Management of Immune-Related Adverse Events from Immune-Checkpoint Inhibitors in Advanced or Metastatic Renal Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
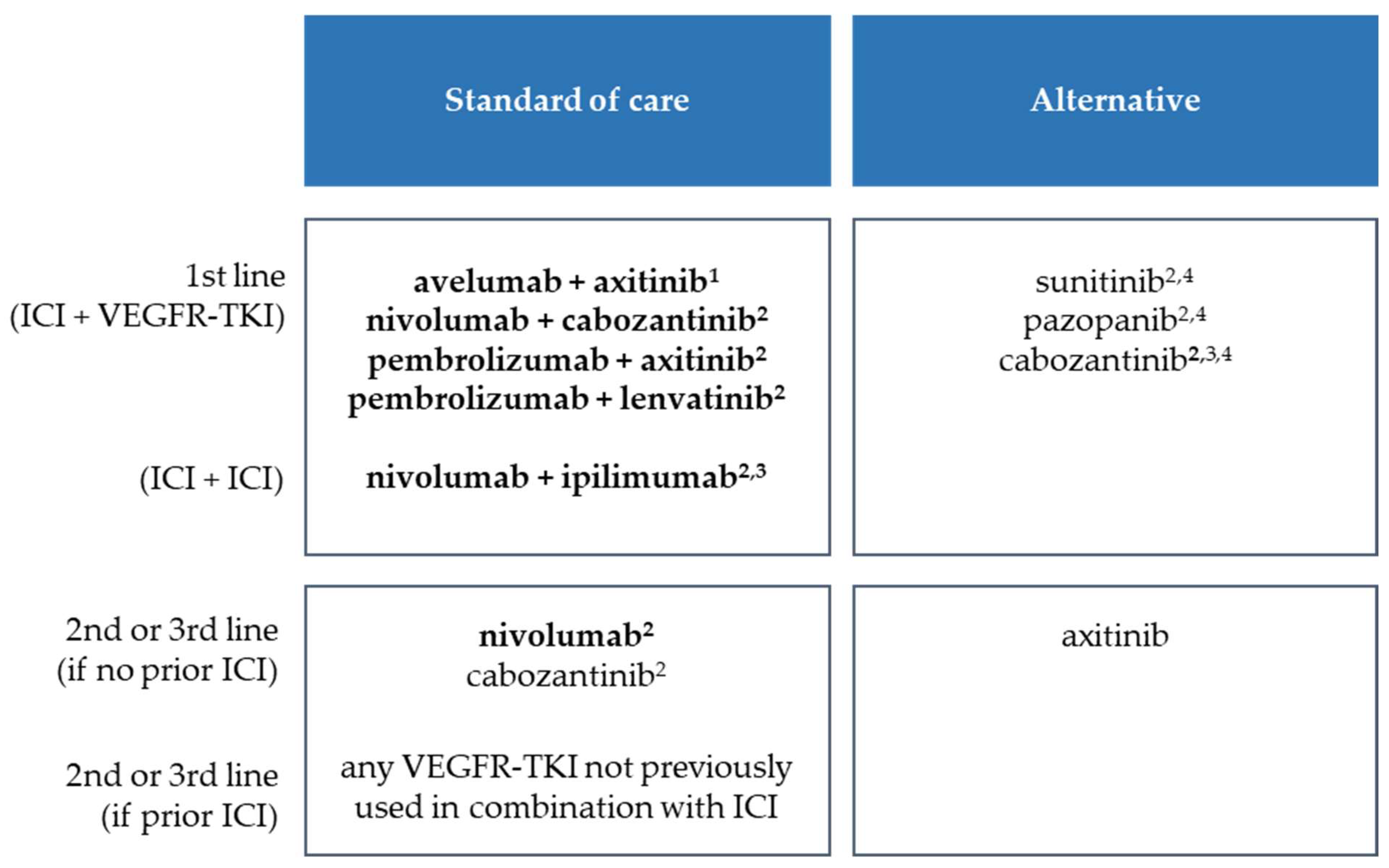
