The Impact of KRAS Mutational Status on Long-Term Survival following Liver Resection for Hilar Cholangiocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Pathologic Data
2.3. KRAS Mutation Analysis
2.4. Adjuvant Chemotherapy
2.5. Primary Outcome
2.6. Secondary Outcome
2.7. Statistical Analysis
3. Results
3.1. Pathology
3.2. KRAS Mutation Analysis
3.3. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakeeb, A.; Pitt, H.A.; Sohn, T.A.; Coleman, J.; Abrams, R.A.; Piantadosi, S.; Hruban, R.H.; Lillemoe, K.D.; Yeo, C.J.; Cameron, J.L. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann. Surg. 1996, 224, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, G.; Giuliante, F.; Ardito, F.; Giovannini, I.; Aldrighetti, L.; Belli, G.; Bresadola, F.; Calise, F.; Dalla Valle, R.; D’Amico, D.F.; et al. Italian Chapter of the International Hepato-Pancreato-Biliary Association. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: Results of an Italian multicenter analysis of 440 patients. Arch. Surg. 2012, 147, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Ebata, T.; Kamiya, J.; Nishio, H.; Nagasaka, T.; Nimura, Y.; Nagino, M. The concept of perihilar cholangiocarcinoma is valid. Br. J. Surg. 2009, 96, 926–934. [Google Scholar] [CrossRef]
- Mizuno, T.; Ebata, T.; Nagino, M. Advanced hilar cholangiocarcinoma: An aggressive surgical approach for the treatment of advanced hilar cholangiocarcinoma: Perioperative management, extended procedures, and multidisciplinary approaches. Surg. Oncol. 2020, 33, 201–206. [Google Scholar] [CrossRef]
- Kambakamba, P.; Linecker, M.; Slankamenac, K.; DeOliveira, M.L. Lymph node dissection in resectable perihilar cholangiocarcinoma: A systematic review. Am. J. Surg. 2015, 210, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Giuliante, F.; Ardito, F.; Guglielmi, A.; Aldrighetti, L.; Ferrero, A.; Calise, F.; Giulini, S.M.; Jovine, E.; Breccia, C.; De Rose, A.M.; et al. Association of Lymph Node Status with Survival in Patients after Liver Resection for Hilar Cholangiocarcinoma in an Italian Multicenter Analysis. JAMA Surg. 2016, 151, 916–922. [Google Scholar] [CrossRef]
- Montal, R.; Sia, D.; Montironi, C.; Leow, W.Q.; Esteban-Fabró, R.; Pinyol, R.; Torres-Martin, M.; Bassaganyas, L.; Moeini, A.; Peix, J.; et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J. Hepatol. 2020, 73, 315–327. [Google Scholar] [CrossRef]
