Platinum Plus Tegafur–Uracil versus Platinum Alone during Concurrent Chemoradiotherapy in Patients with Nonmetastatic Nasopharyngeal Carcinoma: A Propensity-Score-Matching Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment Methods
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Survival Outcomes
3.3. Safety Profiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lo, K.W.; To, K.F.; Huang, D.P. Focus on nasopharyngeal carcinoma. Cancer Cell 2004, 5, 423–428. [Google Scholar] [CrossRef]
- Chen, Y.P.; Chan, A.T.C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Tsang, C.M.; Lui, V.W.Y.; Bruce, J.P.; Pugh, T.J.; Lo, K.W. Translational genomics of nasopharyngeal cancer. Semin. Cancer Biol. 2019, 61, 84–100. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today (accessed on 11 August 2020).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2020, 68, 394–424. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Nasopharynx. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/4-Nasopharynx-fact-sheet.pdf (accessed on 21 February 2021).
- Beyene, E.T.; Ketema, S.G.; Alebachew, A.N.; Saleh, M.Y.; Gebremariam, T.A. Descriptive epidemiology of nasopharyngeal carcinoma at Tikur Anbessa Hospital, Ethiopia. BMC Cancer 2021, 21, 540. [Google Scholar] [CrossRef]
- Chua, M.L.K.; Wee, J.T.S.; Hui, E.P.; Chan, A.T.C. Nasopharyngeal carcinoma. Lancet 2016, 387, 1012–1024. [Google Scholar] [CrossRef]
- OuYang, P.Y.; Zhang, L.N.; Lan, X.W.; Xie, C.; Zhang, W.W.; Wang, Q.X.; Su, Z.; Tang, J.; Xie, F.Y. The significant survival advantage of female sex in nasopharyngeal carcinoma: A propensity-matched analysis. Br. J. Cancer 2015, 112, 1554–1561. [Google Scholar] [CrossRef]
- Tang, L.Q.; Li, C.F.; Li, J.; Chen, W.H.; Chen, Q.Y.; Yuan, L.X.; Lai, X.P.; He, Y.; Xu, Y.X.; Hu, D.P.; et al. Establishment and Validation of Prognostic Nomograms for Endemic Nasopharyngeal Carcinoma. J. Natl. Cancer Inst. 2015, 108, djv291. [Google Scholar] [CrossRef]
- Wu, S.G.; Lian, C.L.; Wang, J.; Zhang, W.W.; Sun, J.Y.; Lin, Q.; He, Z.Y. The effect of histological subtypes on survival outcome in nasopharyngeal carcinoma after extensive follow up. Ann. Transl. Med. 2019, 7, 768. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Liu, L.Z.; Wei, W.H.; Deng, Y.M.; Li, Y.Z.; Liu, X.W. Radiologic criteria of retropharyngeal lymph node metastasis in nasopharyngeal carcinoma treated with radiation therapy. Radiology 2010, 255, 605–612. [Google Scholar] [CrossRef]
