Patterns of Healthcare Utilization Leading to Diagnosis of Young-Onset Colorectal Cancer (yCRC): Population-Based Case-Control Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Source
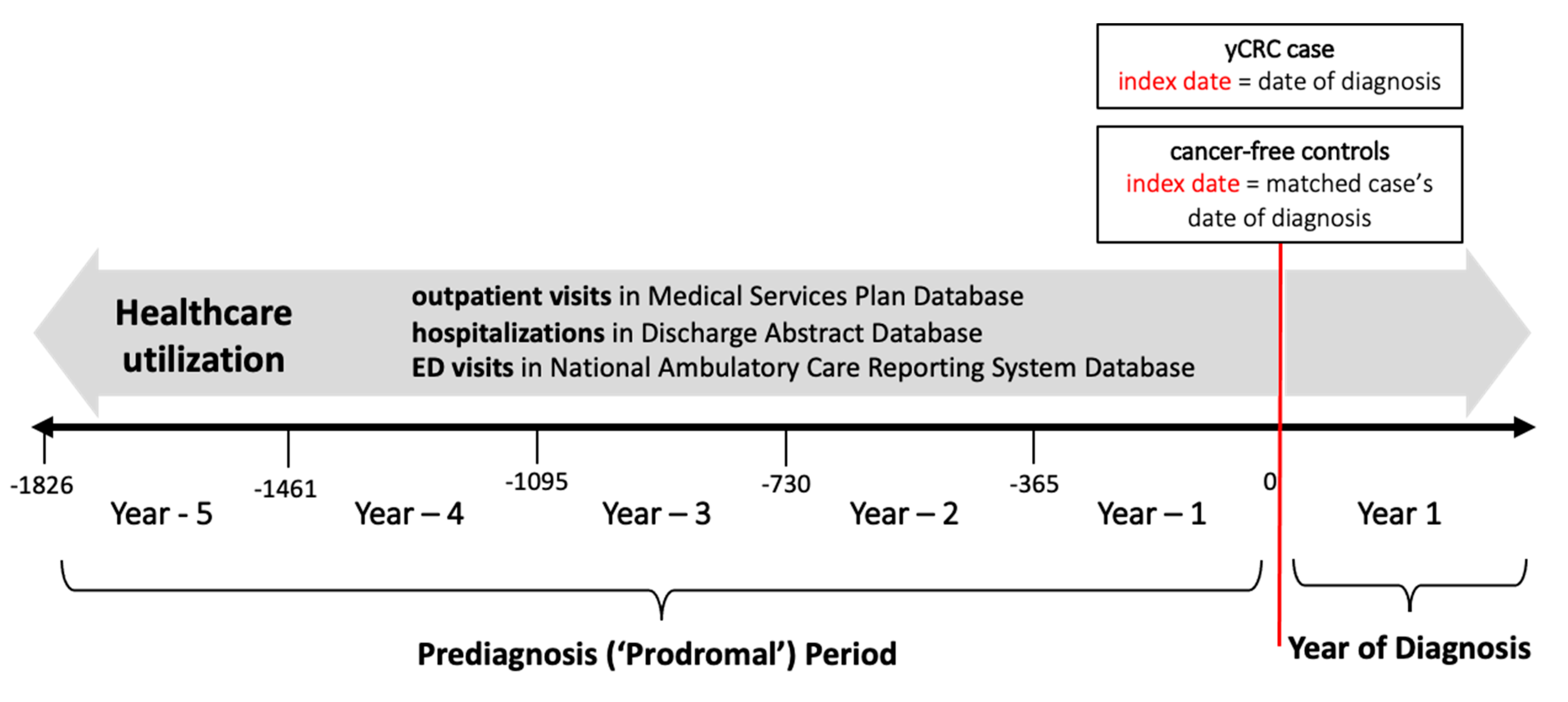
2.2. Study Design
2.3. Healthcare Utilization
2.4. Presenting Complaints
2.5. Statistical Analysis
3. Results
3.1. Demographics
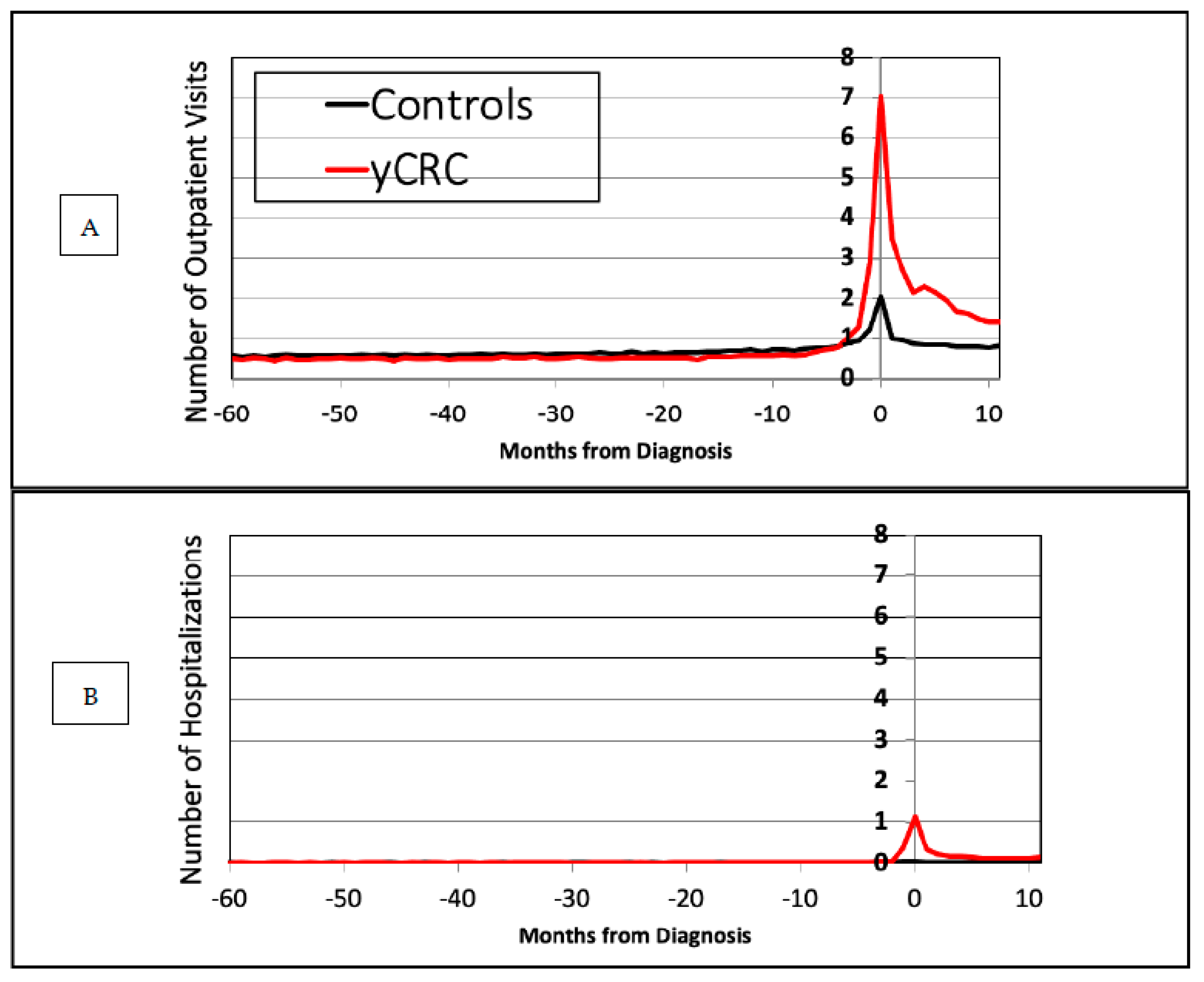
3.2. Health Care Utilization
3.3. Presenting Complaints
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canada PHA of Colorectal Cancer in Canada. Available online: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/colorectal-cancer.html (accessed on 9 December 2020).
- American Cancer Society Guideline for Colorectal Cancer Screening. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/acs-recommendations.html (accessed on 13 August 2019).