2. Current Therapeutic Situation in mRCC with Respect to Immune Checkpoint Inhibitors
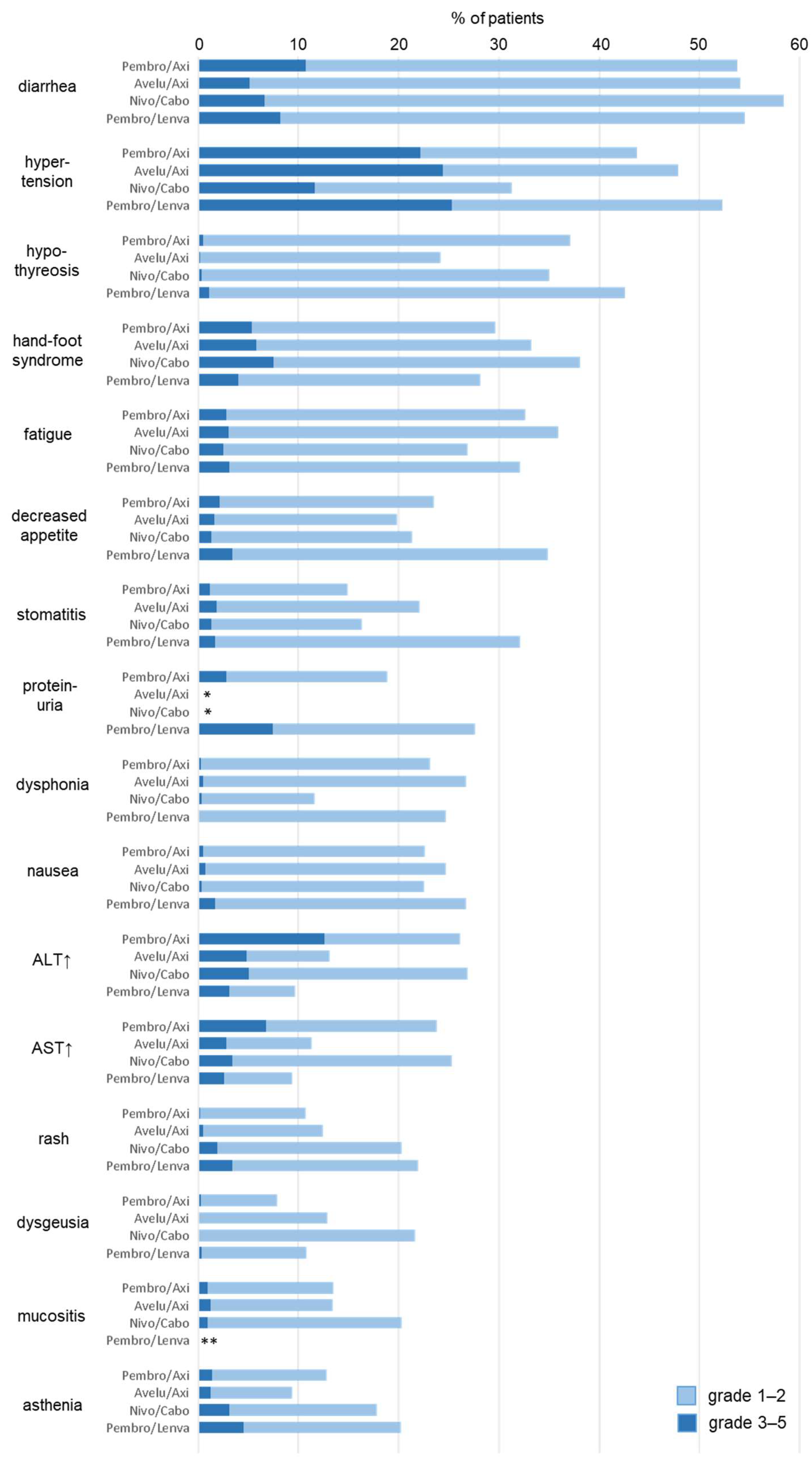
3. Frequencies of Treatment- and Immune-Related Adverse Events
