The Nervous System as a Regulator of Cancer Hallmarks: Insights into Therapeutic Implications
Abstract
Simple Summary
Abstract
1. A Bit of History
2. Emotional State, Personality and Cancer
3. Participation of the Nervous System in Physiological Proliferative Processes
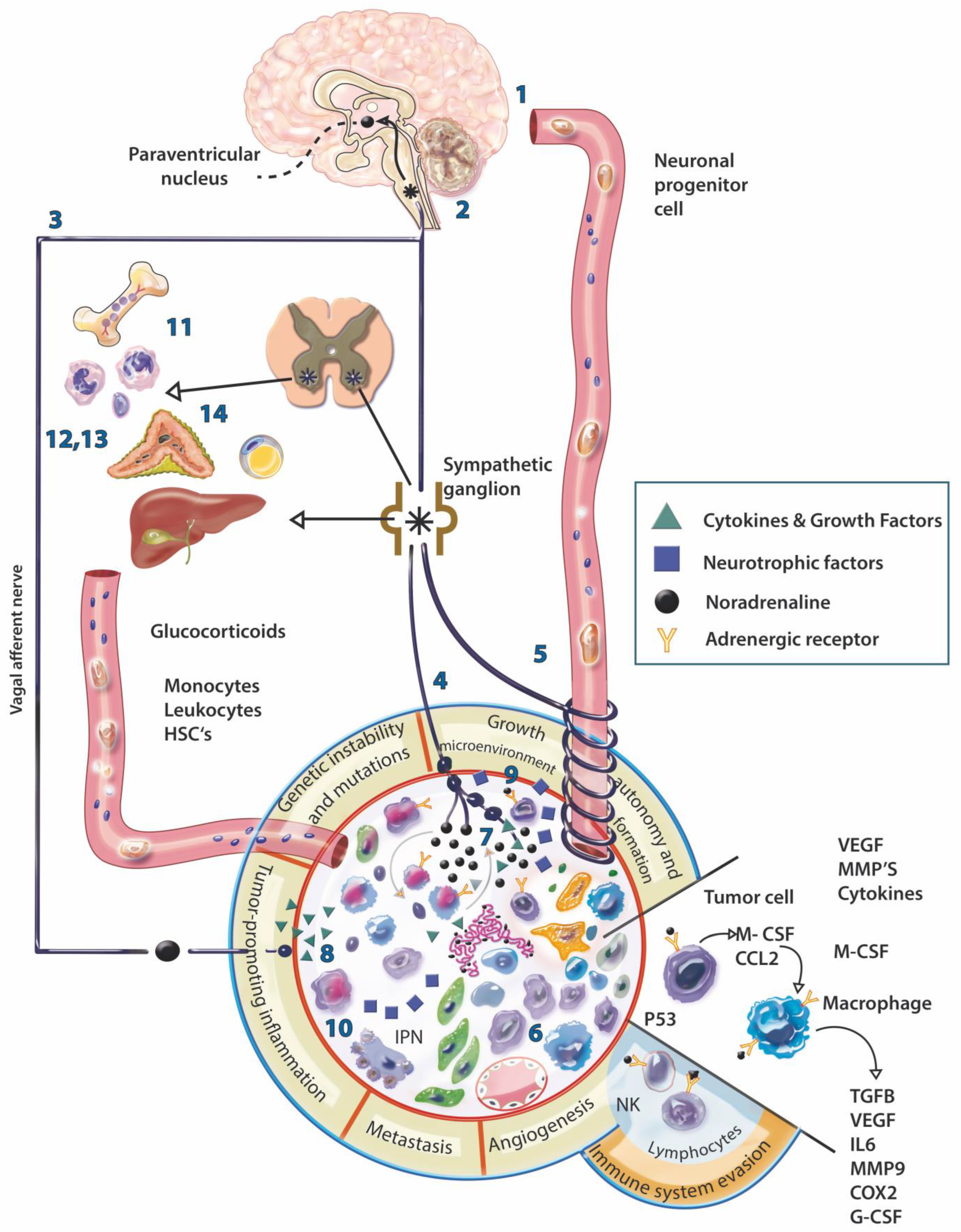
4. Evidence of the Involvement of the Nervous System in Cancer
5. Tumor Innervation
6. Nerve Diversity in Varied Tumor Types
7. Neurotumoral Communication
8. Neuromodulation of Immunity
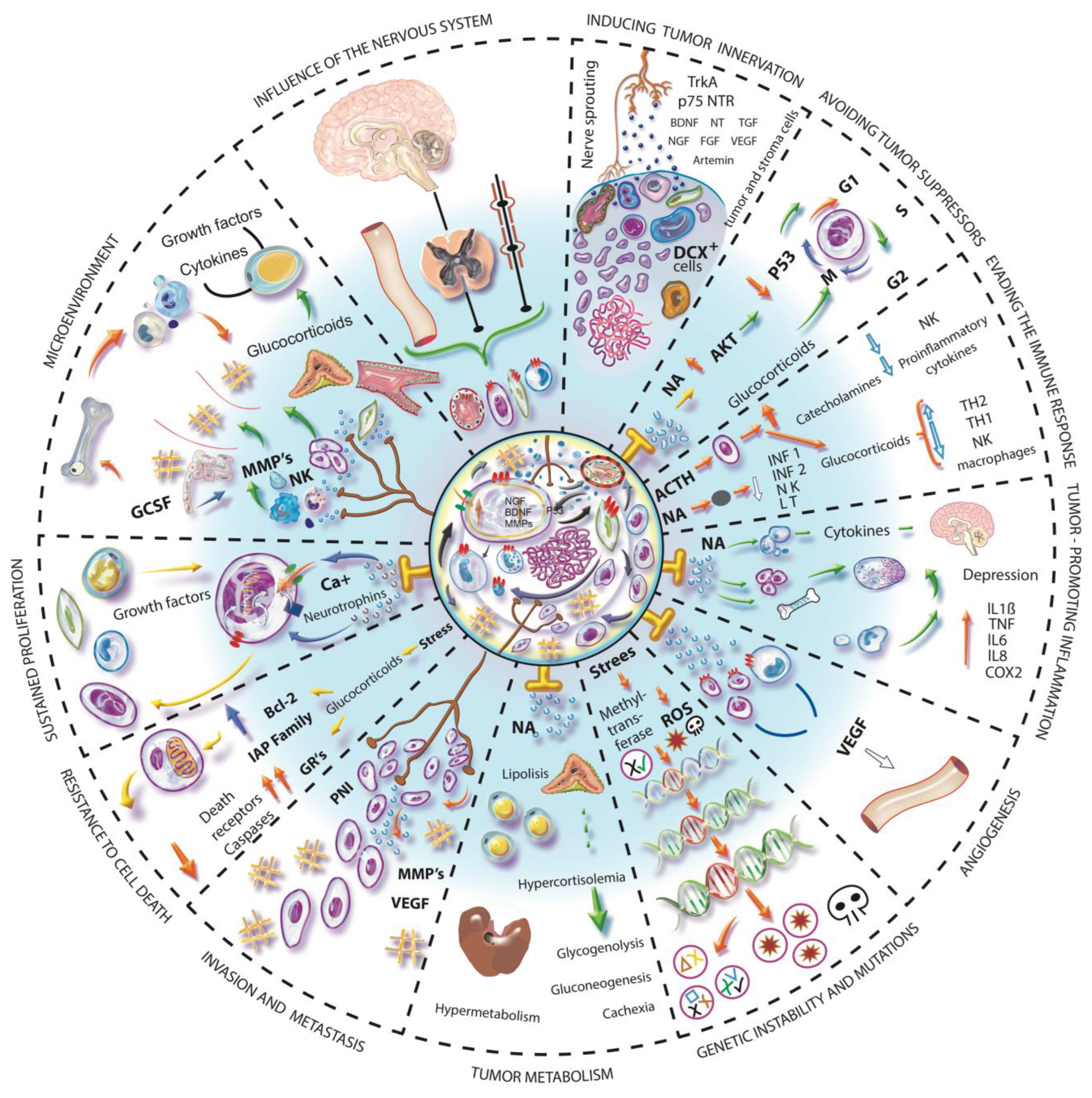
9. Intrinsic Properties of Malignant Tumors and Their Mechanisms Related to the Nervous System
9.1. Evasion of Tumor Suppressors, Genetic Instability and Mutation
9.2. Evasion of the Immune System
9.3. Tumor Associated Inflammation
9.4. Angiogenesis
9.5. Invasion and Metastasis
10. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Young, H.H. On the Presence of Nerves in Tumors and of Other Structures in Them as Revealed by a Modification of Ehrlich’s Method of “Vital Staining” with Methylene Blue. J. Exp. Med. 1897, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ryrie, G.M. On the significance of nerve fibres in human malignant neoplasms. J. Pathol. Bacteriol. 1933, 36, 13–18. [Google Scholar] [CrossRef]
- Shapiro, D.M.; Warren, S. Cancer Innervation. Cancer Res. 1949, 9, 707–711. [Google Scholar]
- Tarlau, M.; Smalheiser, I. Personality patterns in patients with malignant tumors of the breast and cervix; an exploratory study. Psychosom. Med. 1951, 13, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Leshan, L.L.; Gassmann, M.L. Some Observations on Psychotherapy with Patients Suffering from Neoplastic Disease. Am. J. Psychother. 1958, 12, 723–734. [Google Scholar] [CrossRef]
- Hinzey, A.; Gaudier-Diaz, M.M.; Lustberg, M.B.; DeVries, A.C. Breast cancer and social environment: Getting by with a little help from our friends. Breast Cancer Res. 2016, 18, 54. [Google Scholar] [CrossRef]
- Kim, G.M.; Kim, S.J.; Song, S.K.; Kim, H.R.; Kang, B.D.; Noh, S.H.; Chung, H.C.; Kim, K.R.; Rha, S.Y. Prevalence and prognostic implications of psychological distress in patients with gastric cancer. BMC Cancer 2017, 17, 283. [Google Scholar] [CrossRef]
- Batty, G.D.; Russ, T.C.; MacBeath, M.; Stamatakis, E.; Kivimäki, M. Psychological distress in relation to site specific cancer mortality: Pooling of unpublished data from 16 prospective cohort studies. BMJ 2017, 356, j108. [Google Scholar] [CrossRef]
- Sloan, E.K.; Priceman, S.J.; Cox, B.F.; Yu, S.; Pimentel, M.A.; Tangkanangnukul, V.; Arevalo, J.M.; Morizono, K.; Karanikolas, B.D.; Wu, L.; et al. Sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010, 70, 7042–7052. [Google Scholar] [CrossRef]
- Azpiroz, A.; De Miguel, Z.; Fano, E.; Vegas, O. Relations between different coping strategies for social stress, tumor development and neuroendocrine and immune activity in male mice. Brain Behav. Immun. 2008, 22, 690–698. [Google Scholar] [CrossRef]
- Fox, B.H. The role of psychological factors in cancer incidence and prognosis. Oncology 1995, 9, 245–253, discussion 253–256. [Google Scholar] [PubMed]
- Chida, Y.; Hamer, M.; Wardle, J.; Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008, 5, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Trosko, J.E.; Chang, C.C.; Upham, B.L.; Tai, M.H. Ignored hallmarks of carcinogenesis: Stem Cells and Cell-Cell communication. Ann. N. Y. Acad. Sci. 2004, 1028, 192–201. [Google Scholar] [CrossRef]
- Soto, A.; Sonnenschein, C. The tissue organization field theory of cancer: A testable replacement for the somatic mutation theory. Bioessays 2011, 33, 332–340. [Google Scholar] [CrossRef]