- Chen, L.; Mao, Y.P.; Xie, F.Y.; Liu, L.Z.; Sun, Y.; Tian, L.; Tang, L.L.; Lin, A.H.; Li, L.; Ma, J. The seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma is prognostically useful for patients treated with intensity-modulated radiotherapy from an endemic area in China. Radiother. Oncol. 2012, 104, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Colevas, A.D.; Yom, S.S.; Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.J.; et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 1. J. Natl. Compr. Cancer Netw. 2018, 16, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Xu, C.; Chen, Y.P.; Guo, R.; Mao, Y.P.; Sun, Y.; Ma, J.; Tang, L.L. Optimizing the cumulative cisplatin dose during radiotherapy in nasopharyngeal carcinoma: Dose-effect analysis for a large cohort. Oral Oncol. 2019, 89, 102–106. [Google Scholar] [CrossRef]
- Chan, A.T.; Leung, S.F.; Ngan, R.K.; Teo, P.M.; Lau, W.H.; Kwan, W.H.; Hui, E.P.; Yiu, H.Y.; Yeo, W.; Cheung, F.Y.; et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J. Natl. Cancer Inst. 2005, 97, 536–539. [Google Scholar] [CrossRef]
- Chen, J.H.; Huang, W.Y.; Ho, C.L.; Chao, T.Y.; Lee, J.C. Evaluation of oral tegafur-uracil as metronomic therapy following concurrent chemoradiotherapy in patients with non-distant metastatic TNM stage IV nasopharyngeal carcinoma. Head Neck 2019, 41, 3775–3782. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, X.; Wei, Y.; Deng, H.; Ma, L.; Chen, Z. Activity and Safety of Tegafur, Gimeracil, and Oteracil Potassium for Nasopharyngeal Carcinoma: A Systematic Review and Meta-Analysis. J. Oncol. 2021, 2021, 6690275. [Google Scholar] [CrossRef]
- Blanchard, P.; Lee, A.; Marguet, S.; Leclercq, J.; Ng, W.T.; Ma, J.; Chan, A.T.; Huang, P.Y.; Benhamou, E.; Zhu, G.; et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: An update of the MAC-NPC meta-analysis. Lancet Oncol. 2015, 16, 645–655. [Google Scholar] [CrossRef]
