Benefits from Adjuvant Chemotherapy in Patients with Resected Non-Small Cell Lung Cancer: Possibility of Stratification by Gene Amplification of ACTN4 According to Evaluation of Metastatic Ability
Abstract
Simple Summary
Abstract
1. Introduction
2. Adjuvant Chemotherapy in NSCLC
3. Current Biomarker Candidates for Perioperative Patients with NSCLC
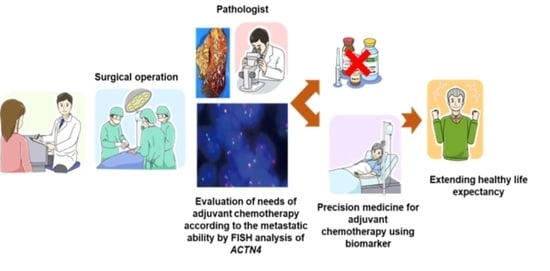
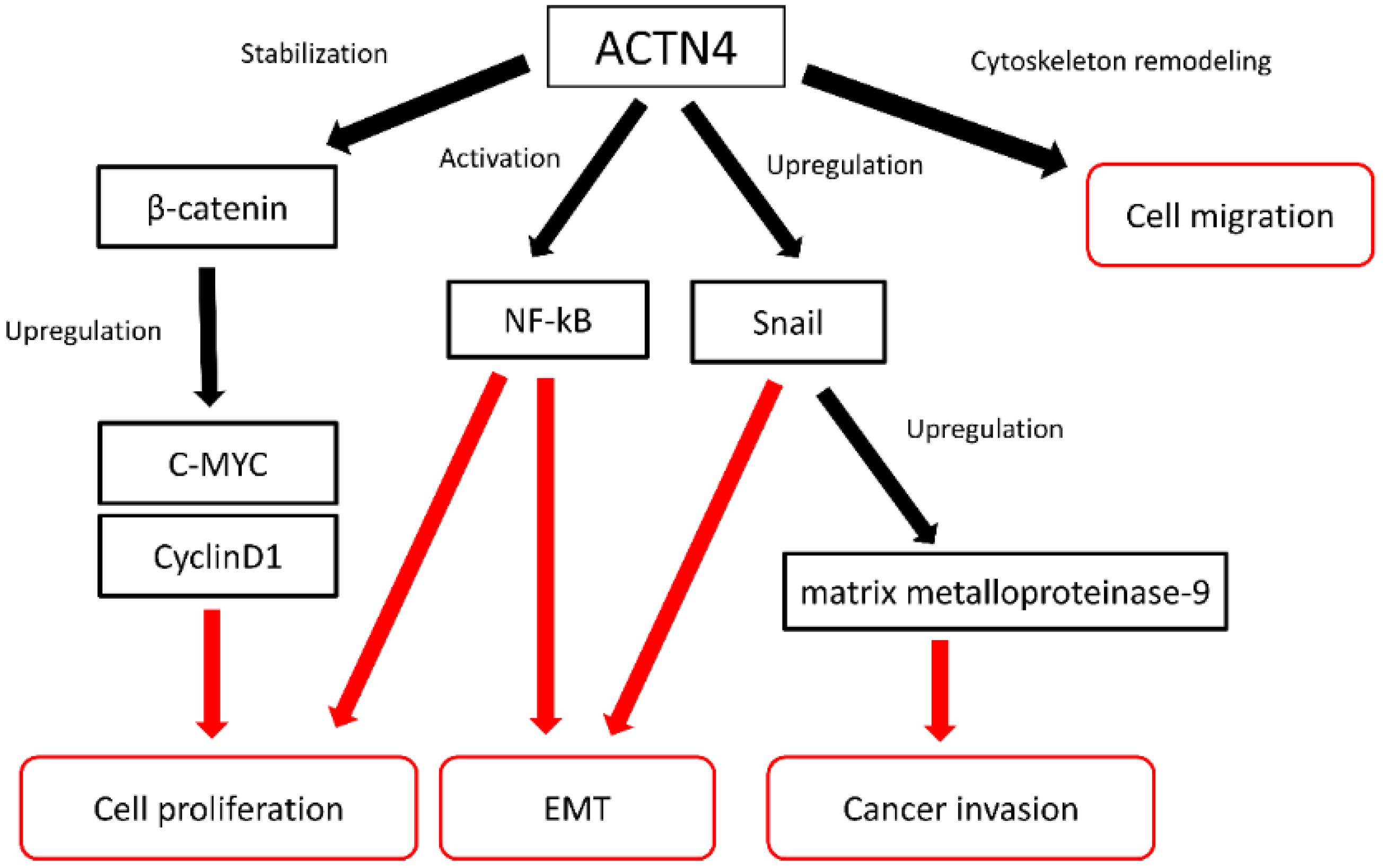
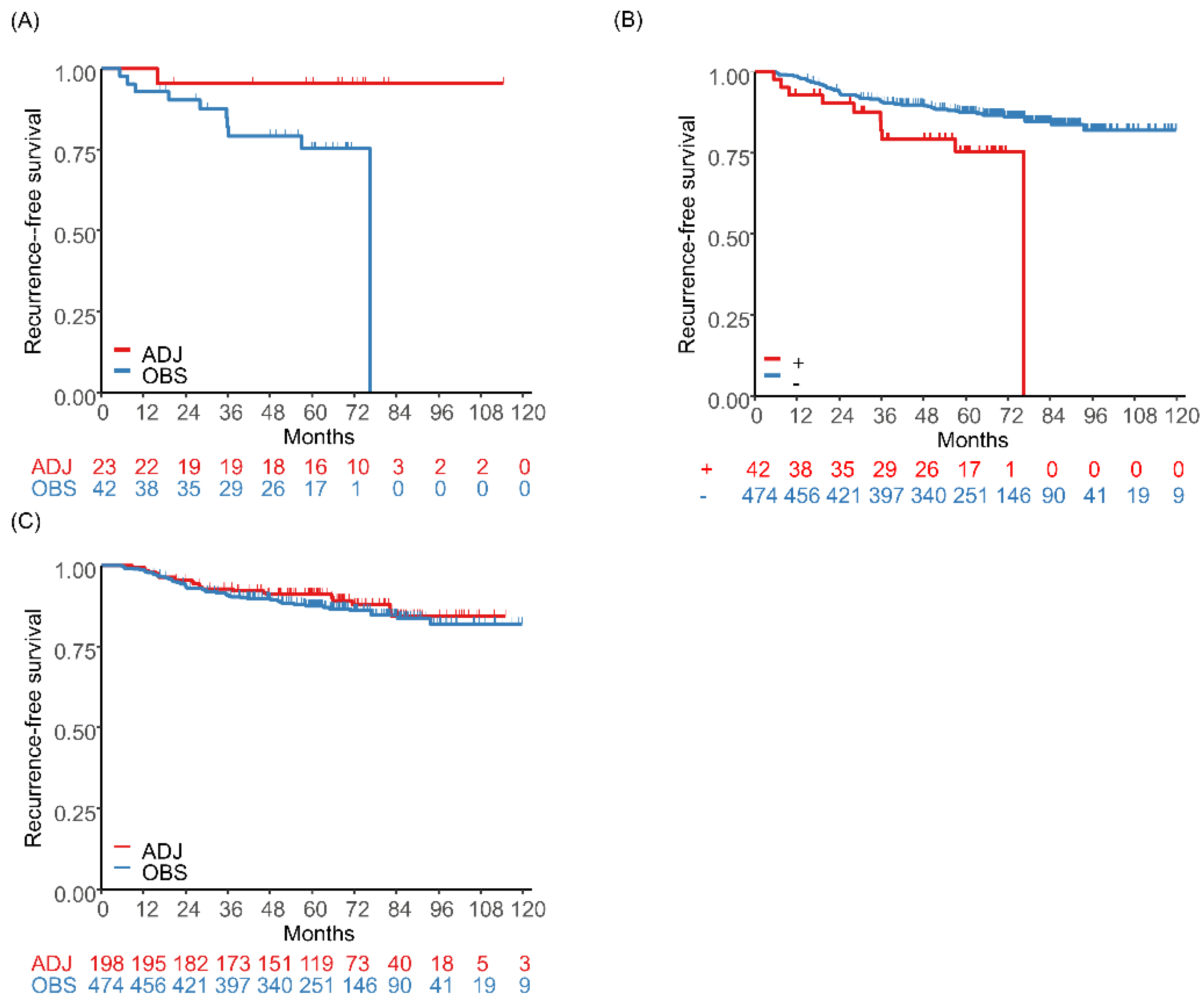
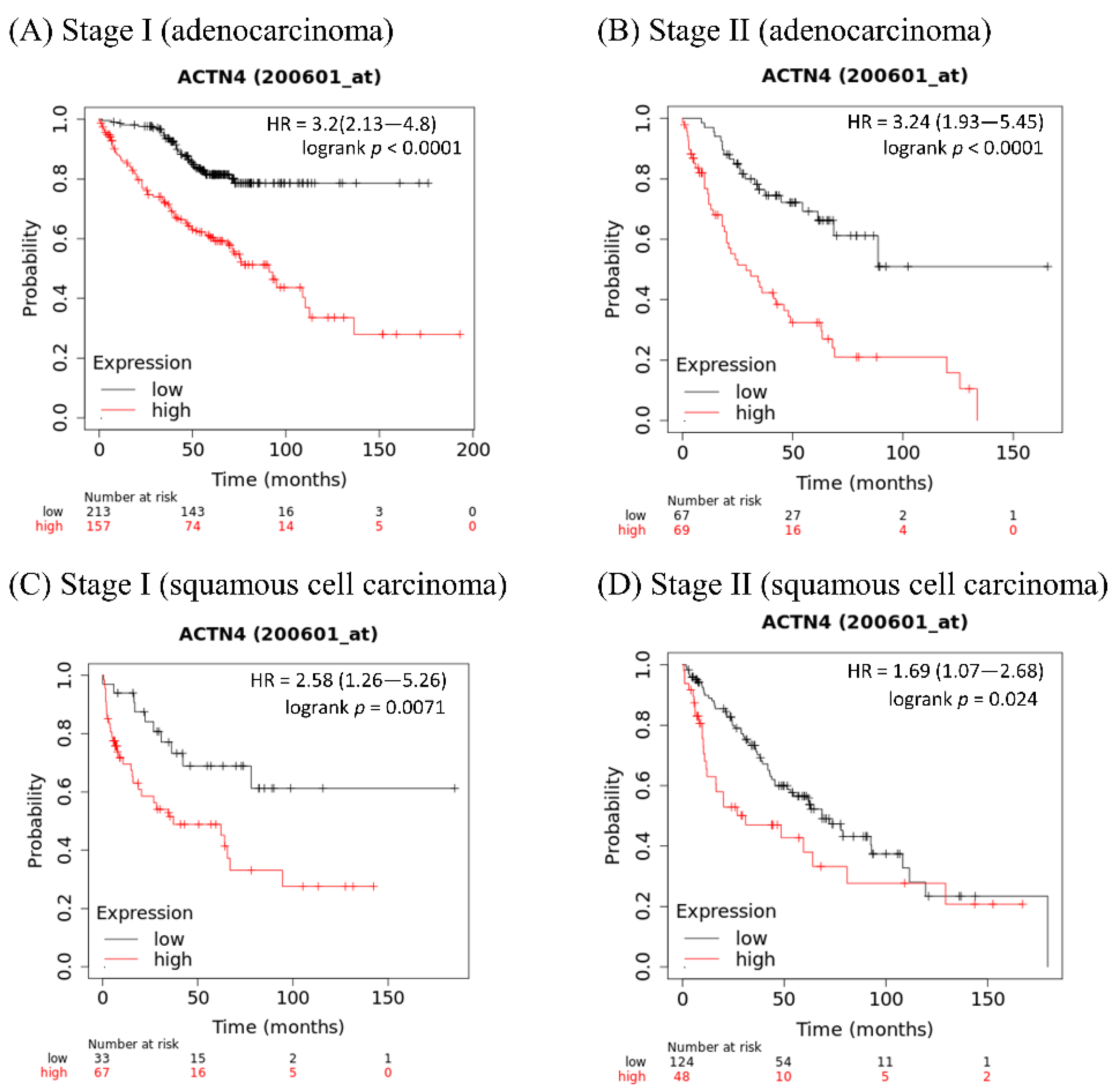
4. ACTN4 as a Biomarker for Evaluation of Metastatic Ability
5. Summary of the Advantages and Limitations of Perioperative Biomarkers
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Vansteenkiste, J.; Wauters, E.; Reymen, B.; Ackermann, C.J.; Peters, S.; De Ruysscher, D. Current status of immune checkpoint inhibition in early-stage NSCLC. Ann. Oncol. 2019, 30, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming [Eighth] Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.-P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.-Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer [IMpower010]: A randomised; multicentre; open-label; phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Ng, R.; Hasan, B.; Mittmann, N.; Florescu, M.; Shepherd, F.A.; Ding, K.; Butts, C.A.; Cormier, Y.; Darling, G.; Goss, G.D.; et al. Economic analysis of NCIC CTG JBR.10: A randomized trial of adjuvant vinorelbine plus cisplatin compared with observation in early stage non-small-cell lung cancer--a report of the Working Group on Economic Analysis, and the Lung Disease Site Group, National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007, 25, 2256–2261. [Google Scholar]
- Zafar, S.Y.; Peppercorn, J.M.; Schrag, D.; Taylor, D.H.; Goetzinger, A.M.; Zhong, X.; Abernethy, A.P. The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist 2013, 18, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Tran, G.; Zafar, S.Y. Financial toxicity and implications for cancer care in the era of molecular and immune therapies. Ann. Transl. Med. 2018, 6, 166. [Google Scholar] [CrossRef]
- Robles, A.I.; Arai, E.; Mathé, E.A.; Okayama, H.; Schetter, A.J.; Brown, D.; Petersen, D.; Bowman, E.D.; Noro, R.; Welsh, J.A.; et al. An Integrated Prognostic Classifier for Stage I Lung Adenocarcinoma Based on mRNA, microRNA, and DNA Methylation Biomarkers. J. Thorac. Oncol. 2015, 10, 1037–1048. [Google Scholar]
- Zuo, S.; Wei, M.; Zhang, H.; Chen, A.; Wu, J.; Wei, J.; Dong, J. A robust six-gene prognostic signature for prediction of both disease-free and overall survival in non-small cell lung cancer. J. Transl. Med. 2019, 17, 152. [Google Scholar] [PubMed]
- Li, B.; Cui, Y.; Diehn, M.; Li, R. Development and Validation of an Individualized Immune Prognostic Signature in Early-Stage Nonsquamous Non-Small Cell Lung Cancer. JAMA Oncol. 2017, 3, 1529–1537. [Google Scholar] [CrossRef]
- Shahid, M.; Choi, T.G.; Nguyen, M.N.; Matondo, A.; Jo, Y.H.; Yoo, J.Y.; Nguyen, N.N.Y.; Yun, H.R.; Kim, J.; Akter, S.; et al. An 8-gene signature for prediction of prognosis and chemoresponse in non-small cell lung cancer. Oncotarget 2016, 7, 86561–86572. [Google Scholar]