- Levin, M. Morphogenetic fields in embryogenesis, regeneration, and cancer: Non-local control of complex patterning. BioSystems 2012, 109, 243–261. [Google Scholar] [CrossRef]
- Xuan, C.; Shamonki, J.M.; Chung, A.; Dinome, M.L.; Chung, M.; Sieling, P.A.; Lee, D.J. Microbial dysbiosis is associated with human breast cancer. PLoS ONE 2014, 9, e83744. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef]
- Lobikin, M.; Chernet, B.; Lobo, D.; Levin, M. Resting Potential, Oncogene-induced Tumorigenesis, and Metastasis: The Bioelectric Basis of Cancer in vivo. Phys. Biol. 2012, 9, 065002. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion channels and the hallmarks of cancer. Trends Mol. Med. 2010, 16, 107–121. [Google Scholar] [CrossRef]
- Huber, S.M. Oncochannels. Cell Calcium. 2013, 53, 241–255. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Bernstein, J.; Gronostaj, M. Psychological Stress and Cellular Aging in Cancer: A Meta-Analysis. Oxid. Med. Cell. Longev. 2019, 2019, 1270397. [Google Scholar] [CrossRef] [PubMed]
- Betof, A.S.; Dewhirst, M.W.; Jones, L.W. Effects and potential mechanisms of excercise training on cancer progression: A translational perspective. Brain Behav. Immun. 2013, 30, S75–S87. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.; Koranteng, F. Availability, accessibility, and impact of social support on breast cancer treatment among breast cancer patients in Kumasi, Ghana: A qualitative study. PLoS ONE 2020, 15, e0231691. [Google Scholar] [CrossRef] [PubMed]
- Montes-Nogueira, I.; Gutiérrez-Ospina, G.; Romo-González, T. Towards a Psychoneuroimmunendocrine Hypothesis of Breast Cancer. Adv. Neuroimmune Biol. 2017, 6, 153–160. [Google Scholar] [CrossRef]
- Cavigelli, S.A.; Bennett, J.M.; Michael, K.C.; Cousino, L. Female temperament, tumor development and life span: Relation to glucocorticoid and tumor necrosis factor α levels in rats. Brain Behav. Immun. 2008, 727–735. [Google Scholar] [CrossRef][Green Version]
- Heidt, T.; Sager, H.B.; Courties, G.; Dutta, P.; Iwamoto, Y.; Zaltsman, A.; von Zur Muhlen, C.; Bode, C.; Fricchione, G.L.; Denninger, J.; et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 2014, 20, 754–758. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Galván-Hernández, C.I.; Toscano-Márquez, B.; Gutiérrez-Ospina, G. Modulatory Role of Sensory Innervation on Hair Follicle Stem Cell Progeny during Wound Healing of the Rat Skin. PLoS ONE 2012, 7, e36421. [Google Scholar] [CrossRef]
- Sharifpanah, F.; Saliu, F.; Bekhite, M.M.; Wartenberg, M.; Sauer, H. β-Adrenergic receptor antagonists inhibit vasculogenesis of embryonic stem cells by downregulation of nitric oxide generation and interference with VEGF signalling. Cell Tissue Res. 2014, 358, 443–452. [Google Scholar] [CrossRef]
- Spiegel, A.; Shivtiel, S.; Kalinkovich, A.; Ludin, A.; Netzer, N.; Goichberg, P.; Azaria, Y.; Resnick, I.; Hardan, I.; Ben-Hur, H.; et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat. Immunol. 2007, 8, 1123–1131. [Google Scholar] [CrossRef]
- Katayama, Y.; Battista, M.; Kao, W.M.; Hidalgo, A.; Peired, A.J.; Thomas, S.A.; Frenette, P.S. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006, 124, 407–421. [Google Scholar] [CrossRef]
- Liang, W.; Zhuo, X.; Tang, Z. Calcitonin gene-related peptide stimulates proliferation and osteogenic differentiation of osteoporotic rat-derived bone mesenchymal stem cells. Mol. Cell. Biochem. 2015, 402, 101–110. [Google Scholar] [CrossRef]