- Ribassin-Majed, L.; Marguet, S.; Lee, A.W.M.; Ng, W.T.; Ma, J.; Chan, A.T.C.; Huang, P.Y.; Zhu, G.; Chua, D.T.T.; Chen, Y.; et al. What Is the Best Treatment of Locally Advanced Nasopharyngeal Carcinoma? An Individual Patient Data Network Meta-Analysis. J. Clin. Oncol. 2017, 35, 498–505. [Google Scholar] [CrossRef]
- Zhu, Q.; Hu, H.; Tang, L.Q.; You, R.; Zhao, J.J.; Weng, D.S.; Pan, Q.Z.; Chen, C.L.; Zhou, Z.Q.; Tang, Y.; et al. Weekly versus triweekly cisplatin plus intensity-modulated radiotherapy in locally advanced nasopharyngeal carcinoma: A propensity score analysis with a large cohort. J. Cancer 2018, 9, 3447–3455. [Google Scholar] [CrossRef]
- Szturz, P.; Wouters, K.; Kiyota, N.; Tahara, M.; Prabhash, K.; Noronha, V.; Castro, A.; Licitra, L.; Adelstein, D.; Vermorken, J.B. Weekly Low-Dose Versus Three-Weekly High-Dose Cisplatin for Concurrent Chemoradiation in Locoregionally Advanced Non-Nasopharyngeal Head and Neck Cancer: A Systematic Review and Meta-Analysis of Aggregate Data. Oncologist 2017, 22, 1056–1066. [Google Scholar] [CrossRef]
- Lin, J.C.; Jan, J.S.; Hsu, C.Y.; Liang, W.M.; Jiang, R.S.; Wang, W.Y. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: Positive effect on overall and progression-free survival. J. Clin. Oncol. 2003, 21, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Haddad, R.; Posner, M.; Machtay, M. Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist 2008, 13, 886–898. [Google Scholar] [CrossRef]
- Yan, P.; Yin, H.; Guo, W.; Sun, X.; Li, F.; Huang, S.; Bian, X.; Wang, F.; Zhang, F.; Wang, B.; et al. Raltitrexed versus 5-fluorouracil with cisplatin and concurrent radiotherapy for locally advanced nasopharyngeal carcinoma: An open labeled, randomized, controlled, and multicenter clinical trial. Cancer Med. 2020, 9, 6166–6172. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Li, P.; Qian, Y.J.; Wu, X.; Xie, L.; Wang, F.; Zhang, H.; Liu, L. A retrospective paired study: Efficacy and toxicity of nimotuzumab versus cisplatin concurrent with radiotherapy in nasopharyngeal carcinoma. BMC Cancer 2016, 16, 946. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Huang, J.; Wu, Q.; Zhu, G.; Wang, X.; Wen, F.; Zhang, P.; Zhang, N.; Li, Q. Concurrent chemoradiotherapy with nedaplatin versus cisplatin in stage II-IVB nasopharyngeal carcinoma: A cost-effectiveness analysis. Oral Oncol. 2019, 93, 15–20. [Google Scholar] [CrossRef]