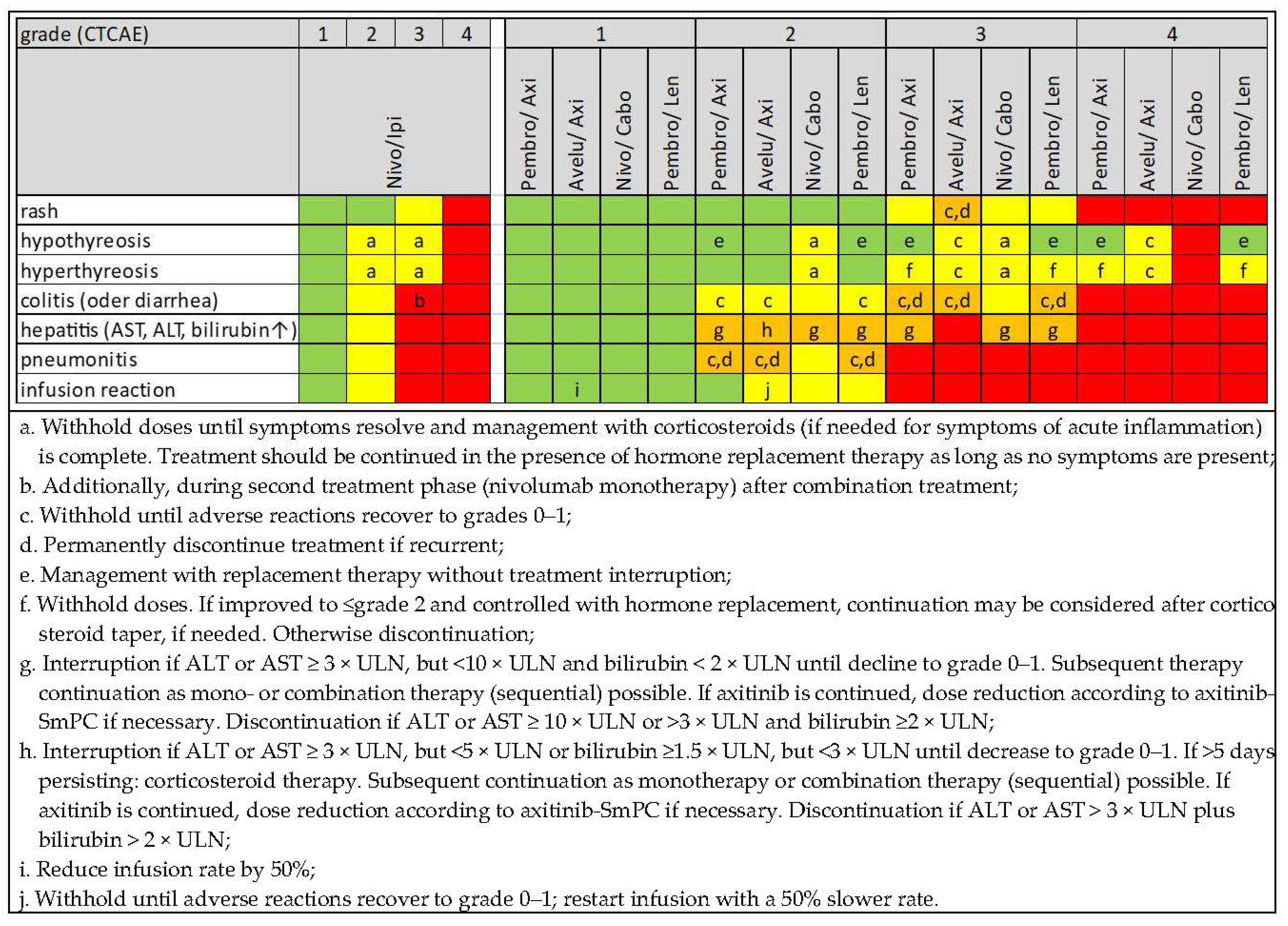
4. Early Diagnosis, Differential Diagnosis and Management of Immune-Related Adverse Events
- Diarrhoea/colitis: stool culture for exclusion of pathogenic agents.