- Powell, N.D.; Sloan, E.K.; Bailey, M.T.; Arevalo, J.M.; Miller, G.E.; Chen, E.; Kobor, M.S.; Reader, B.F.; Sheridan, J.F.; Cole, S.W. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc. Natl. Acad. Sci. USA 2013, 110, 16574–16579. [Google Scholar] [CrossRef]
- Collins, S.; Sarzani, R.; Bordicchia, M. Coordinate control of adipose ‘browning’ and energy expenditure by β-adrenergic and natriuretic peptide signalling. Int. J. Obes. Suppl. 2014, 4 (Suppl. 1), S17–S20. [Google Scholar] [CrossRef]
- Boilly, B.; Faulknerx, S.; Jobling, P.; Hondermarck, H. Nerve dependence: From regeneration to cancer. Cancer Cell 2017, 31, 342–354. [Google Scholar] [CrossRef]
- Makita, T.; Sucov, H.M.; Gariepy, C.E.; Yanagisawa, M.; Ginty, D.D. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature 2008, 452, 759–763. [Google Scholar] [CrossRef]
- Mitchell, B.S.; Schumacher, U.; Stauber, V.V.; Kaiserling, E. Are breast tumours innervated? Immunohistological investigations using antibodies against the neuronal marker protein gene product 9.5 (PGP 9.5) in benign and malignant breast lesions. Eur. J. Cancer 1994, 30, 1100–1103. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, Y.; Liang, X.; Du, G.; Liu, L.; Lu, L.; Dong, J.; Han, H.; Zhang, G. The clinicopathological significance of neurogenesis in breast cancer. BMC Cancer 2014, 14, 484. [Google Scholar] [CrossRef]
- Lü, S.H.; Zhou, Y.; Que, H.P.; Liu, S.J. Peptidergic innervation of human esophageal and cardiac carcinoma. World J. Gastroenterol. 2003, 9, 399–403. [Google Scholar] [CrossRef]
- Chamary, V.L.; Robson, T.; Loizidou, M.; Boulos, P.B.; Burnstock, G. Progressive loss of perivascular nerves adjacent to colorectal cancer. Eur. J. Surg. Oncol. 2000, 26, 588–593. [Google Scholar] [CrossRef]
- Nagy, J.A.; Chang, S.H.; Shih, S.C.; Dvorak, A.M.; Dvorak, H.F. Heterogeneity of the tumor vasculature. Semin. Thromb. Hemost. 2010, 36, 321–331. [Google Scholar] [CrossRef]
- Saloman, J.L.; Albers, K.M.; Rhim, A.D.; Davis, B.M. Can Stopping Nerves, Stop Cancer? Trends Neurosci. 2016, 39, 880–889. [Google Scholar] [CrossRef]
- Driver, J.A. Inverse association between cancer and neurodegenerative disease: Review of the epidemiologic and biological evidence. Biogerontology 2014, 15, 547–557. [Google Scholar] [CrossRef]
- Wefel, J.S.; Vardy, J.; Ahles, T.; Schagen, S.B. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011, 12, 703–708. [Google Scholar] [CrossRef]
- Rutledge, A.; Jobling, P.; Walker, M.M.; Denham, J.W.; Hondermarck, H. Spinal Cord Injuries and Nerve Dependence in Prostate Cancer. Trends Cancer 2017, 3, 812–815. [Google Scholar] [CrossRef]
- Raimondi, S.; Botteri, E.; Munzone, E.; Cipolla, C.; Rotmensz, N.; DeCensi, A.; Gandini, S. Use of beta-blockers, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers and breast cancer survival: Systematic review and meta-analysis. Int. J. Cancer 2016, 139, 212–219. [Google Scholar] [CrossRef]
- Barron, T.I.; Connolly, R.M.; Sharp, L.; Bennett, K.; Visvanathan, K. Beta blockers and breast cancer mortality: A population- based study. J. Clin. Oncol. 2011, 29, 2635–2644. [Google Scholar] [CrossRef]
- Melhem-Bertrandt, A.; Chavez-Macgregor, M.; Lei, X.; Brown, E.N.; Lee, R.T.; Meric-Bernstam, F.; Sood, A.K.; Conzen, S.D.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2011, 29, 2645–2652. [Google Scholar] [CrossRef]
- Kokolus, K.M.; Zhang, Y.; Sivik, J.M.; Schmeck, C.; Zhu, J.; Repasky, E.A.; Drabick, J.J.; Schell, T.D. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology 2017, 7, e1405205. [Google Scholar] [CrossRef]