- Xu, W.; Jia, G.; Davie, J.R.; Murphy, L.; Kratzke, R.; Banerji, S. A 10-Gene Yin Yang Expression Ratio Signature for Stage IA and IB Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2016, 11, 2150–2160. [Google Scholar] [PubMed]
- Leng, S.; Do, K.; Yingling, C.M.; Picchi, M.A.; Wolf, H.J.; Kennedy, T.C.; Feser, W.J.; Baron, A.E.; Franklin, W.A.; Brock, M.V.; et al. Defining a gene promoter methylation signature in sputum for lung cancer risk assessment. Clin. Cancer Res. 2012, 18, 3387–3395. [Google Scholar] [PubMed]
- Chen, H.-Y.; Yu, S.-L.; Chen, C.-H.; Chang, G.-C.; Chen, C.-Y.; Yuan, A.; Cheng, C.-L.; Wang, C.-H.; Terng, H.-J.; Kao, S.-F.; et al. A five-gene signature and clinical outcome in non-small-cell lung cancer. N. Engl. J. Med. 2007, 356, 11–20. [Google Scholar]
- Chen, D.-T.; Hsu, Y.-L.; Fulp, W.J.; Coppola, D.; Haura, E.B.; Yeatman, T.J.; Cress, W.D. Prognostic and predictive value of a malignancy-risk gene signature in early-stage non-small cell lung cancer. J. Natl. Cancer Inst. 2011, 103, 1859–1870. [Google Scholar]
- Xie, Y.; Xiao, G.; Coombes, K.R.; Behrens, C.; Solis, L.M.; Raso, G.; Girard, L.; Erickson, H.S.; Roth, J.; Heymach, J.V.; et al. Robust gene expression signature from formalin-fixed paraffin-embedded samples predicts prognosis of non-small-cell lung cancer patients. Clin. Cancer Res. 2011, 17, 5705–5714. [Google Scholar] [PubMed]
- Subramanian, J.; Simon, R. Gene expression-based prognostic signatures in lung cancer: Ready for clinical use? J. Natl. Cancer Inst. 2010, 102, 464–474. [Google Scholar]
- Seike, M.; Yanaihara, N.; Bowman, E.D.; Zanetti, K.A.; Budhu, A.; Kumamoto, K.; Mechanic, L.E.; Matsumoto, S.; Yokota, J.; Shibata, T.; et al. Use of a cytokine gene expression signature in lung adenocarcinoma and the surrounding tissue as a prognostic classifier. J. Natl. Cancer Inst. 2007, 99, 1257–1269. [Google Scholar]
- Okayama, H.; Kohno, T.; Ishii, Y.; Shimada, Y.; Shiraishi, K.; Iwakawa, R.; Furuta, K.; Tsuta, K.; Shibata, T.; Yamamoto, S.; et al. Identification of genes upregulated in ALK-positive and EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res. 2012, 72, 100–111. [Google Scholar] [PubMed]
- Zheng, Z.; Chen, T.; Li, X.; Haura, E.; Sharma, A.; Bepler, G. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N. Engl. J. Med. 2007, 356, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Olaussen, K.A.; Dunant, A.; Fouret, P.; Brambilla, E.; André, F.; Haddad, V.; Taranchon, E.; Filipits, M.; Pirker, R.; Popper, H.H.; et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N. Engl. J. Med. 2006, 355, 983–991. [Google Scholar]
- Friboulet, L.; Olaussen, K.A.; Pignon, J.-P.; Shepherd, F.A.; Tsao, M.-S.; Graziano, S.; Kratzke, R.; Douillard, J.-Y.; Seymour, L.; Pirker, R.; et al. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N. Engl. J. Med. 2013, 368, 1101–1110. [Google Scholar]