- Patel, P.; De, P. Trends in colorectal cancer incidence and related lifestyle risk factors in 15–49-year-olds in Canada, 1969–2010. Cancer Epidemiol. 2016, 42, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.R.; Heer, E.; Sutherland, R.L.; Ruan, Y.; Tinmouth, J.; Heitman, S.J.; Hilsden, R.J. National Trends in Colorectal Cancer Incidence Among Older and Younger Adults in Canada. JAMA Netw. Open 2019, 2, e198090. [Google Scholar] [CrossRef] [PubMed]
- Howren, A.; Sayre, E.C.; Loree, J.M.; Gill, S.; Brown, C.J.; Raval, M.J.; Farooq, A.; De Vera, M.A. Trends in the incidence of young-onset colorectal cancer with a focus on years approaching screening age: A population-based longitudinal study. JNCI J. Natl. Cancer Inst. 2021, 113, 863–868. [Google Scholar] [CrossRef]
- Arhi, C.S.; Ziprin, P.; Bottle, A.; Burns, E.M.; Aylin, P.; Darzi, A. Colorectal cancer patients under the age of 50 experience delays in primary care leading to emergency diagnoses: A population-based study. Colorectal Dis. 2019, 21, 1270–1278. [Google Scholar] [CrossRef]
- Survey of Young-Onset Colorectal Cancer Patients Suggests Misdiagnosis May Be Common.pdf|Powered by Box. Available online: https://aacr.ent.box.com/s/oz6p61ezuw0233urwwzsmvjnzrbwooi8 (accessed on 12 January 2021).
- Scott, R.B.; Rangel, L.E.; Osler, T.M.; Hyman, N.H. Rectal cancer in patients under the age of 50 years: The delayed diagnosis. Am. J. Surg. 2016, 211, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.W.; Sundaram, V.; Chew, T.A.; Ladabaum, U. Advanced-Stage Colorectal Cancer in Persons Younger Than 50 Years Not Associated with Longer Duration of Symptoms or Time to Diagnosis. Clin. Gastroenterol. Hepatol. 2017, 15, 728–737.e3. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.; Brennan, K.; Karim, S.; Nanji, S.; Patel, S.V.; Booth, C.M. Disease Characteristics, Clinical Management, and Outcomes of Young Patients with Colon Cancer: A Population-based Study. Clin. Colorectal Cancer 2018, 17, e651–e661. [Google Scholar] [CrossRef] [PubMed]
- Home|Population Data BC. Available online: https://www.popdata.bc.ca/ (accessed on 2 June 2021).
- British Columbia Ministry of Health. MEDICAL SERVICES PLAN DATABASE (MSP). Medical Services Plan (MSP) Payment Information File. Population Data BC. Data Extract. MOH. 2019. Available online: https://www.popdata.bc.ca/data (accessed on 1 January 2022).
- Canadian Institute for Health Information. Discharge Abstract Database (Hospital Separations). Population Data BC. Data Extract. MOH. 2019. Available online: https://www.popdata.bc.ca/ (accessed on 1 January 2022).
- BC Vital Statistics Agency. Vital Statistics Deaths. Population Data BC. Data Extract BC Vital Statistics Agency. 2019. Available online: https://www2.gov.bc.ca/assets/gov/birth-adoption-death-marriage-and-divorce/statistics-reports/death-reports/deaths-by-lha-2019.pdf (accessed on 1 January 2022).
- British Columbia Ministry of Health. National Ambulatory Care Reporting System (NACRS). Population Data BC. Data Extract. British Columbia Ministry of Health. 2019. Available online: http://www.popdata.bc.ca/data (accessed on 1 January 2022).
- BC Cancer Registry Data. BC Cancer. Data Extract. BC Cancer. 2019. Available online: http://www.bccancer.bc.ca/health-professionals/professional-resources/bc-cancer-registry (accessed on 1 January 2022).
- International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3). Available online: https://www.who.int/standards/classifications/other-classifications/international-classification-of-diseases-for-oncology (accessed on 2 June 2021).
- ICD-ICD-9-CM-International Classification of Diseases, Ninth Revision, Clinical Modification. 2019. Available online: https://www.cdc.gov/nchs/icd/icd9cm.htm (accessed on 2 June 2021).
- ICD-ICD-10-CM-International Classification of Diseases, Tenth Revision, Clinical Modification. 2021. Available online: https://www.cdc.gov/nchs/icd/icd10cm.htm (accessed on 2 June 2021).
- 1 Recommendations Organised by Site of Cancer|Suspected Cancer: Recognition and Referral|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/ng12/chapter/1-Recommendations-organised-by-site-of-cancer#lower-gastrointestinal-tract-cancers (accessed on 19 February 2021).