- Renz, B.W.; Takahashi, R.; Tanaka, T.; Macchini, M.; Hayakawa, Y.; Dantes, Z.; Maurer, H.C.; Chen, X.; Jiang, Z.; Westphalen, C.B.; et al. β2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell 2018, 33, 75–90.e7, Erratum in: Cancer Cell. 2018, 34, 863–867. [Google Scholar] [CrossRef]
- Nilsson, M.B.; Sun, H.; Diao, L.; Tong, P.; Liu, D.; Li, L.; Fan, Y.; Poteete, A.; Lim, S.O.; Howells, K.; et al. Stress hormones promote EGFR inhibitor resistance in NSCLC: Implications for combinations with β-blockers. Sci. Transl. Med. 2017, 9, eaao4307. [Google Scholar] [CrossRef]
- Pasquier, E.; Street, J.; Pouchy, C.; Carre, M.; Gifford, A.J.; Murray, J.; Norris, M.D.; Trahair, T.; Andre, N.; Kavallaris, M. β-blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastoma. Br. J. Cancer 2013, 108, 2485–2494. [Google Scholar] [CrossRef]
- Rico, M.; Baglioni, M.; Bondarenko, M.; Laluce, N.C.; Rozados, V.; André, N.; Carré, M.; Scharovsky, O.G.; Menacho Márquez, M. Metformin and propranolol combination prevents cancer progression and metastasis in different breast cancer models. Oncotarget 2017, 8, 2874–2889. [Google Scholar] [CrossRef]
- Marchesi, F.; Piemonti, L.; Mantovani, A.; Allavena, P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010, 21, 77–82. [Google Scholar] [CrossRef]
- Chen, J.W.; Bhandari, M.; Astill, D.S.; Wilson, T.G.; Kow, L.; Brooke-Smith, M.; Toouli, J.; Padbury, R.T. Predicting patient survival after pancreaticoduodenectomy for malignancy: Histopathological criteria based on perineural infiltration and lymphovascular invasion. HPB J. 2010, 12, 101–108. [Google Scholar] [CrossRef]
- Yao, J.; Li, W.Y.; Li, S.G.; Feng, X.S.; Gao, S.G. Midkine promotes perineural invasion in human pancreatic cancer. WJG 2014, 20, 3018–3024. [Google Scholar] [CrossRef]
- Qian, X.; Nguyen, D.T.; Dong, Y.; Sinikovic, B.; Kaufmann, A.M.; Myers, J.N.; Albers, A.E.; Graviss, E.A. Prognostic Score Predicts Survival in HPV-Negative Head and Neck Squamous Cell Cancer Patients. Int. J. Biol. Sci. 2019, 15, 1336–1344. [Google Scholar] [CrossRef]
- Seefeld, P.H.; Bargen, J.A. The spread of carcinoma of the rectum: Invasion of lymphatics, veins and nerves. Ann. Surg. 1943, 118, 76–90. [Google Scholar] [CrossRef]
- Chablani, P.; Nguyen, P.; Pan, X.; Robinson, A.; Walston, S.; Wu, C.; Frankel, W.L.; Chen, W.; Bekaii-Saab, T.; Chakravarti, A.; et al. Perineural Invasion Predicts for Distant Metastasis in Locally Advanced Rectal Cancer Treated with Neoadjuvant Chemoradiation and Surgery. Am. J. Clin. Oncol. 2017, 40, 561–568. [Google Scholar] [CrossRef]
- Mirkin, K.A.; Hollenbeak, C.S.; Mohamed, A.; Jia, Y.; El-Deiry, W.S.; Messaris, E. Impact of perineural invasion on survival in node negative colon cancer. Cancer Biol. Ther. 2017, 18, 740–745. [Google Scholar] [CrossRef]
- Ceyhan, G.O.; Demir, I.E.; Altintas, B.; Rauch, U.; Thiel, G.; Müller, M.W.; Giese, N.A.; Friess, H.; Schäfer, K.H. Neural invasion in pancreatic cancer: A mutual tropism between neurons and cancer cells. Biochem. Biophys. Res. Commun. 2008, 374, 442–447. [Google Scholar] [CrossRef]
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer: A review of the literature. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef]
- Magnon, C. Role of the autonomic nervous system in tumorigenesis and metastasis. Mol. Cell. Oncol. 2015, 2, e975643. [Google Scholar] [CrossRef]
- Chakravarthy, R.; Mnich, K.; Gorman, A.M. Nerve growth factor (NGF)-mediated regulation of p75 (NTR) expression contributes to chemotherapeutic resistance in triple negative breast cancer cells. Biochem. Biophys. Res. Commun. 2016, 478, 1541–1547. [Google Scholar] [CrossRef]
- Mancino, M.; Ametller, E.; Gascón, P.; Almendro, V. The neuronal influence on tumor progression. Biochim. Et Biophys. Acta 2011, 1816, 105–118. [Google Scholar] [CrossRef]