- Honda, K.; Yamada, T.; Endo, R.; Ino, Y.; Gotoh, M.; Tsuda, H.; Yamada, Y.; Chiba, H.; Hirohashi, S. Actinin-4; a novel actin-bundling protein associated with cell motility and cancer invasion. J. Cell Biol. 1998, 140, 1383–1393. [Google Scholar] [PubMed]
- Hayashida, Y.; Honda, K.; Idogawa, M.; Ino, Y.; Ono, M.; Tsuchida, A.; Aoki, T.; Hirohashi, S.; Yamada, T. E-cadherin regulates the association between beta-catenin and actinin-4. Cancer Res. 2005, 65, 8836–8845. [Google Scholar] [PubMed]
- Honda, K. The biological role of actinin-4 [ACTN4] in malignant phenotypes of cancer. Cell Biosci. 2015, 5, 41. [Google Scholar] [PubMed]
- Kikuchi, S.; Honda, K.; Tsuda, H.; Hiraoka, N.; Imoto, I.; Kosuge, T.; Umaki, T.; Onozato, K.; Shitashige, M.; Yamaguchi, U.; et al. Expression and gene amplification of actinin-4 in invasive ductal carcinoma of the pancreas. Clin. Cancer Res. 2008, 14, 5348–5356. [Google Scholar]
- Yamamoto, S.; Tsuda, H.; Honda, K.; Onozato, K.; Takano, M.; Tamai, S.; Imoto, I.; Inazawa, J.; Yamada, T.; Matsubara, O. Actinin-4 gene amplification in ovarian cancer: A candidate oncogene associated with poor patient prognosis and tumor chemoresistance. Mod. Pathol. 2009, 22, 499–507. [Google Scholar]
- Sugano, T.; Yoshida, M.; Masuda, M.; Ono, M.; Tamura, K.; Kinoshita, T.; Tsuda, H.; Honda, K.; Gemma, A.; Yamada, T. Prognostic impact of ACTN4 gene copy number alteration in hormone receptor-positive; HER2-negative; node-negative invasive breast carcinoma. Br. J. Cancer 2020, 122, 1811–1817. [Google Scholar]
- Miura, N.; Kamita, M.; Kakuya, T.; Fujiwara, Y.; Tsuta, K.; Shiraishi, H.; Takeshita, F.; Ochiya, T.; Shoji, H.; Huang, W.; et al. Efficacy of adjuvant chemotherapy for non-small cell lung cancer assessed by metastatic potential associated with ACTN4. Oncotarget 2016, 7, 33165–33178. [Google Scholar]
- Shiraishi, H.; Fujiwara, Y.; Kakuya, T.; Tsuta, K.; Motoi, N.; Miura, N.; Watabe, Y.; Watanabe, S.-I.; Noro, R.; Nagashima, K.; et al. Actinin-4 protein overexpression as a predictive biomarker in adjuvant chemotherapy for resected lung adenocarcinoma. Biomark. Med. 2017, 11, 721–731. [Google Scholar] [PubMed]
- Noro, R.; Honda, K.; Nagashima, K.; Motoi, N.; Kunugi, S.; Matsubayashi, J.; Takeuchi, S.; Shiraishi, H.; Okano, T.; Kashiro, A.; et al. Alpha-actinin-4 [ACTN4] gene amplification is a predictive biomarker for adjuvant chemotherapy with tegafur/uracil in stage I lung adenocarcinomas. Cancer Sci. 2022, 113, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, R.; Bergman, B.; Dunant, A.; Le Chevalier, T.; Pignon, J.-P.; Vansteenkiste, J.; International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N. Engl. J. Med. 2004, 350, 351–360. [Google Scholar]
- Douillard, J.-Y.; Rosell, R.; De Lena, M.; Carpagnano, F.; Ramlau, R.; Gonzáles-Larriba, J.L.; Grodzki, T.; Pereira, J.R.; Le Groumellec, A.; Lorusso, V.; et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer [Adjuvant Navelbine International Trialist Association [ANITA]]: A randomised controlled trial. Lancet Oncol. 2006, 7, 719–727. [Google Scholar]