- Lee, H.; Wang, K.; Johnson, A.; Jones, D.M.; Ali, S.M.; Elvin, J.A.; Yelensky, R.; Lipson, D.; Miller, V.A.; Stephens, P.J.; et al. Comprehensive genomic profiling of extrahepatic cholangiocarcinoma reveals a long tail of therapeutic targets. J. Clin. Pathol. 2016, 69, 403–408. [Google Scholar] [CrossRef]
- Lowery, M.A.; Ptashkin, R.; Jordan, E.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: Potential targets for intervention. Clin. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef]
- Ruzzenente, A.; Fassan, M.; Conci, S.; Simbolo, M.; Lawlor, R.T.; Pedrazzani, C.; Capelli, P.; D’Onofrio, M.; Iacono, C.; Scarpa, A.; et al. Cholangiocarcinoma Heterogeneity Revealed by Multigene Mutational Profiling: Clinical and Prognostic Relevance in Surgically Resected Patients. Ann. Surg. Oncol. 2016, 23, 1699–1707. [Google Scholar] [CrossRef]
- Strasberg, S.M.; Belghiti, J.; Clavien, P.A. Terminology Committee of the IHPBA: Terminology of liver anatomy and resections. HPB 2000, 2, 333–339. [Google Scholar] [CrossRef]
- Giuliante, F.; Ardito, F.; Aldrighetti, L.; Ferrero, A.; Pinna, A.D.; De Carlis, L.; Cillo, U.; Jovine, E.; Portolani, N.; Gruttadauria, S.; et al. Italian Association of HepatoBilioPancreatic Surgeons-AICEP. Surgery 2021, 170, 383–389. [Google Scholar] [CrossRef]
- Neuhaus, P.; Jonas, S.; Bechstein, W.O.; Lohmann, R.; Radke, C.; Kling, N.; Wex, C.; Lobeck, H.; Hintze, R. Extended resections for hilar cholangiocarcinoma. Ann. Surg. 1999, 230, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Bismuth, H.; Corlette, M.B. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg. Gynecol. Obstet. 1975, 140, 170–178. [Google Scholar] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Ruzzenente, A.; Bagante, F.; Ardito, F.; Campagnaro, T.; Scoleri, I.; Conci, S.; Iacono, C.; Giuliante, F.; Guglielmi, A. Comparison of the 7th and 8th editions of the American Joint Committee on Cancer Staging Systems for perihilar cholangiocarcinoma. Surgery 2018, 164, 244–250. [Google Scholar] [CrossRef]
- Basso, M.; Strippoli, A.; Orlandi, A.; Martini, M.; Calegari, M.A.; Schinzari, G.; Di Salvatore, M.; Cenci, T.; Cassano, A.; Larocca, L.M.; et al. KRAS mutational status affects oxaliplatin-based chemotherapy independently from basal mRNA ERCC-1 expression in metastatic colorectal cancer patients. Br. J. Cancer 2013, 108, 115–120. [Google Scholar] [CrossRef][Green Version]