| Variables | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| CCRT-UP | CCRT-P | p | CCRT-UP | CCRT-P | p | |
| N = 44 | N = 74 | N = 44 | N = 44 | |||
| Gender | 0.376 | 0.999 | ||||
| Male | 30 (68%) | 56 (76%) | 30 (68%) | 30 (68%) | ||
| Female | 14 (32%) | 18 (24%) | 14 (32%) | 14 (32%) | ||
| Age | 0.082 | 0.999 | ||||
| ≦60 years | 32 (73%) | 42 (57%) | 32 (73%) | 32 (73%) | ||
| >60 years | 12 (27%) | 32 (43%) | 12 (27%) | 12 (27%) | ||
| ECOG PS | 0.952 | 0.999 | ||||
| 0–1 | 41 (93%) | 67 (91%) | 41 (93%) | 41 (93%) | ||
| 2 | 3 (7%) | 7 (9%) | 3 (7%) | 3 (7%) | ||
| Renal function | 0.211 | 0.697 | ||||
| CCr ≥ 60 mL/min | 36 (82%) | 51 (69%) | 36 (82%) | 34 (77%) | ||
| CCr < 60 mL/min | 8 (18%) | 23 (31%) | 8 (18%) | 10 (23%) | ||
| T stage | 0.633 | 0.851 | ||||
| T1–T2 | 24 (55%) | 37 (50%) | 24 (55%) | 23 (52%) | ||
| T3–T4 | 20 (45%) | 37 (50%) | 20 (45%) | 21 (48%) | ||
| N stage | 0.481 | 0.892 | ||||
| N0–N1 | 11 (25%) | 23 (31%) | 11 (25%) | 10 (23%) | ||
| N2–N3 | 33 (75%) | 51 (69%) | 33 (75%) | 34 (77%) | ||
| Stage | 0.615 | 0.869 | ||||
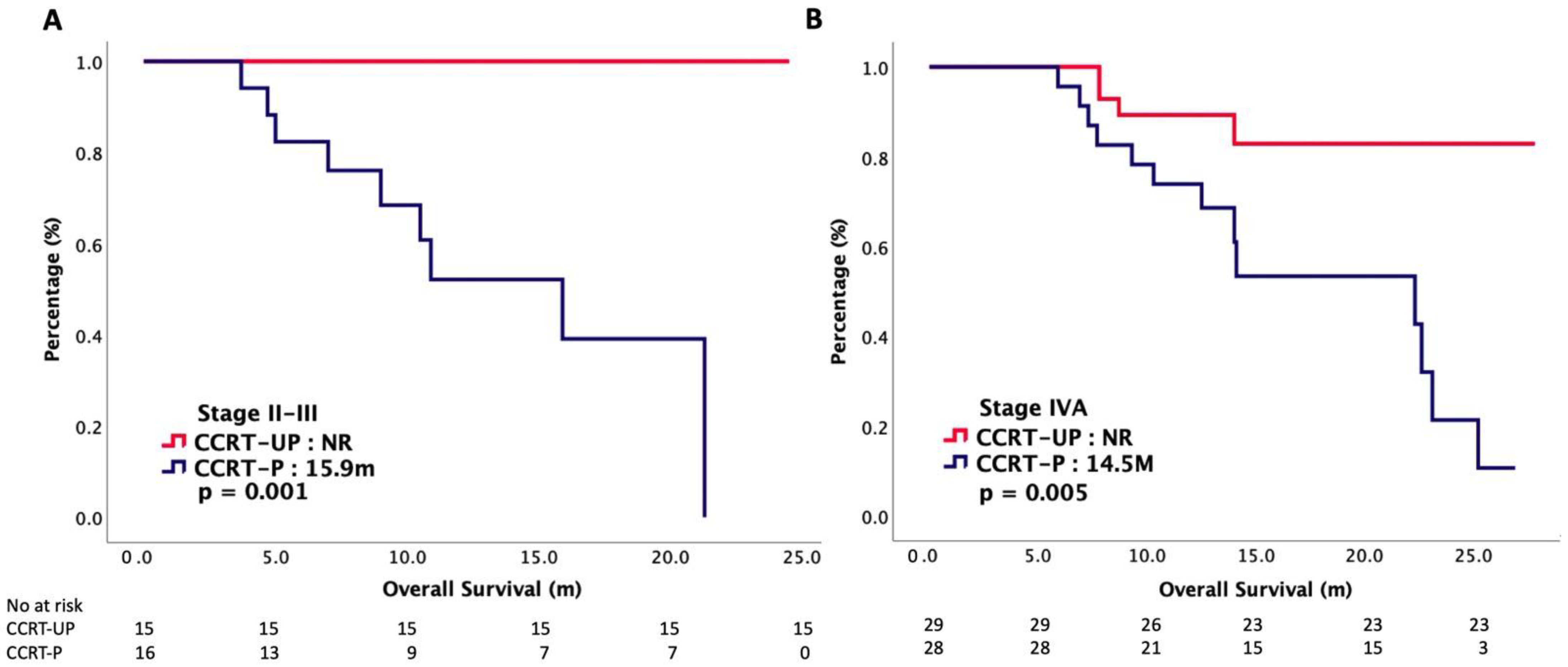
| II–III | 15 (34%) | 29 (39%) | 15 (34%) | 16 (36%) | ||
| IVA | 29 (66%) | 45 (61%) | 29 (66%) | 28 (64%) | ||
| Oncologic Outcomes | CCRT-UP N = 44 | CCRT-P N = 44 | p |
|---|---|---|---|
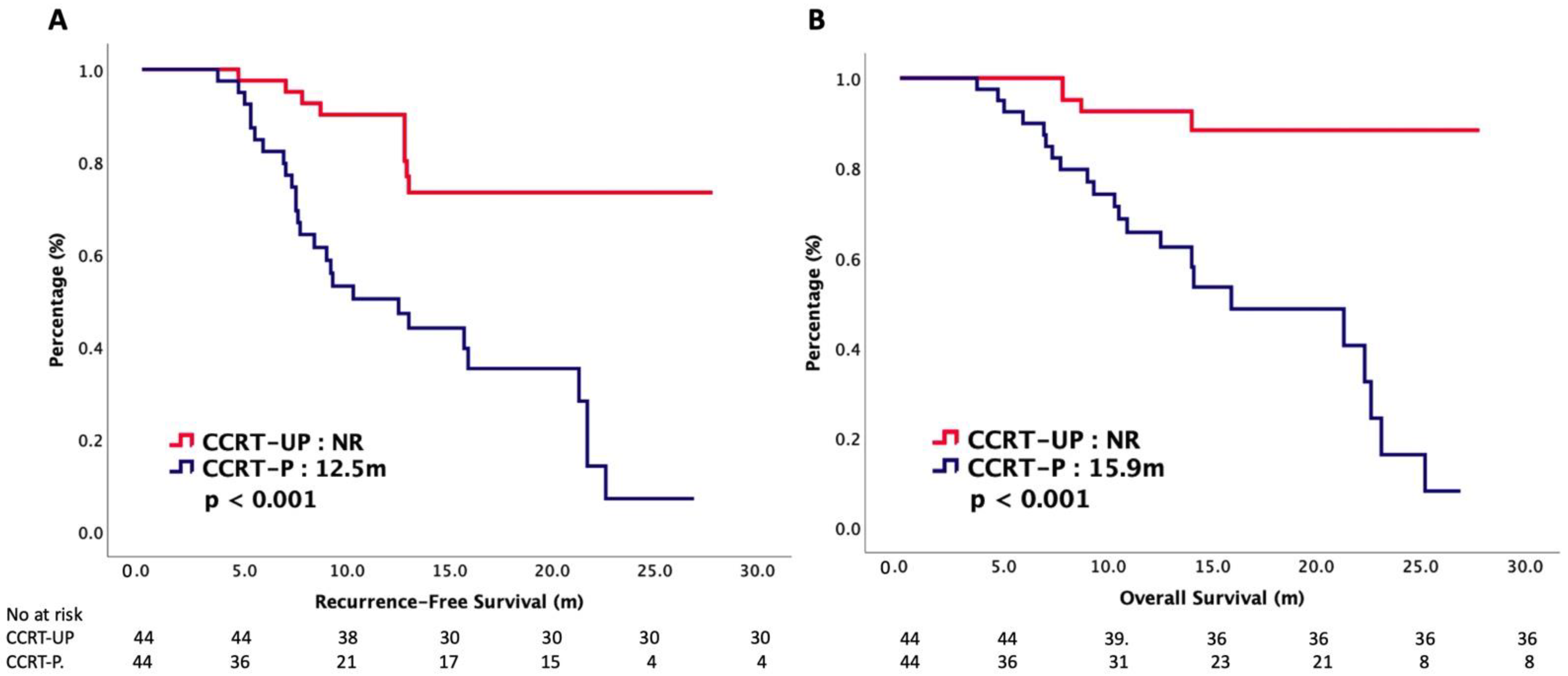
| mRFS (m) | NR | 12.5 | < 0.001 |
| mOS (m) | NR | 15.9 | < 0.001 |
| CR (%) | 17 (39) | 13 (30) | |
| PR (%) | 23 (52) | 25 (57) | |
| SD (%) | 1(2) | 2 (5) | |
| PD (%) | 3 (7) | 4 (8) | |
| ORR (%) | 40 (91) | 32 (87) | 0.368 |
| DCR (%) | 41 (93) | 38 (92) | 0.773 |
| Variables | RFS | OS | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Gender (female vs. male) | 0.81 (0.38–1.72) | 0.574 | 0.64 (0.24–1.69) | 0.362 |
| Age (≦60 years vs. >60 years) | 0.68 (0.33–1.40) | 0.300 | 0.74 (0.31–1.79) | 0.499 |
| ECOG PS (0–1 vs. 2) | 0.78 (0.24–1.85) | 0.420 | 0.87 (0.21–1.84) | 0.515 |
| Renal function (CCr ≥ 60 vs. < 60) | 0.49 (0.21–1.23) | 0.211 | 0.45 (0.17–1.21) | 0.114 |
| T stage (T1–T2 vs. T3–T4) | 0.82 (0.42–1.59) | 0.561 | 0.79 (0.36–1.73) | 0.549 |
| N stage (N0–N1 vs. N2–N3) | 0.75 (0.21–2.71) | 0.666 | 0.71 (0.19–2.73) | 0.619 |