- Winton, T.; Livingston, R.; Johnson, D.; Rigas, J.; Johnston, M.; Butts, C.; Cormier, Y.; Goss, G.; Inculet, R.; Vallieres, E.; et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N. Engl. J. Med. 2005, 352, 2589–2597. [Google Scholar] [PubMed]
- Hamada, C.; Tsuboi, M.; Ohta, M.; Fujimura, S.; Kodama, K.; Imaizumi, M.; Wada, H. Effect of postoperative adjuvant chemotherapy with tegafur-uracil on survival in patients with stage IA non-small cell lung cancer: An exploratory analysis from a meta-analysis of six randomized controlled trials. J. Thorac. Oncol. 2009, 4, 1511–1516. [Google Scholar] [PubMed]
- Hamada, C.; Tanaka, F.; Ohta, M.; Fujimura, S.; Kodama, K.; Imaizumi, M.; Wada, H. Meta-analysis of postoperative adjuvant chemotherapy with tegafur-uracil in non-small-cell lung cancer. J. Clin. Oncol. 2005, 23, 4999–5006. [Google Scholar]
- Kelly, K.; Altorki, N.K.; Eberhardt, W.E.E.; O’Brien, M.E.R.; Spigel, D.R.; Crinò, L.; Tsai, C.-M.; Kim, J.-H.; Cho, E.K.; Hoffman, P.C.; et al. Adjuvant Erlotinib Versus Placebo in Patients with Stage IB-IIIA Non-Small-Cell Lung Cancer [RADIANT]: A Randomized; Double-Blind; Phase III Trial. J. Clin. Oncol. 2015, 33, 4007–4014. [Google Scholar] [PubMed]
- Zhong, W.-Z.; Wang, Q.; Mao, W.-M.; Xu, S.-T.; Wu, L.; Shen, Y.; Liu, Y.-Y.; Chen, C.; Cheng, Y.; Xu, L.; et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA [N1-N2] EGFR-mutant NSCLC [ADJUVANT/CTONG1104]: A randomised; open-label; phase 3 study. Lancet Oncol. 2018, 19, 139–148. [Google Scholar] [CrossRef]
- Tada, H.; Mitsudomi, T.; Misumi, T.; Sugio, K.; Tsuboi, M.; Okamoto, I.; Iwamoto, Y.; Sakakura, N.; Sugawara, S.; Atagi, S.; et al. Randomized Phase III Study of Gefitinib Versus Cisplatin Plus Vinorelbine for Patients with Resected Stage II-IIIA Non-Small-Cell Lung Cancer with EGFR Mutation [IMPACT]. J. Clin. Oncol. 2022, 40, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.-E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer [CheckMate 9LA]: An international; randomised; open-label; phase 3 trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Blumenthal, G.M.; Bunn, P.A., Jr.; Chaft, J.E.; McCoach, C.E.; Perez, E.A.; Scagliotti, G.V.; Carbone, D.P.; Aerts, H.J.W.L.; Aisner, D.L.; Bergh, J.; et al. Current Status and Future Perspectives on Neoadjuvant Therapy in Lung Cancer. J. Thorac. Oncol. 2018, 13, 1818–1831. [Google Scholar] [CrossRef]
- Soh, J.; Hamada, A.; Fujino, T.; Mitsudomi, T. Perioperative Therapy for Non-Small Cell Lung Cancer with Immune Checkpoint Inhibitors. Cancers 2021, 13, 4035. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Kandioler, D.; Stamatis, G.; Eberhardt, W.; Kappel, S.; Zöchbauer-Müller, S.; Kührer, I.; Mittlböck, M.; Zwrtek, R.; Aigner, C.; Bichler, C.; et al. Growing clinical evidence for the interaction of the p53 genotype and response to induction chemotherapy in advanced non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2008, 135, 1036–1041. [Google Scholar] [CrossRef][Green Version]
- Scoccianti, C.; Vesin, A.; Martel, G.; Olivier, M.; Brambilla, E.; Timsit, J.-F.; Tavecchio, L.; Brambilla, C.; Field, J.K.; Hainaut, P.; et al. Prognostic value of TP53; KRAS and EGFR mutations in nonsmall cell lung cancer: The EUELC cohort. Eur. Respir. J. 2012, 40, 177–184. [Google Scholar] [CrossRef]