- Natalicchio, M.I.; Improta, G.; Zupa, A.; Cursio, O.E.; Stampone, E.; Possidente, L.; Teresa Gerardi, A.M.; Vita, G.; Martini, M.; Cassano, A.; et al. Pyrosequencing evaluation of low-frequency KRAS mutant alleles for EGF receptor therapy selection in metastatic colorectal carcinoma. Future Oncol. 2014, 10, 713–723. [Google Scholar] [CrossRef]
- Strippoli, A.; Cocomazzi, A.; Basso, M.; Cenci, T.; Ricci, R.; Pierconti, F.; Cassano, A.; Fiorentino, V.; Barone, C.; Bria, E.; et al. c-MYC Expression Is a Possible Keystone in the Colorectal Cancer Resistance to EGFR Inhibitors. Cancers 2020, 12, 638. [Google Scholar] [CrossRef]
- Ardito, F.; Razionale, F.; Salvatore, L.; Cenci, T.; Vellone, M.; Basso, M.; Panettieri, E.; Calegari, M.A.; Tortora, G.; Martini, M.; et al. Discordance of KRAS Mutational Status between Primary Tumors and Liver Metastases in Colorectal Cancer: Impact on Long-Term Survival Following Radical Resection. Cancers 2021, 13, 2148. [Google Scholar] [CrossRef]
- Mehrad, M.; Roy, S.; LaFramboise, W.A.; Petrosko, P.; Miller, C.; Incharoen, P.; Dacic, S. KRAS mutation is predictive of outcome in patients with pulmonary sarcomatoid carcinoma. Histopathology 2018, 73, 207–214. [Google Scholar] [CrossRef]
- Sa, J.K.; Kim, J.; Kang, S.; Kim, S.W.; Song, T.; Shim, S.H.; Choi, M.C.; No, J.H.; Song, J.; Kim, D.; et al. Somatic genomic landscape of East Asian epithelial ovarian carcinoma and its clinical implications from prospective clinical sequencing: A Korean Gynecologic Oncology Group study (KGOG 3047). Int. J. Cancer 2022, 151, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.C.; Conduit, C.; Loveland, K.L.; Thomas, B.; Lewin, J.; Tran, B. Genetics of testicular cancer: A review. Curr. Opin. Urol. 2022, 32, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.R.; Schultheis, A.M.; Yu, H.; Mandelker, D.; Ladanyi, M.; Büttner, R. Precision medicine in non-small cell lung cancer: Current applications and future directions. Semin. Cancer Biol. 2022, 84, 184–198. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, G.M.; Lowery, M.A. Moving the Needle on Precision Medicine in Pancreatic Cancer. J. Clin. Oncol. 2022, 40, 2693–2705. [Google Scholar] [CrossRef]
- Fakih, M.; Tu, H.; Hsu, H.; Aggarwal, S.; Chan, E.; Rehn, M.; Chia, V.; Kopetz, S. Real-World Study of Characteristics and Treatment Outcomes among Patients with KRAS p.G12C-Mutated or Other KRAS Mutated Metastatic Colorectal Cancer. Oncologist 2022, 27, 663–674. [Google Scholar] [CrossRef]