- Bradshaw, R.A.; Pundavela, J.; Biarc, J.; Chalkley, R.J.; Burlingame, A.L.; Hondermarck, H. NGF and ProNGF: Regulation of neuronal and neoplastic responses through receptor signaling. Adv. Biol. Regul. 2015, 58, 16–27. [Google Scholar] [CrossRef]
- Entschladen, F.; Palm, D.; Lang, K.; Drell, T.L., 4th; Zaenker, K.S. Neoneurogenesis: Tumors may initiate their own innervation by the release of neurotrophic factors in analogy to lymphangiogenesis and neoangiogenesis. Med. Hypotheses 2006, 67, 33–35. [Google Scholar] [CrossRef]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef]
- Mauffrey, P.; Tchitchek, N.; Barroca, V.; Bemelmans, A.P.; Firlej, V.; Allory, Y.; Roméo, P.H.; Magnon, C. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 2019, 569, 672–678. [Google Scholar] [CrossRef]
- Blacklock, A.D.; Smith, P.G. Estrogen increases calcitonin gene-related peptide-immunoreactive sensory innervation of rat mammary gland. J. Neurobiol. 2004, 59, 192–204. [Google Scholar] [CrossRef]
- Godlewski, J.; Łakomy, I.M. Changes in vasoactive intestinal peptide, pituitary adenylate cyclase-activating polypeptide and neuropeptide Y-ergic structures of the enteric nervous system in the carcinoma of the human large intestine. Folia Histochem. Cytobiol. 2010, 48, 208–216. [Google Scholar] [CrossRef]
- Albo, D.; Akay, C.L.; Marshall, C.L.; Wilks, J.A.; Verstovsek, G.; Liu, H.; Agarwal, N.; Berger, D.H.; Ayala, G.E. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer 2011, 117, 4834–4845. [Google Scholar] [CrossRef]
- Narayan, P.; Flynn, J.; Zhang, Z.; Gillespie, E.F.; Mueller, B.; Xu, A.J.; Cuaron, J.; McCormick, B.; Khan, A.J.; Cahlon, O.; et al. Perineural invasion as a risk factor for locoregional recurrence of invasive breast cancer. Sci. Rep. 2021, 11, 12781. [Google Scholar] [CrossRef]
- Pagella, P.; Catón, J.; Meisel, C.T.; Mitsiadis, T.A. Ameloblastomas Exhibit Stem Cell Potential, Possess Neurotrophic Properties, and Establish Connections with Trigeminal Neurons. Cells 2020, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.W.; Dill, T.; Griffin, N.; Jobling, P.; Faulkner, S.; Paul, J.W.; King, S.; Smith, R.; Hondermarck, H. Innervation of papillary thyroid cancer and its association with extra-thyroidal invasion. Sci. Rep. 2020, 10, 1539. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.E.; Behrendt, C.A.; Glatzel, M.; Hagel, C. Vascular Innervation in Benign Neurofibromas of Patients with Neurofibromatosis Type 1. Anticancer Res. 2015, 35, 6509–6516. [Google Scholar]
- Palm, D.; Entschladen, F. Neoneurogenesis and the neuro-neoplastic synapse. Neuronal Act. Tumor Tissue 2007, 39, 91–98. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Q.; Lu, Z.; Wang, L.; Ding, L.; Xia, L.; Zhang, H.; Wang, M.; Chen, Y.; Li, G. Role of the nervous system in cancers: A review. Cell Death Discov. 2021, 7, 76. [Google Scholar] [CrossRef]
- Díaz-Alonso, J.; Nicoll, R.A. AMPA receptor trafficking and LTP: Carboxy-termini, amino-termini and TARPs. Neuropharmacology 2021, 197, 108710. [Google Scholar] [CrossRef]
- Mravec, B.; Gidron, Y.; Hulin, I. Neurobiology of cancer: Interactions between nervous, endocrine and immune systems as a base for monitoring and modulating the tumorigenesis by the brain. Semin. Cancer Biol. 2008, 18, 150–163. [Google Scholar] [CrossRef]
- Cole, S.W.; Nagaraja, A.S.; Lutgendorf, S.K.; Green, P.A.; Sood, A.K. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer 2015, 15, 563–572. [Google Scholar] [CrossRef]