- Hepatitis: liver ultrasound in case of increased transaminases (cholestasis, progression of liver metastasis?), hepatitis serology, testing for potentially hepatotoxic drugs/nutritional supplements.
5. Common irAE per Entity
5.1. Skin and Mucosal Toxicity
5.2. Hepatobiliary Toxicities
5.3. Gastrointestinal Toxicities
5.4. Endocrinological and Metabolic Toxicity
5.5. Pulmonary Toxicity
5.6. Renal Toxicity
5.7. Cardiac Toxicity
5.8. Neurological Toxicity
6. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Ljungberg, B.; Albiges, L.; Bedke, J.; Bex, A.; Capitanio, U.; Giles, R.H.; Hora, M.; Klatte, T.; Lam, T.; Marconi, L.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma. 2022. Available online: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Renal-Cell-Carinoma-2022.pdf (accessed on 22 July 2022).
- Baxi, S.; Yang, A.; Gennarelli, R.L.; Khan, N.; Wang, Z.; Boyce, L.; Korenstein, D. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ 2018, 360, k793. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.W.; Razak, A.R.; Bedard, P.L.; Siu, L.L.; Hansen, A.R. A systematic review of immune-related adverse event reporting in clinical trials of immune checkpoint inhibitors. Ann. Oncol. 2015, 26, 1824–1829. [Google Scholar] [CrossRef] [PubMed]
- Albiges, L.; Tannir, N.M.; Burotto, M.; McDermott, D.; Plimack, E.R.; Barthélémy, P.; Porta, C.; Powles, T.; Donskov, F.; George, S.; et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 2020, 5, e001079. [Google Scholar] [CrossRef] [PubMed]
- Apolo, A.B.; Powles, T.; Burotto, M.; Bourlon, M.T.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; Suarez, C.; Porta, C.; Barrios, C.H.; et al. Nivolumab plus cabozantinib (N + C) versus sunitinib (S) for advanced renal cell carcinoma (aRCC): Outcomes by baseline disease characteristics in the phase 3 CheckMate 9ER trial. J. Clin. Oncol. 2021, 39, 4553. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Motzer, R.J.; Rini, B.I.; Haanen, J.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Gravis-Mescam, G.; Uemura, M.; Lee, J.L.; et al. Updated efficacy results from the JAVELIN Renal 101 trial: First-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann. Oncol. 2020, 31, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.; Alekseev, B.; Rha, S.Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Waddell, T.; Gafanov, R.; Pouliot, F.; Nosov, D.; Melichar, B.; Soulieres, D.; Borchiellini, D.; et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma (ccRCC): Results from 42-month follow-up of KEYNOTE-426. J. Clin. Oncol. 2021, 39, 4500. [Google Scholar] [CrossRef]
- Larkin, J.; Fishman, M.; Wood, L.; Negrier, S.; Olivier, K.; Pyle, L.; Gorbunova, V.; Jonasch, E.; Andrews, L.; Staehler, M. Axitinib for the treatment of metastatic renal cell carcinoma: Recommendations for therapy management to optimize outcomes. Am. J. Clin. Oncol. 2014, 37, 397–403. [Google Scholar] [CrossRef]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Powles, T.; Plimack, E.R.; Soulières, D.; Waddell, T.; Stus, V.; Gafanov, R.; Nosov, D.; Pouliot, F.; Melichar, B.; Vynnychenko, I.; et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): Extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 21, 1563–1573. [Google Scholar] [CrossRef]