- Andersen, J.B.; Spee, B.; Blechacz, B.R.; Avital, I.; Komuta, M.; Barbour, A.; Conner, E.A.; Gillen, M.C.; Roskams, T.; Roberts, L.R.; et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology 2012, 142, 1021–1031. [Google Scholar] [CrossRef]
- Zheng, Y.; Qin, Y.; Gong, W.; Li, H.; Li, B.; Wang, Y.; Chao, B.; Zhao, S.; Liu, L.; Yao, S.; et al. Specific genomic alterations and prognostic analysis of perihilar cholangiocarcinoma and distal cholangiocarcinoma. J. Gastrointest. Oncol. 2021, 12, 2631–2642. [Google Scholar] [CrossRef]
- Sturm, P.D.; Baas, I.O.; Clement, M.J.; Nakeeb, A.; Johan, G.; Offerhaus, A.; Hruban, R.H.; Pitt, H.A. Alterations of the p53 tumor-suppressor gene and K-ras oncogene in perihilar cholangiocarcinomas from a high-incidence area. Int. J. Cancer 1998, 78, 695–698. [Google Scholar] [CrossRef]

| Primer | Sequence |
|---|---|
| A50 | TGTTCTAATATAGTCACATTTTCATT |
| A51 | TCCTGCACCAGTAATATGC |
| 646 | GCCTGCTGAAAATGACTGAAT |
| 647 | TTATCTGTATCAAAGAATGGTC |
| Variable | No. (%)/Median [Range] |
|---|---|
| Age | 65 [33–80] |
| Gender; (men/women) | 32/22 |
| Extent of biliary involvement | |
| (Bismuth-Corlette classification) | |
| Type 1 | 1 (1.8) |
| Type 2 | 4 (7.4) |
| Type 3 | 48 (89.0) |
| Type 3a | 24 (50.0) |
| Type 3b | 24 (50.0) |
| Type 4 | 1 (1.8) |
| Preoperative biliary drainage | |
| Yes | 32 (59.3) |
| No | 22 (40.7) |
| Percutaneous approach | 22 (68.75) |
| Endoscopic approach | 10 (31.25) |
| Preoperative right portal vein embolization | 16/23 right-sided hepatectomies (69.6) |
| Type of liver resection | |
| Right-sided hepatectomy | 23 (42.6) |
| Right hepatectomy | 7 |
| Right hepatectomy with S4 | 2 |
| Right hepatectomy with S1 | 7 |
| Right hepatectomy with S4-1 | 7 |
| Left-sided hepatectomy | 31 (57.4) |
| Left hepatectomy | 6 |
| Left hepatectomy with S1 | 25 |
| Associated caudate lobe resection | 39 (72.2) |
| Pedicle clamping | 30 (55.5) |
| Intraoperative blood transfusions | 10 (18.5) |
| Postoperative complications | 22 (40.7) |
| Adjuvant chemotherapy | 20 (37.0) |
| Variable | No. (%)/Mean ± SD [Range] |
|---|---|
| Margin status | |
| R0 | 39 (72.2) |
| R1 | 15 (27.8) |
| Perineural invasion | 37 (68.5) |
| Caudate lobe invasion | 12/39 caudate lobe resection (30.8) |
| T stage, 8th edition | |
| T1 | 3 (5.6) |
| T2a | 8 (14.8) |
| T2b | 35 (64.8) |