- Flierl, M.A.; Rittirsch, D.; Nadeau, B.A.; Chen, A.J.; Sarma, J.V.; Zetoune, F.S.; McGuire, S.R.; List, R.P.; Day, D.E.; Hoesel, L.M.; et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature 2007, 449, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psych. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, D.; Han, R.; Zhang, C.; Jin, W.N.; Wood, K.; Liu, Q.; Shi, F.D.; Hao, J. Acetylcholine-producing NK cells attenuate CNS inflammation via modulation of infiltrating monocytes/macrophages. Proc. Natl. Acad. Sci. USA 2017, 114, E6202–E6211. [Google Scholar] [CrossRef]
- Rosas-Ballina, M.; Olofsson, P.S.; Ochani, M.; Valdés-Ferrer, S.I.; Levine, Y.A.; Reardon, C.; Tusche, M.W.; Pavlov, V.A.; Andersson, U.; Chavan, S.; et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011, 334, 98–101. [Google Scholar] [CrossRef]
- Torres-Rosas, R.; Yehia, G.; Peña, G.; Mishra, P.; del Rocio Thompson-Bonilla, M.; Moreno-Eutimio, M.A.; Arriaga-Pizano, L.A.; Isibasi, A.; Ulloa, L. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat. Med. 2014, 20, 291–295. [Google Scholar] [CrossRef]
- Kim, T.H.; Ly, C.; Christodoulides, A.; Nowell, C.J.; Gunning, P.W.; Sloan, E.K.; Rowat, A.C. Stress hormone signaling through β-adrenergic receptors regulates macrophage mechanotype and function. FASEB 2019, 33, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- De, R.; Mazumder, S.; Sarkar, S.; Debsharma, S.; Siddiqui, A.A.; Saha, S.J.; Banerjee, C.; Nag, S.; Saha, D.; Bandyopadhyay, U. Acute mental stress induces mitochondrial bioenergetic crisis and hyper-fission along with aberrant mitophagy in the gut mucosa in rodent model of stress-related mucosal disease. Free Radic. Biol. Med. 2017, 113, 424–438. [Google Scholar] [CrossRef]
- Flint, M.S.; Baum, A.; Chambers, W.H.; Jenkins, F.J. Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinology 2007, 32, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.; Asami, S.; Nagata, S.; Miyata, M.; Kasai, H. Relationships between perceived workload, stress and oxidative DNA damage. Int. Arch. Occup. Environ. Health 2001, 74, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.R.; Sachs, B.D.; Caron, M.G.; Lefkowitz, R.J. Pharmacological blockade of a β(2)AR-β-arrestin-1 signaling cascade prevents the accumulation of DNA damage in a behavioral stress model. Cell Cycle 2013, 12, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Glaser, R.; Thorn, B.E.; Tarr, K.L.; Kiecolt-Glaser, J.K.; D’Ambrosio, S.M. Effects of stress on methyltransferase synthesis: An important DNA repair enzyme. Health Psychol. 1985, 4, 403–412. [Google Scholar] [CrossRef]
- Hanoun, M.; Maryanovich, M.; Arnal-Estapé, A.; Frenette, P.S. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron 2015, 86, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.; Caron, M.G.; Lefkowitz, R.J. Beta-adrenergic receptors in hamster smooth muscle cells are transcriptionally regulated by glucocorticoids. J. Biol. Chem. 1988, 263, 9067–9070. [Google Scholar] [CrossRef]
- Mak, J.C.; Nishikawa, M.; Barnes, P.J. Glucocorticosteroids increase beta 2-adrenergic receptor transcription in human lung. Am. J. Physiol. 1995, 268 Pt 1, L41–L46. [Google Scholar] [CrossRef]
- De Lorenzo, B.H.; de Oliveira Marchioro, L.; Greco, C.R.; Suchecki, D. Sleep-deprivation reduces NK cell number and function mediated by β-adrenergic signalling. Psychoneuroendocrinology 2015, 57, 134–143. [Google Scholar] [CrossRef]
- Bellinger, D.L.; Millar, B.A.; Perez, S.; Carter, J.; Wood, C.; ThyagaRajan, S.; Molinaro, C.; Lubahn, C.; Lorton, D. Sympathetic modulation of immunity: Relevance to disease. Cell. Immunol. 2008, 252, 27–56. [Google Scholar] [CrossRef]