- Motzer, R.J.; Choueiri, T.K.; Powles, T.; Burotto, M.; Bourlon, M.T.; Hsieh, J.J.; Maruzzo, M.; Shah, A.Y.; Suarez, C.; Barrios, C.H.; et al. Nivolumab + cabozantinib (NIVO + CABO) versus sunitinib (SUN) for advanced renal cell carcinoma (aRCC): Outcomes by sarcomatoid histology and updated trial results with extended follow-up of CheckMate 9ER. J. Clin. Oncol. 2021, 39, 308. [Google Scholar] [CrossRef]
- Motzer, R.J.; Rini, B.I.; McDermott, D.F.; Aren Frontera, O.; Hammers, H.J.; Carducci, M.A.; Salman, P.; Escudier, B.; Beuselinck, B.; Amin, A.; et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: Extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019, 20, 1370–1385. [Google Scholar] [CrossRef]
- Heinzerling, L.; de Toni, E.N.; Schett, G.; Hundorfean, G.; Zimmer, L. Checkpoint Inhibitors. Dtsch. Arztebl. Int. 2019, 116, 119–126. [Google Scholar] [CrossRef]
- Khan, O.F.; Monzon, J. Diagnosis, monitoring, and management of adverse events from immune checkpoint inhibitor therapy. Curr. Oncol. 2020, 27, S43–S50. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulieres, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Bedke, J.; Rini, B.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Soulieres, D.; Melichar, B.; et al. Health-related quality-of-life (HRQoL) analysis from KEYNOTE-426: Pembrolizumab (pembro) plus axitinib (axi) vs sunitinib for advanced renal cell carcinoma (RCC). In Proceedings of the 35th Annual EAU Congress, Virtual, 17–19 July 2020. [Google Scholar]
- Choueiri, T.K.; Eto, M.; Kopyltsov, E.; Rha, S.Y.; Porta, C.; Motzer, R.; Grünwald, V.; Hutson, T.E.; Méndez-Vidal, M.J.; Hong, S.H.; et al. Phase 3 CLEAR Trial in Advanced Renal Cell Carcinoma: Outcomes in Subgroups and Toxicity Update. In Proceedings of the ESMO 2021, Virtual, 16–21 September 2021. [Google Scholar]
- Fabi, A.; Bhargava, R.; Fatigoni, S.; Guglielmo, M.; Horneber, M.; Roila, F.; Weis, J.; Jordan, K.; Ripamonti, C.I.; ESMO Guidelines Committee. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann. Oncol. 2020, 31, 713–723. [Google Scholar] [CrossRef]
- Bossi, P.; Antonuzzo, A.; Cherny, N.I.; Rosengarten, O.; Pernot, S.; Trippa, F.; Schuler, U.; Snegovoy, A.; Jordan, K.; Ripamonti, C.I.; et al. Diarrhoea in adult cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29 (Suppl. S4), iv126–iv142. [Google Scholar] [CrossRef]
- Razvi, Y.; Chan, S.; McFarlane, T.; McKenzie, E.; Zaki, P.; DeAngelis, C.; Pidduck, W.; Bushehri, A.; Chow, E.; Jerzak, K.J. ASCO, NCCN, MASCC/ESMO: A comparison of antiemetic guidelines for the treatment of chemotherapy-induced nausea and vomiting in adult patients. Support. Care Cancer 2019, 27, 87–95. [Google Scholar] [CrossRef]
- Rosello, S.; Blasco, I.; Garcia Fabregat, L.; Cervantes, A.; Jordan, K.; Committee, E.G. Management of infusion reactions to systemic anticancer therapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv260. [Google Scholar] [CrossRef] [PubMed]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.I.; Committee, E.G. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv166–iv191. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Cardinale, D.; Suter, T.; Plataniotis, G.; de Azambuja, E.; Sandri, M.T.; Criscitiello, C.; Goldhirsch, A.; Cipolla, C.; Roila, F.; et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2012, 23 (Suppl. S7), vii155–vii166. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; Committee, E.G. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv264–iv266. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Suzman, D.L.; Pelosof, L.; Rosenberg, A.; Avigan, M.I. Hepatotoxicity of immune checkpoint inhibitors: An evolving picture of risk associated with a vital class of immunotherapy agents. Liver Int. 2018, 38, 976–987. [Google Scholar] [CrossRef]