| T3 | 4 (7.4) |
| T4 | 4 (7.4) |
| Harvested lymph node | 5.7 ± 4.9 [1–20] |
| Lymph node status | |
| Negative | 43 (79.6) |
| N1 | 10 (18.5) |
| N2 | 1 (1.9) |
| Metastatic lymph nodes (among the total number of documented positive lymph nodes) | 2.1 ± 1.5 [1–6] |
| KRAS Mutation | No. (%) |
|---|---|
| Codon 12 | 8 (14.8) |
| p.G12D | 5 (9.3) |
| p.G12V | 3 (5.5) |
| Codon 13 | 2 (3.7) |
| p.G13D | 2 (3.7) |
| p.(Gln61Xaa) | 2 (3.7) |
| Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|
| Variable | No. (%) | 5-Year OS (%) | p-Value | HR (95% CI) | p-Value |
| Age (yr) | 0.923 | ||||
| <70 | 36 (66.7) | 43.4 | |||
| ≥70 | 18 (33.3) | 39.9 | |||
| Gender | 0.735 | ||||
| Male | 32 (59.3) | 40.9 | |||
| Female | 22 (40.7) | 41.3 | |||
| Bismuth type | 0.994 | ||||
| 1–2 | 5 (9.3) | 33.3 | |||
| 3–4 | 49 (90.7) | 42.4 | |||
| Preoperative biliary drainage | 0.366 | ||||
| Yes | 32 (59.3) | 43.0 | |||
| No | 22 (40.7) | 52.5 | |||
| Preoperative right portal vein embolization | 0.793 | ||||
| Yes | 16 (29.6) | 43.8 | |||
| No | 38 (70.4) | 41.3 | |||
| Type of liver resection | 0.619 | ||||
| Right-sided resection | 23 (42.6) | 50.5 | |||
| Left-sided resection | 31 (57.4) | 36.4 | |||
| Associated caudate lobe resection | 0.416 | ||||
| Yes | 39 (72.2) | 45.4 | |||
| No | 15 (27.8) | 31.8 | |||
| Portal vein resection | 0.394 | ||||
| Yes | 8 (14.8) | 41.7 | |||
| No | 46 (85.2) | 41.8 | |||
| Pedicle clamping | 0.642 | ||||
| Yes | 30 (55.5) | 41.8 | |||
| No | 24 (44.5) | 42.1 | |||
| Intraoperative blood transfusions | 0.547 | ||||
| Yes | 10 (18.5) | 37.0 | |||
| No | 44 (81.5) | 42.3 | |||
| Postoperative complications | 0.760 | ||||
| Yes | 22 (40.7) | 43.3 | |||
| No | 32 (59.3) | 41.2 | |||
| Margin status | 0.464 | ||||
| R0 | 39 (72.2) | 44.6 | |||
| R1 | 15 (27.8) | 33.3 | |||
| Perineural invasion | 0.072 | ||||
| Yes | 37 (68.5) | 49.4 | |||
| No | 17 (31.5) | 25.4 | |||
| Caudate lobe invasion | 0.544 | ||||
| Yes | 12 (30.8) | 59.3 | |||
| No | 27 (69.2) | 39.9 | |||
| T stage | 0.591 | ||||
| T1-T2 | 46 (85.2) | 41.1 | |||
| T3-T4 | 8 (14.8) | 50.0 | |||
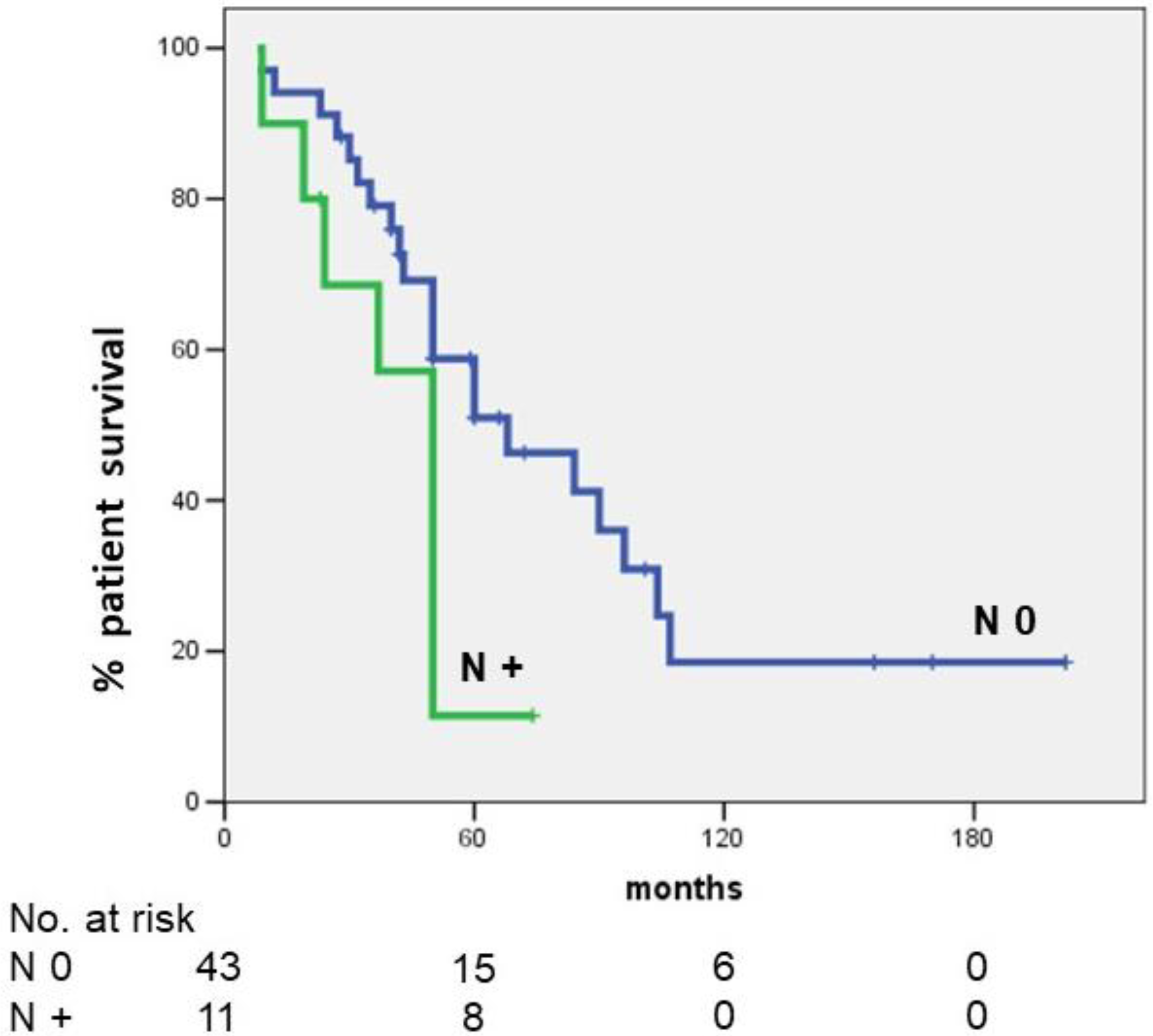
| Lymph node status | 0.039 | 2.805 (1.155–6.810) | 0.023 | ||
| Negative | 43 (79.6) | 51.0 | |||
| Metastatic | 11 (20.4) | 11.4 | |||
| Adjuvant chemotherapy | 0.269 | ||||
| Yes | 20 (37.0) | 50.5 | |||
| No | 34 (63.0) | 37.0 | |||