- Ma, X.; Le Teuff, G.; Lacas, B.; Tsao, M.S.; Graziano, S.; Pignon, J.-P.; Douillard, J.-Y.; Le Chevalier, T.; Seymour, L.; Filipits, M.; et al. Prognostic and Predictive Effect of TP53 Mutations in Patients with Non-Small Cell Lung Cancer from Adjuvant Cisplatin-Based Therapy Randomized Trials: A LACE-Bio Pooled Analysis. J. Thorac. Oncol. 2016, 11, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.H.; Adak, S.; Feins, R.H.; Keller, S.M.; Fry, W.A.; Livingston, R.B.; Hammond, M.E.; Wolf, B.; Sabatini, L.; Jett, J.; et al. Lack of prognostic significance of p53 and K-ras mutations in primary resected non-small-cell lung cancer on E4592: A Laboratory Ancillary Study on an Eastern Cooperative Oncology Group Prospective Randomized Trial of Postoperative Adjuvant Therapy. J. Clin. Oncol. 2001, 19, 448–457. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef]
- Gross, D.J.; Chintala, N.K.; Vaghjiani, R.G.; Grosser, R.; Tan, K.S.; Li, X.; Choe, J.; Li, Y.; Aly, R.G.; Emoto, K.; et al. Tumor and Tumor-Associated Macrophage Programmed Death-Ligand 1 Expression Is Associated with Adjuvant Chemotherapy Benefit in Lung Adenocarcinoma. J. Thorac. Oncol. 2022, 17, 89–102. [Google Scholar] [CrossRef]
- Tsao, M.S.; Le Teuff, G.; Shepherd, F.A.; Landais, C.; Hainaut, P.; Filipits, M.; Pirker, R.; Le Chevalier, T.; Graziano, S.; Kratze, R.; et al. PD-L1 protein expression assessed by immunohistochemistry is neither prognostic nor predictive of benefit from adjuvant chemotherapy in resected non-small cell lung cancer. Ann. Oncol. 2017, 28, 882–889. [Google Scholar] [CrossRef]
- Ohara, S.; Suda, K.; Sakai, K.; Nishino, M.; Chiba, M.; Shimoji, M.; Takemoto, T.; Fujino, T.; Koga, T.; Hamada, A.; et al. Prognostic implications of preoperative versus postoperative circulating tumor DNA in surgically resected lung cancer patients: A pilot study. Transl. Lung Cancer Res. 2020, 9, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.A.; Chabon, J.J.; Lovejoy, A.F.; Newman, A.M.; Stehr, H.; Azad, T.D.; Khodadoust, M.S.; Esfahani, M.S.; Liu, C.L.; Zhou, L.; et al. Early Detection of Molecular Residual Disease in Localized Lung Cancer by Circulating Tumor DNA Profiling. Cancer Discov. 2017, 7, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Abbosh, C.; Birkbak, N.J.; Wilson, G.A.; Jamal-Hanjani, M.; Constantin, T.; Salari, R.; Le Quesne, J.; Moore, D.A.; Veeriah, S.; Rosenthal, R.; et al. Phylogenetic ERCC1 analysis depicts early-stage lung cancer evolution. Nature 2017, 545, 446–451. [Google Scholar] [CrossRef]
- Qiu, B.; Guo, W.; Zhang, F.; Lv, F.; Ji, Y.; Peng, Y.; Chen, X.; Bao, H.; Xu, Y.; Shao, Y.; et al. Dynamic recurrence risk and adjuvant chemotherapy benefit prediction by ctDNA in resected NSCLC. Nat. Commun. 2021, 12, 6770. [Google Scholar] [CrossRef] [PubMed]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef]
- Newman, A.M.; Bratman, S.V.; To, J.; Wynne, J.F.; Eclov, N.C.W.; Modlin, L.A.; Liu, C.L.; Neal, J.W.; Wakelee, H.A.; Merritt, R.E.; et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat. Med. 2014, 20, 548–554. [Google Scholar] [CrossRef]
