic | 364 | 186 (2.8) | 178 (2.1) | ||
| Asian/Pacific Islander | 133 | 68 (1.0) | 65 (0.8) | ||
| Primary site | Tonsil | 10,768 | 4689 (70.4) | 6079 (72.6) | 0.002 |
| Base of tongue | 3548 | 1616 (24.3) | 1932 (23.1) | ||
| Other oropharynx | 720 | 356 (5.3) | 364 (4.4) | ||
| Tumor Category | T1 | 8116 | 3797 (57.0) | 4319 (51.6) | <0.001 |
| T2 | 6920 | 2864 (43.0) | 4056 (48.4) | ||
| Nodal Category | N0 | 2577 | 1768 (26.5) | 809 (9.7) | <0.001 |
| N1 | 11,331 | 4576 (68.7) | 6755 (80.7) | ||
| N2 | 599 | 178 (2.7) | 421 (5.0) | ||
| N3 | 437 | 97 (1.5) | 340 (4.1) | ||
| Tumor Margin Status | Negative | 9640 | 5657 (84.9) | 3983 (47.6) | <0.001 |
| Positive | 4033 | 0 (0.0) | 4033 (48.2) | ||
| Unknown | 1363 | 1004 (15.1) | 359 (4.3) | ||
| Lymphovascular Invasion | Negative | 8556 | 4709 (70.7) | 3847 (45.9) | <0.001 |
| Positive | 2809 | 0 (0.0) | 2809 (33.5) | ||
| Unknown | 3671 | 1952 (29.3) | 1719 (20.5) | ||
| Extranodal Extension | Negative | 6543 | 4090 (61.4) | 2453 (29.3) | <0.001 |
| Positive | 3238 | 0 (0.0) | 3238 (38.7) | ||
| Unknown | 5255 | 2571 (38.6) | 2684 (32.1) | ||
| Level 4/5 Lymph Node | Negative | 12,571 | 6347 (95.3) | 6224 (74.3) | <0.001 |
| Positive | 1710 | 0 (0.0) | 1710 (20.4) | ||
| Unknown | 755 | 314 (4.7) | 441 (5.3) | ||
| Adjuvant Radiotherapy | Yes | 11,804 | 4537 (68.1) | 7267 (86.8) | <0.001 |
| Charlson-Deyo Comorbidity Score | 0 | 12,355 | 5519 (82.9) | 6836 (81.6) | 0.107 |
| 1 | 2021 | 874 (13.1) | 1147 (13.7) | ||
| 2 | 432 | 181 (2.7) | 251 (3.0) | ||
| 3 | 228 | 87 (1.3) | 141 (1.7) | ||
| Primary Payor | Not Insured | 323 | 137 (2.1) | 186 (2.2) | 0.280 |
| Private | 9625 | 4295 (64.5) | 5330 (63.6) | ||
| Medicaid/Medicare | 4904 | 2138 (32.1) | 2766 (33.0) | ||
| Median Household Income | >$63,000 | 5340 | 2452 (42.5) | 2888 (40.0) | 0.031 |
| $48,000–62,999 | 3536 | 1550 (26.9) | 1986 (27.5) | ||
| $38,000–47,999 | 2608 | 1115 (19.3) | 1493 (20.7) | ||
| <$38,000 | 1500 | 651 (11.3) | 849 (11.8) |
| Unadjusted Cox Analysis | Adjusted Cox Analyses | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | aHRAF | 95% CI | p-Value | aHRPSM | 95% CI | p-Value | aHRENE | 95% CI | p-Value | aHRLVI | 95% CI | p-Value | aHRLN4/5 | 95% CI | p-Value | |
| Total HPV-OPC Cohort | n = 15,036 | n = 12,780 | n = 12,780 | n = 12,780 | n = 12,780 | n = 12,780 | ||||||||||||
| Any AF | ||||||||||||||||||
| No | 1.00 | -- | -- | 1.00 | -- | -- | ||||||||||||
| Yes | 1.44 | 1.31–1.58 | <0.001 | 1.56 | 1.40–1.73 | <0.001 | ||||||||||||
| PSM | ||||||||||||||||||
| Negative | 1.00 | -- | -- | 1.00 | -- | -- | ||||||||||||
| Positive | 1.49 | 1.35–1.64 | <0.001 | 1.57 | 1.41–1.75 | <0.001 | ||||||||||||
| ENE | ||||||||||||||||||
| Negative | 1.00 | -- | -- | 1.00 | -- | -- | ||||||||||||
| Positive | 1.79 | 1.59–2.01 | <0.001 | 1.74 | 1.52–2.00 | <0.001 | ||||||||||||
| LVI | ||||||||||||||||||
| Negative | 1.00 | -- | -- | 1.00 | -- | -- | ||||||||||||
| Positive | 1.54 | 1.38–1.71 | <0.001 | 1.45 | 1.29–1.64 | <0.001 | ||||||||||||
| LN4/5 | ||||||||||||||||||
| Negative | 1.00 | -- | -- | 1.00 | -- | -- | ||||||||||||
| Positive | 1.63 | 1.44–1.83 | <0.001 | 1.63 | 1.42–1.86 | <0.001 | ||||||||||||
| AF-HPV-OPC Cohort | n = 8375 | n = 7103 | n = 7103 | n = 7103 | n = 7103 | n = 7103 | ||||||||||||
| Adjuvant RT | ||||||||||||||||||
| No | 1.00 | -- | -- | 1.00 | -- | -- | 1.00 | -- | -- | 1.00 | -- | -- | 1.00 | -- | -- | 1.00 | -- | -- |
| Yes | 0.48 | 0.42–0.55 | <0.001 | 0.60 | 0.51–0.69 | <0.001 | 0.56 | 0.49–0.66 | <0.001 | 0.56 | 0.48–0.65 | <0.001 | 0.60 | 0.52–0.70 | <0.001 | 0.60 | 0.52–0.69 | <0.001 |