- Generalized Linear Models. Routledge & CRC Press. Available online: https://www.routledge.com/Generalized-Linear-Models/McCullagh-Nelder/p/book/9780412317606 (accessed on 18 May 2021).
- Ferrante, J.M.; Lee, J.-H.; McCarthy, E.P.; Fisher, K.J.; Chen, R.; Gonzalez, E.C.; Love-Jackson, K.; Roetzheim, R.G. Primary care utilization and colorectal cancer incidence and mortality among Medicare beneficiaries: A population-based, case-control study. Ann. Intern. Med. 2013, 159, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Dozois, E.J.; Boardman, L.A.; Suwanthanma, W.; Limburg, P.J.; Cima, R.R.; Bakken, J.L.; Vierkant, R.A.; Aakre, J.A.; Larson, D.W. Young-Onset Colorectal Cancer in Patients with No Known Genetic Predisposition. Medicine 2008, 87, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Fields, A.C.; Vise, A.S.; Shabat, G.; Irani, J.L.; Bleday, R.; Goldberg, J.E.; Melnitchouk, N. Anatomic Distribution of Colorectal Adenocarcinoma in Young Patients. Dis. Colon Rectum 2019, 62, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Amberg, A.; Saunders, D.N. Cancer in the news: Bias and quality in media reporting of cancer research. PLoS ONE 2020, 15, e0242133. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force; Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965–1977. [Google Scholar] [CrossRef] [PubMed]
- Kalyta, A.; De Vera, M.A.; Peacock, S.; Telford, J.J.; Brown, C.J.; Donnellan, F.; Gill, S.; Loree, J.M. Canadian Colorectal Cancer Screening Guidelines: Do They Need an Update Given Changing Incidence and Global Practice Patterns? Curr. Oncol. 2021, 28, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Abualkhair, W.H.; Zhou, M.; Ahnen, D.; Yu, Q.; Wu, X.-C.; Karlitz, J.J. Trends in Incidence of Early-Onset Colorectal Cancer in the United States Among Those Approaching Screening Age. JAMA Netw. Open 2020, 3, e1920407. [Google Scholar] [CrossRef]
- Lowery, J.T.; Weber, T.K.; Ahnen, D.J.; Schroy III, P.C.; Levell, C.L.; Smith, R.A. An action plan to address the rising burden of colorectal cancer in younger adults. Colorectal Cancer 2020, 9, CRC24. [Google Scholar] [CrossRef]
- Quan, M.L.; Olivotto, I.A.; Baxter, N.N.; Friedenreich, C.M.; Metcalfe, K.; Warner, E.; MacLennan, K.; Stephen, J.E.; Akbari, M.R.; Howell, D.; et al. A Pan-Canadian Prospective Study of Young Women with Breast Cancer: The Rationale and Protocol Design for the RUBY Study. Curr. Oncol. 2020, 27, 516–523. [Google Scholar] [CrossRef]
| yCRC Cases (n = 2567) | Controls (n = 25,455) | aCRC Cases (n = 32,014) | Controls A (n = 297,399) | |
|---|---|---|---|---|
| Age at diagnosis (years, mean ± SD) | 43.0 ± 5.8 | 43.0 ± 5.8 | 69.2 ± 9.8 | 69.2 ± 9.9 |