- Kedrin, D.; van Rheenen, J.; Hernandez, L.; Condeelis, J.; Segall, J.E. Cell motility and cytoskeletal regulation in invasion and metastasis. J. Mammary Gland Biol. Neoplasia 2007, 12, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Otey, C.A.; Carpen, O. Alpha-actinin revisited: A fresh look at an old player. Cell Motil. Cytoskelet. 2004, 58, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.D.; Ashby, W.J.; Lewis, J.D.; Zijlstra, A. Targeting tumor cell motility to prevent metastasis. Adv. Drug Deliv. Rev. 2011, 63, 568–581. [Google Scholar] [CrossRef]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer. 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Matsumoto, F.; Mori, T.; Miura, N.; Ino, Y.; Onidani, K.; Kobayashi, K.; Matsuzaki, Y.; Yoshimoto, S.; Ikeda, K.; et al. BP180 Is a Prognostic Factor in Head and Neck Squamous Cell Carcinoma. Anticancer Res. 2021, 41, 1089–1099. [Google Scholar] [CrossRef]
- Kakuya, T.; Mori, T.; Yoshimoto, S.; Watabe, Y.; Miura, N.; Shoji, H.; Onidani, K.; Shibahara, T.; Honda, K. Prognostic significance of gene amplification of ACTN4 in stage I and II oral tongue cancer. Int. J. Oral Maxillofac. Surg. 2017, 46, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Yamada, T.; Hayashida, Y.; Idogawa, M.; Sato, S.; Hasegawa, F.; Ino, Y.; Ono, M.; Hirohashi, S. Actinin-4 increases cell motility and promotes lymph node metastasis of colorectal cancer. Gastroenterology 2005, 128, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tsuda, H.; Honda, K.; Kita, T.; Takano, M.; Tamai, S.; Inazawa, J.; Yamada, T.; Matsubara, O. Actinin-4 expression in ovarian cancer: A novel prognostic indicator independent of clinical stage and histological type. Mod. Pathol. 2007, 20, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Watabe, Y.; Mori, T.; Yoshimoto, S.; Nomura, T.; Shibahara, T.; Yamada, T.; Honda, K. Copy number increase of ACTN4 is a prognostic indicator in salivary gland carcinoma. Cancer Med. 2014, 3, 613–622. [Google Scholar] [CrossRef]
- Honda, K. Development of Biomarkers to Predict Recurrence by Determining the Metastatic Ability of Cancer Cells. J. Nippon Med. Sch. 2022, 89, 24–32. [Google Scholar] [CrossRef]
- Miyanaga, A.; Honda, K.; Tsuta, K.; Masuda, M.; Yamaguchi, U.; Fujii, G.; Miyamoto, A.; Shinagawa, S.; Miura, N.; Tsuda, H.; et al. Diagnostic and prognostic significance of the alternatively spliced ACTN4 variant in high-grade neuroendocrine pulmonary tumours. Ann. Oncol. 2013, 24, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Yamada, T.; Seike, M.; Hayashida, Y.; Idogawa, M.; Kondo, T.; Ino, Y.; Hirohashi, S. Alternative splice variant of actinin-4 in small cell lung cancer. Oncogene 2004, 23, 5257–5262. [Google Scholar] [CrossRef][Green Version]
- Morris, H.T.; Machesky, L.M. Actin cytoskeletal control during epithelial to mesenchymal transition: Focus on the pancreas and intestinal tract. Br. J. Cancer 2015, 112, 613–620. [Google Scholar] [CrossRef]