| Unadjusted (n = 1678) | Adjusted (n = 1678) | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| Age | 1.040 | 1.026–1.053 | <0.001 | 1.014 | 0.999–1.030 | 0.064 | |
| Race | White | 1.000 | -- | -- | |||
| Black | 1.509 | 0.945–2.408 | 0.085 | ||||
| Hispanic | 0.748 | 0.278–2.009 | 0.564 | ||||
| Asian/Pacific Islander | 1.737 | 0.431–6.992 | 0.437 | ||||
| Primary site | Tonsil | 1.000 | -- | -- | |||
| Base of tongue | 1.120 | 0.863–1.454 | 0.395 | ||||
| Other oropharynx | 1.539 | 0.944–2.509 | 0.084 | ||||
| Tumor Category | T1 | 1.000 | -- | -- | 1.000 | -- | -- |
| T2 | 1.607 | 1.255–2.057 | <0.001 | 1.425 | 1.105–1.838 | 0.006 | |
| Nodal Category | N0 | 1.000 | -- | -- | |||
| N1 | 0.890 | 0.611–1.297 | 0.544 | ||||
| N2 | 1.203 | 0.553–2.618 | 0.641 | ||||
| N3 | 0.807 | 0.371–1.755 | 0.588 | ||||
| Tumor Margin Status | Negative | 1.000 | -- | -- | 1.000 | -- | -- |
| Positive | 1.308 | 1.003–1.705 | 0.048 | 1.581 | 1.188–2.103 | 0.002 | |
| Lymphovascular Invasion | Negative | 1.000 | -- | -- | 1.000 | -- | -- |
| Positive | 1.010 | 0.791–1.288 | 0.939 | 1.241 | 0.959–1.608 | 0.101 | |
| Extranodal Extension | Negative | 1.000 | -- | -- | 1.000 | -- | -- |
| Positive | 1.701 | 1.333–2.171 | <0.001 | 1.753 | 1.348–2.279 | <0.001 | |
| Level 4/5 Lymph Node | Negative | 1.000 | -- | -- | 1.000 | -- | -- |
| Positive | 1.184 | 0.895–1.566 | 0.237 | 1.266 | 0.954–1.681 | 0.103 | |
| Adjuvant Radiotherapy | No | 1.000 | -- | -- | 1.000 | -- | -- |
| Yes | 0.548 | 0.425–0.705 | <0.001 | 0.552 | 0.428–0.713 | <0.001 | |
| Charlson-Deyo Comorbidity Score | 0 | 1.000 | -- | -- | 1.000 | -- | -- |
| 1 | 1.701 | 1.270–2.277 | <0.001 | 1.442 | 1.073–1.937 | 0.015 | |
| 2 | 1.645 | 0.841–3.218 | 0.146 | 1.277 | 0.648–2.516 | 0.481 | |
| 3 | 3.972 | 2.544–6.203 | <0.001 | 2.723 | 1.727–4.294 | <0.001 | |
| Primary Payor | Private insurance | 1.000 | -- | -- | 1.000 | -- | -- |
| Medicare/Medicaid/Gov. | 2.716 | 2.065–3.571 | <0.001 | 2.083 | 1.521–2.851 | <0.001 | |
| Not insured | 2.884 | 1.566–5.313 | 0.001 | 2.971 | 1.605–5.502 | 0.001 | |
| Median Household Income | >$63,000 | 1.000 | -- | -- | |||
| $48,000–$62,999 | 1.409 | 1.014–1.957 | 0.041 | ||||
| $38,000–$47,999 | 1.325 | 0.934–1.880 | 0.115 | ||||
| <$38,000 | 1.988 | 1.392–2.838 | <0.001 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, S.I.; Faraji, F.; Pang, J.; Mell, L.K.; Califano, J.A.; Orosco, R.K. Adjuvant Radiotherapy in Surgically Treated HPV-Positive Oropharyngeal Carcinoma with Adverse Pathological Features. Cancers 2022, 14, 4515. https://doi.org/10.3390/cancers14184515
Soliman SI, Faraji F, Pang J, Mell LK, Califano JA, Orosco RK. Adjuvant Radiotherapy in Surgically Treated HPV-Positive Oropharyngeal Carcinoma with Adverse Pathological Features. Cancers. 2022; 14(18):4515. https://doi.org/10.3390/cancers14184515
Chicago/Turabian StyleSoliman, Shady I., Farhoud Faraji, John Pang, Loren K. Mell, Joseph A. Califano, and Ryan K. Orosco. 2022. "Adjuvant Radiotherapy in Surgically Treated HPV-Positive Oropharyngeal Carcinoma with Adverse Pathological Features" Cancers 14, no. 18: 4515. https://doi.org/10.3390/cancers14184515
APA StyleSoliman, S. I., Faraji, F., Pang, J., Mell, L. K., Califano, J. A., & Orosco, R. K. (2022). Adjuvant Radiotherapy in Surgically Treated HPV-Positive Oropharyngeal Carcinoma with Adverse Pathological Features. Cancers, 14(18), 4515. https://doi.org/10.3390/cancers14184515