- Chmiel, K.D.; Suan, D.; Liddle, C.; Nankivell, B.; Ibrahim, R.; Bautista, C.; Thompson, J.; Fulcher, D.; Kefford, R. Resolution of severe ipilimumab-induced hepatitis after antithymocyte globulin therapy. J. Clin. Oncol. 2011, 29, e237-240. [Google Scholar] [CrossRef]
- Arnaud-Coffin, P.; Maillet, D.; Gan, H.K.; Stelmes, J.J.; You, B.; Dalle, S.; Péron, J. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int. J. Cancer 2019, 145, 639–648. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Allolio, B.; Arlt, W.; Barthel, A.; Don-Wauchope, A.; Hammer, G.D.; Husebye, E.S.; Merke, D.P.; Murad, M.H.; Stratakis, C.A.; et al. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 364–389. [Google Scholar] [CrossRef]
- Castillero, F.; Castillo-Fernandez, O.; Jimenez-Jimenez, G.; Fallas-Ramirez, J.; Peralta-Alvarez, M.P.; Arrieta, O. Cancer immunotherapy-associated hypophysitis. Future Oncol. 2019, 15, 3159–3169. [Google Scholar] [CrossRef] [Green Version]
- Grimm, M.O.; Oppel-Heuchel, H.; Foller, S. Treatment with PD-1/PD-L1 and CTLA-4 immune checkpoint inhibitors: Immune-mediated side effects. Urologe A 2018, 57, 543–551. [Google Scholar] [CrossRef]
- Delasos, L.; Bazewicz, C.; Sliwinska, A.; Lia, N.L.; Vredenburgh, J. New onset diabetes with ketoacidosis following nivolumab immunotherapy: A case report and review of literature. J. Oncol. Pharm. Pract. 2020, 27, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Inthasot, V.; Bruyneel, M.; Muylle, I.; Ninane, V. Severe pulmonary infections complicating nivolumab treatment for lung cancer: A report of two cases. Acta Clin. Belg. 2020, 75, 308–310. [Google Scholar] [CrossRef]
- Herrmann, S.M.; Perazella, M.A. Immune Checkpoint Inhibitors and Immune-Related Adverse Renal Events. Kidney Int. Rep. 2020, 5, 1139–1148. [Google Scholar] [CrossRef]
- Spallarossa, P.; Sarocchi, M.; Tini, G.; Arboscello, E.; Toma, M.; Ameri, P.; Porto, I. How to Monitor Cardiac Complications of Immune Checkpoint Inhibitor Therapy. Front. Pharmacol. 2020, 11, 972. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Tummala, S.; de Groot, J. Neurologic Toxicities of Cancer Immunotherapies: A Review. Curr. Neurol. Neurosci. Rep. 2020, 20, 27. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leucht, K.; Ali, N.; Foller, S.; Grimm, M.-O. Management of Immune-Related Adverse Events from Immune-Checkpoint Inhibitors in Advanced or Metastatic Renal Cell Carcinoma. Cancers 2022, 14, 4369. https://doi.org/10.3390/cancers14184369
Leucht K, Ali N, Foller S, Grimm M-O. Management of Immune-Related Adverse Events from Immune-Checkpoint Inhibitors in Advanced or Metastatic Renal Cell Carcinoma. Cancers. 2022; 14(18):4369. https://doi.org/10.3390/cancers14184369
Chicago/Turabian StyleLeucht, Katharina, Nalyan Ali, Susan Foller, and Marc-Oliver Grimm. 2022. "Management of Immune-Related Adverse Events from Immune-Checkpoint Inhibitors in Advanced or Metastatic Renal Cell Carcinoma" Cancers 14, no. 18: 4369. https://doi.org/10.3390/cancers14184369
APA StyleLeucht, K., Ali, N., Foller, S., & Grimm, M.-O. (2022). Management of Immune-Related Adverse Events from Immune-Checkpoint Inhibitors in Advanced or Metastatic Renal Cell Carcinoma. Cancers, 14(18), 4369. https://doi.org/10.3390/cancers14184369