| KRAS mutation status | 0.003 | 5.384 (1.755–16.519) | 0.003 | ||
| wild-type | 42 (77.8) | 49.2 | |||
| mutated | 12 (22.2) | 0 | |||
| Time period | 0.097 | ||||
| 2001–2010 | 25 (46.3) | 46.8 | |||
| 2011–2019 | 29 (53.7) | 40.3 | |||
| Recurrence | 0.011 | ||||
| Yes | 33 (61.1) | 30.8 | |||
| No | 21 (38.9) | 72.7 |
| Variable, No. (%) | KRAS Mutated (No. 12) | KRAS Wild-Type (No. 42) | p-Value |
|---|---|---|---|
| Age ≥ 70 | 4/12 (33.3) | 14/42 (33.3) | 1 |
| Male | 9/12 (75.0) | 23/42 (55.0) | 0.208 |
| Preoperative biliary drainage | 6/12 (50.0) | 26/42 (61.9) | 0.459 |
| Right-sided resection | 7/12 (58.3) | 16/42 (38.1) | 0.211 |
| R0 resection | 10/12 (83.3) | 29/42 (69.0) | 0.329 |
| T stage (T3–T4) | 1/12 (8.3) | 7/42 (16.7) | 0.473 |
| Lymph node metastases | 3/12 (25.0) | 8/42 (19.0) | 0.651 |
| Presence of perineural invasion | 8/12 (66.7) | 29/42 (69.0) | 0.875 |
| Caudate lobe invasion | 4/9 (44.4) | 8/30 (26.7) | 0.310 |
| Bismuth type 3–4 | 12/12 (100) | 37/42 (88.1) | 0.209 |
| Type of recurrence (data available on 21 pts.) | 0.548 | ||
| Local recurrence | 1/4 (25.0) | 7/17 (41.2) | |
| Systemic recurrence | 3/4 (75.0) | 10/17 (58.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardito, F.; Razionale, F.; Campisi, A.; Carlino, A.; Vellone, M.; Vani, S.; Larocca, L.M.; Giuliante, F. The Impact of KRAS Mutational Status on Long-Term Survival following Liver Resection for Hilar Cholangiocarcinoma. Cancers 2022, 14, 4370. https://doi.org/10.3390/cancers14184370
Ardito F, Razionale F, Campisi A, Carlino A, Vellone M, Vani S, Larocca LM, Giuliante F. The Impact of KRAS Mutational Status on Long-Term Survival following Liver Resection for Hilar Cholangiocarcinoma. Cancers. 2022; 14(18):4370. https://doi.org/10.3390/cancers14184370
Chicago/Turabian StyleArdito, Francesco, Francesco Razionale, Andrea Campisi, Angela Carlino, Maria Vellone, Simone Vani, Luigi M. Larocca, and Felice Giuliante. 2022. "The Impact of KRAS Mutational Status on Long-Term Survival following Liver Resection for Hilar Cholangiocarcinoma" Cancers 14, no. 18: 4370. https://doi.org/10.3390/cancers14184370
APA StyleArdito, F., Razionale, F., Campisi, A., Carlino, A., Vellone, M., Vani, S., Larocca, L. M., & Giuliante, F. (2022). The Impact of KRAS Mutational Status on Long-Term Survival following Liver Resection for Hilar Cholangiocarcinoma. Cancers, 14(18), 4370. https://doi.org/10.3390/cancers14184370