- López-Novoa, J.M.; Nieto, M.A. Inflammation and EMT: An alliance towards organ fibrosis and cancer progression. EMBO Mol. Med. 2009, 1, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Travers, T.; Camacho, C.J.; Wells, A. The carboxyl tail of alpha-actinin-4 regulates its susceptibility to m-calpain and thus functions in cell migration and spreading. Int. J. Biochem. Cell Biol. 2013, 45, 1051–1063. [Google Scholar] [CrossRef]
- Ma, S.Y.; Park, J.-H.; Jung, H.; Ha, S.-M.; Kim, Y.; Park, D.H.; Lee, D.H.; Lee, S.; Chu, I.-H.; Jung, S.Y.; et al. Snail maintains metastatic potential, cancer stem-like properties, and chemoresistance in mesenchymal mouse breast cancer TUBO--P2J cells. Oncol. Rep. 2017, 38, 1867–1876. [Google Scholar] [CrossRef][Green Version]
- An, H.-T.; Yoo, S.; Ko, J. α-Actinin-4 induces the epithelial-to-mesenchymal transition and tumorigenesis via regulation of Snail expression and β-catenin stabilization in cervical cancer. Oncogene 2016, 35, 5893–5904. [Google Scholar] [CrossRef]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-signaling pathway in cancer. OncoTargets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hsu, K.-S.; Lim, J.H.; Bruggeman, L.A.; Kao, H.-Y. α-Actinin 4 potentiates nuclear factor κ-light-chain-enhancer of activated B-cell (NF-κB) activity in podocytes independent of its cytoplasmic actin binding function. J. Biol. Chem. 2015, 290, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.A.; Azoitei, N.; Baumann, B.; Grünert, S.; Sommer, A.; Pehamberger, H.; Kraut, N.; Beug, H.; Wirth, T. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Investig. 2004, 114, 569–581. [Google Scholar] [CrossRef]
- Noro, R.; Honda, K.; Tsuta, K.; Ishii, G.; Maeshima, A.M.; Miura, N.; Furuta, K.; Shibata, T.; Tsuda, H.; Ochiai, A.; et al. Distinct outcome of stage I lung adenocarcinoma with ACTN4 cell motility gene amplification. Ann. Oncol. 2013, 24, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Noro, R.; Ishigame, T.; Walsh, N.; Shiraishi, K.; Robles, A.I.; Ryan, B.M.; Schetter, A.J.; Bowman, E.D.; Welsh, J.A.; Seike, M.; et al. A Two-Gene Prognostic Classifier for Early-Stage Lung Squamous Cell Carcinoma in Multiple Large-Scale and Geographically Diverse Cohorts. J. Thorac. Oncol. 2017, 12, 65–76. [Google Scholar] [CrossRef]
- Yamagata, N.; Shyr, Y.; Yanagisawa, K.; Edgerton, M.; Dang, T.P.; Gonzalez, A.; Nadaf, S.; Larsen, P.; Roberts, J.R.; Nesbitt, J.C.; et al. A training-testing approach to the molecular classification of resected non-small cell lung cancer. Clin. Cancer Res. 2003, 9, 4695–4704. [Google Scholar]
- Lánczky, A.; Győrffy, B. Web-Based Survival Analysis Tool Tailored for Medical Research [KMplot]: Development and Implementation. J. Med. Internet Res. 2021, 23, e27633. [Google Scholar] [CrossRef]
| Histology | Stage | Adjuvant Chemotherapy | Evaluation Methods | |
|---|---|---|---|---|
| Miura et al. (2016) [32] | NSCLC | IB-II | CDDP + VNR | mRNA expression |
| Shiraishi et al. (2017) [33] | Ad | II-IIIA | CDDP + VNR | Protein expression |