| Clinical stage (stage II–III vs. IVA) | 0.57 (0.27–1.22) | 0.150 | 0.86 (0.38–1.96) | 0.721 |
| CCRT (CCRT-UP vs. CCRT-P) | 0.21 (0.09–0.48) | <0.001 | 0.17 (0.06–0.52) | 0.002 |
| Adverse Events | CCRT-UP N = 44 | CCRT-P N = 44 | p |
|---|---|---|---|
| Hematologic events (n (%)) | |||
| Neutropenia | 4 (9) | 3 (7) | 0.566 |
| Febrile neutropenia | 1 (2) | 1 (2) | 0.999 |
| Anemia | 3 (7) | 2 (5) | 0.514 |
| Nonhematologic events (n (%)) | |||
| Skin rash | 2 (5) | 2 (5) | 0.999 |
| Fatigue | 3 (7) | 2 (5) | 0.523 |
| Diarrhea | 2 (5) | 2 (5) | 0.999 |
| Vomiting | 6 (14) | 5 (11) | 0.423 |
| Anorexia | 4 (9) | 5 (11) | 0.589 |
| Oral mucositis | 7 (16) | 5 (11) | 0.257 |
| Hand–foot syndrome | 1 (2) | 1 (2) | 0.999 |
| Peripheral neuropathy | 2 (5) | 3 (7) | 0.549 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lien, C.-F.; Wang, C.-C.; Yang, C.-C.; Wang, C.-C.; Hwang, T.-Z.; Shih, Y.-C.; Yeh, S.-A.; Hsieh, M.-C. Platinum Plus Tegafur–Uracil versus Platinum Alone during Concurrent Chemoradiotherapy in Patients with Nonmetastatic Nasopharyngeal Carcinoma: A Propensity-Score-Matching Analysis. Cancers 2022, 14, 4511. https://doi.org/10.3390/cancers14184511
Lien C-F, Wang C-C, Yang C-C, Wang C-C, Hwang T-Z, Shih Y-C, Yeh S-A, Hsieh M-C. Platinum Plus Tegafur–Uracil versus Platinum Alone during Concurrent Chemoradiotherapy in Patients with Nonmetastatic Nasopharyngeal Carcinoma: A Propensity-Score-Matching Analysis. Cancers. 2022; 14(18):4511. https://doi.org/10.3390/cancers14184511
Chicago/Turabian StyleLien, Ching-Feng, Chien-Chung Wang, Chuan-Chien Yang, Chih-Chun Wang, Tzer-Zen Hwang, Yu-Chen Shih, Shyh-An Yeh, and Meng-Che Hsieh. 2022. "Platinum Plus Tegafur–Uracil versus Platinum Alone during Concurrent Chemoradiotherapy in Patients with Nonmetastatic Nasopharyngeal Carcinoma: A Propensity-Score-Matching Analysis" Cancers 14, no. 18: 4511. https://doi.org/10.3390/cancers14184511
APA StyleLien, C.-F., Wang, C.-C., Yang, C.-C., Wang, C.-C., Hwang, T.-Z., Shih, Y.-C., Yeh, S.-A., & Hsieh, M.-C. (2022). Platinum Plus Tegafur–Uracil versus Platinum Alone during Concurrent Chemoradiotherapy in Patients with Nonmetastatic Nasopharyngeal Carcinoma: A Propensity-Score-Matching Analysis. Cancers, 14(18), 4511. https://doi.org/10.3390/cancers14184511