- Mohammadpour, H.; MacDonald, C.R.; Qiao, G.; Chen, M.; Dong, B.; Hylander, B.L.; McCarthy, P.L.; Abrams, S.I.; Repasky, E.A. β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J. Clin. Investig. 2019, 129, 5537–5552. [Google Scholar] [CrossRef]
- Raposo, T.P.; Arias-Pulido, H.; Chaher, N.; Fiering, S.N.; Argyle, D.J.; Prada, J.; Pires, I.; Queiroga, F.L. Comparative aspects of canine and human inflammatory breast cancer. Semin. Oncol. 2017, 44, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.I.; Silva-Carvalho, R.; Pires, I.; Prada, J.; Bianchini, R.; Jensen-Jarolim, E.; Queiroga, F.L. A Comparative Approach of Tumor-Associated Inflammation in Mammary Cancer between Humans and Dogs. BioMed Res. Int. 2016, 2016, 4917387. [Google Scholar] [CrossRef] [PubMed]
- Aliper, A.M.; Frieden-Korovkina, V.P.; Buzdin, A.; Roumiantsev, S.A.; Zhavoronkov, A. A role for G-CSF and GM-CSF in nonmyeloid cancers. Cancer Med. 2014, 3, 737–746. [Google Scholar] [CrossRef]
- Bujak, J.K.; Kosmala, D.; Szopa, I.M.; Majchrzak, K.; Bednarczyk, P. Inflammation, Cancer and Immunity-Implication of TRPV1 Channel. Front. Oncol. 2019, 9, 1087. [Google Scholar] [CrossRef]
- Sauzay, C.; Voutetakis, K.; Chatziioannou, A.; Chevet, E.; Avril, T. CD90/Thy-1, a Cancer-Associated Cell Surface Signaling Molecule. Front. Cell Dev. Biol. 2019, 7, 66. [Google Scholar] [CrossRef]
- Glumac, P.M.; LeBeau, A.M. The role of CD133 in cancer: A concise review. Clin. Transl. Med. 2018, 7, 18. [Google Scholar] [CrossRef]
- Huan, H.B.; Wen, X.D.; Chen, X.J.; Wu, L.; Wu, L.L.; Zhang, L.; Yang, D.P.; Zhang, X.; Bie, P.; Qian, C.; et al. Sympathetic nervous system promotes hepatocarcinogenesis by modulating inflammation through activation of alpha1-adrenergic receptors of Kupffer cells. Brain Behav. Immun. 2017, 59, 118–134. [Google Scholar] [CrossRef]
- Horvathova, L.; Padova, A.; Tillinger, A.; Osacka, J.; Bizik, J.; Mravec, B. Sympathectomy reduces tumor weight and affects expression of tumor-related genes in melanoma tissue in the mouse. Stress 2016, 19, 528–534. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; He, Z.; Yin, K.; Li, B.; Zhang, L.; Xu, Z. Chronic stress promotes gastric cancer progression and metastasis: An essential role for ADRB2. Cell Death Dis. 2019, 10, 788. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W. Beta-adrenergic signaling on neuroendocrine differentiation, angiogenesis, and metastasis in prostate cancer progression. Asian J. Androl. 2019, 21, 253–259. [Google Scholar] [CrossRef]
- Kilpatrick, L.E.; Alcobia, D.C.; White, C.W.; Peach, C.J.; Glenn, J.R.; Zimmerman, K.; Kondrashov, A.; Pfleger, K.; Ohana, R.F.; Robers, M.B.; et al. Complex Formation between VEGFR2 and the β2-Adrenoceptor. Cell Chem. Biol. 2019, 26, 830–841.e9. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Sarkar, C.; Chakroborty, D.; Nagy, J.; Mitra, R.B.; Dasgupta, P.S.; Mukhopadhyay, D. Ablation of peripheral dopaminergic nerves stimulates malignant tumor growth by inducing vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis. Cancer Res. 2004, 64, 5551–5555. [Google Scholar] [CrossRef]
- Peters, M.; Meijer, C.; Fehrmann, R.; Walenkamp, A.; Kema, I.P.; de Vries, E.; Hollema, H.; Oosting, S.F. Serotonin and Dopamine Receptor Expression in Solid Tumours Including Rare Cancers. Pathol. Oncol. Res. 2020, 26, 1539–1547. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, C.; Gao, F.; Li, H. The effect and mechanism of dopamine D1 receptors on the proliferation of osteosarcoma cells. Mol. Cell. Biochem. 2017, 430, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, L.; Chen, N.; Niu, C.; Li, Z.; Hu, J.; Cui, J. Nerves in the Tumor Microenvironment: Origin and Effects. Front. Cell Dev. Biol. 2020, 8, 601738. [Google Scholar] [CrossRef]