| Noro et al. (2021) [34] | Ad | IA/IB | UFT | Gene amplification |
| Miyanaga et al. (2013) [75] | HGNT | resected | Not specified | cDNA sequencing |
| Noro et al. (2013) [85] | Ad | IA-IB | Not specified | Gene amplification |
| Noro et al. (2017) [86] | Sq | I-II | Not specified | Gene expression |
| Yamagata et al. (2003) [87] | NSCLC | resected | Not specified | cDNA microarrays |
| Biomarker | Function | Advantage | Limitation |
|---|---|---|---|
| Gene expression signature | Gene combinations for poor prognosis and poor chemotherapeutic response | More accurate prognostication of a signature from multiple genes compared with individual genes alone | Statistical validation and reproducibility of the signatures/Not a predictor for MRD |
| ERCC1 | Removal of DNA intrastrand crosslinks by nucleotide excision repair | Predictor for the efficacy of cisplatin | Negative results in randomized phase III clinical trials/Not a predictor for MRD |
| TP53 | Prevention and suppression of abnormal cell proliferation through mechanisms including cell cycle arrest, apoptosis, and DNA repair | One of the most frequently mutated genes in lung cancer regardless of histologic type | Not a predictor for MRD |
| PD-L1 | Binding to its receptor PD-1 expressed by T cells and other immune cells to regulate immune responses | Predictor for the efficacy of anti-PD-1/PD-L1 antibody | Not a predictor for MRD |
| ctDNA | Tumor-derived DNA released in the blood | Possibility of MRD detection | Cost/Not a predictor for the efficacy of the specific chemotherapeutic agents |
| ACTN4 | Involvement in cancer invasion and metastatic potential | Evaluating tumor metastatic potential and cancer invasiveness | Not a predictor for the efficacy of specific chemotherapeutic agents |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tozuka, T.; Noro, R.; Seike, M.; Honda, K. Benefits from Adjuvant Chemotherapy in Patients with Resected Non-Small Cell Lung Cancer: Possibility of Stratification by Gene Amplification of ACTN4 According to Evaluation of Metastatic Ability. Cancers 2022, 14, 4363. https://doi.org/10.3390/cancers14184363
Tozuka T, Noro R, Seike M, Honda K. Benefits from Adjuvant Chemotherapy in Patients with Resected Non-Small Cell Lung Cancer: Possibility of Stratification by Gene Amplification of ACTN4 According to Evaluation of Metastatic Ability. Cancers. 2022; 14(18):4363. https://doi.org/10.3390/cancers14184363
Chicago/Turabian StyleTozuka, Takehiro, Rintaro Noro, Masahiro Seike, and Kazufumi Honda. 2022. "Benefits from Adjuvant Chemotherapy in Patients with Resected Non-Small Cell Lung Cancer: Possibility of Stratification by Gene Amplification of ACTN4 According to Evaluation of Metastatic Ability" Cancers 14, no. 18: 4363. https://doi.org/10.3390/cancers14184363
APA StyleTozuka, T., Noro, R., Seike, M., & Honda, K. (2022). Benefits from Adjuvant Chemotherapy in Patients with Resected Non-Small Cell Lung Cancer: Possibility of Stratification by Gene Amplification of ACTN4 According to Evaluation of Metastatic Ability. Cancers, 14(18), 4363. https://doi.org/10.3390/cancers14184363