- Armaiz-Pena, G.N.; Allen, J.K.; Cruz, A.; Stone, R.L.; Nick, A.M.; Lin, Y.G.; Han, L.Y.; Mangala, L.S.; Villares, G.J.; Vivas-Mejia, P.; et al. Src activation by β-adrenoreceptors is a key switch for tumour metastasis. Nat. Commun. 2013, 4, 1403. [Google Scholar] [CrossRef]
- Cheng, Y.; Gao, X.H.; Li, X.J.; Cao, Q.H.; Zhao, D.D.; Zhou, J.R.; Wu, H.X.; Wang, Y.; You, L.J.; Yang, H.B.; et al. Depression promotes prostate cancer invasion and metastasis via a sympathetic-cAMP-FAK signaling pathway. Oncogene 2018, 37, 2953–2966. [Google Scholar] [CrossRef]
- Choy, C.; Raytis, J.L.; Smith, D.D.; Duenas, M.; Neman, J.; Jandial, R.; Lew, M.W. Inhibition of β2-adrenergic receptor reduces triple-negative breast cancer brain metastases: The potential benefit of perioperative β-blockade. Oncol. Rep. 2016, 35, 3135–3142. [Google Scholar] [CrossRef]
- Shaashua, L.; Shabat-Simon, M.; Haldar, R.; Matzner, P.; Zmora, O.; Shabtai, M.; Sharon, E.; Allweis, T.; Barshack, I.; Hayman, L.; et al. Perioperative COX-2 and β-Adrenergic Blockade Improves Metastatic Biomarkers in Breast Cancer Patients in a Phase-II Randomized Trial. Clin. Cancer Res. 2017, 23, 4651–4661. [Google Scholar] [CrossRef]
- Rivero, E.M.; Piñero, C.P.; Gargiulo, L.; Entschladen, F.; Zänker, K.; Bruzzone, A.; Lüthy, I.A. The β 2-Adrenergic Agonist Salbutamol Inhibits Migration, Invasion and Metastasis of the Human Breast Cancer MDA-MB- 231 Cell Line. Curr. Cancer Drug Targets 2017, 17, 756–766. [Google Scholar] [CrossRef]
- Bloom, A.P.; Jimenez-Andrade, J.M.; Taylor, R.N.; Castañeda-Corral, G.; Kaczmarska, M.J.; Freeman, K.T.; Coughlin, K.A.; Ghilardi, J.R.; Kuskowski, M.A.; Mantyh, P.W. Breast cancer-induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J. Pain 2011, 12, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Andrade, J.M.; Ghilardi, J.R.; Castañeda-Corral, G.; Kuskowski, M.A.; Mantyh, P.W. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain 2011, 152, 2564–2574. [Google Scholar] [CrossRef]
- Elefteriou, F. Role of sympathetic nerves in the establishment of metastatic breast cancer cells in bone. J. Bone Oncol. 2016, 5, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Welch, D.R.; Hurst, D.R. Defining the Hallmarks of Metastasis. Cancer Res. 2019, 79, 3011–3027. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, C.; Soto, A.M. Over a century of cancer research: Inconvenient truths and promising leads. PLoS Biol. 2020, 18, e3000670. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Juárez, K.V.; Queiroga, F.L.; Romero-Romero, L.P. The Nervous System as a Regulator of Cancer Hallmarks: Insights into Therapeutic Implications. Cancers 2022, 14, 4372. https://doi.org/10.3390/cancers14184372
Torres-Juárez KV, Queiroga FL, Romero-Romero LP. The Nervous System as a Regulator of Cancer Hallmarks: Insights into Therapeutic Implications. Cancers. 2022; 14(18):4372. https://doi.org/10.3390/cancers14184372
Chicago/Turabian StyleTorres-Juárez, Karla V., Felisbina Luisa Queiroga, and Laura P. Romero-Romero. 2022. "The Nervous System as a Regulator of Cancer Hallmarks: Insights into Therapeutic Implications" Cancers 14, no. 18: 4372. https://doi.org/10.3390/cancers14184372
APA StyleTorres-Juárez, K. V., Queiroga, F. L., & Romero-Romero, L. P. (2022). The Nervous System as a Regulator of Cancer Hallmarks: Insights into Therapeutic Implications. Cancers, 14(18), 4372. https://doi.org/10.3390/cancers14184372