| Age at diagnosis (groupings) | <30: 91 (3.5%) 30–39: 474 (18.5%) 40–49: 2002 (78.0%) | <30: 1133 (4.5%) 30–39: 4706 (18.5%) 40–49: 19,616 (77.1%) | 50–59: 6010 (18.8%) 60–69: 10,096 (31.5%) 70–79: 11,144 (34.8%) ≥80: 4764 (14.9%) | 50–59: 56,959 (19.2%) 60–69: 92,464 (31.1%) 70–79: 103,663 (34.9%) ≥80: 44,225 (14.9%) |
| Sex | ||||
| Female | 1273 (49.6%) | 12,381 (48.6%) | 15,151 (47.3%) | 140,549 (47.3%) |
| Male | 1294 (50.4%) | 13,074 (51.4%) | 16,863 (52.7%) | 156,762 (52.7%) |
| Neighbourhood Income Quintile | ||||
| Quintile 1 | 483 (18.8%) | 5300 (20.8%) | 6981 (21.8%) | 62,612 (21.1%) |
| Quintile 2 | 492 (19.2%) | 5307 (20.9%) | 6399 (20.0%) | 59,850 (20.1%) |
| Quintile 3 | 551 (21.5%) | 5055 (19.9%) | 6360 (19.9%) | 58,072 (19.5%) |
| Quintile 4 | 549 (21.4%) | 5183 (20.4%) | 6127 (19.1%) | 56,952 (19.2%) |
| Quintile 5 | 492 (19.2%) | 4610 (18.1%) | 6147 (19.2%) | 59,825 (20.1%) |
| Residence | ||||
| Rural | 319 (12.4%) | 2760 (10.8%) | 4708 (14.7%) | 41,373 (13.9%) |
| Urban | 2248 (87.6%) | 22,695 (89.2%) | 27,306 (85.3%) | 255,938 (86.1%) |
| Comorbidities B | ||||
| Hypertension | 235 (9.2%) | 2663 (10.5%) | 12,447 (38.9%) | 109,285 (36.8%) |
| Anxiety | 51 (2.0%) | 656 (2.6%) | 518 (1.6%) | 3863 (1.3%) |
| Depression | 249 (9.7%) | 3421 (13.4%) | 2419 (7.6%) | 20,577 (6.9%) |
| Diabetes | 155 (6.0%) | 1462 (5.7%) | 6310 (19.7%) | 48,965 (16.5%) |
| Dyslipidemia | 73 (2.8%) | 836 (3.3%) | 5391 (16.8%) | 49,169 (16.5%) |
| Inflammatory Bowel Disease | 174 (6.8%) | 217 (0.9%) | 690 (2.2%) | 1555 (0.5%) |
| Tumor Site | ||||
| Left Colon | 1040 (40.5%) | 13,192 (41.2%) | ||
| Right Colon | 260 (10.1%) | 5088 (15.9%) | ||
| Transverse Colon | 122 (4.8%) | 1854 (5.8%) | ||
| Rectal | 1067 (41.6%) | 10,347 (32.3%) | ||
| Unknown | 78 (3.0%) | 1533 (4.8%) | ||
| Prediagnosis Period | Year of Diagnosis | |||||
|---|---|---|---|---|---|---|
| Year-5 | Year-4 | Year-3 | Year-2 | Year-1 | Year 1 | |
| Outpatient visits | ||||||
| yCRC (median) | 3 | 3 | 4 | 4 | 8 | 25 |
| yCRC (mean) | 5.80 ± 8.06 | 5.85 ± 8.29 | 6.08 ± 9.01 | 6.23 ± 9.26 | 10.80 ± 10.16 | 29.41 ± 21.21 |
| Control (median) | 4 | 4 | 4 | 5 | 6 | 7 |
| Control (mean) | 6.72 ± 9.70 | 6.99 ± 10.21 | 7.28 ± 10.65 | 7.76 ± 11.44 | 9.64 ± 12.99 | 11.0 ± 14.87 |
| Hospitalizations | ||||||
| yCRC (median) | 0 | 0 | 0 | 0 | 0 | 3 |
| yCRC (mean) | 0.13 ± 0.44 | 0.14 ± 0.49 | 0.15 ± 0.52 | 0.15 ± 0.54 | 0.60 ± 0.88 | 2.95 ± 1.89 |
| Control (median) | 0 | 0 | 0 | 0 | 0 | 0 |
| Control (mean) | 0.13 ± 0.49 | 0.14 ± 0.49 | 0.14 ± 0.49 | 0.14 ± 0.51 | 0.20 ± 0.67 | 0.25 ± 0.70 |
| Emergency visits | ||||||
| yCRC (median) | 0 | 0 | 0 | 0 | 0 | 0 |
| yCRC (mean) | 0.0051 ± 0.098 | 0.022 ± 0.44 | 0.035 ± 0.44 | 0.051 ± 0.37 | 0.21 ± 0.73 | 0.48 ± 1.42 |
| Control (median) | 0 | 0 | 0 | 0 | 0 | 0 |
| Control (mean) | 0.0044 ± 0.081 | 0.024 ± 0.37 | 0.043 ± 0.48 | 0.067 ± 0.56 | 0.12 ± 0.75 | 0.15 ± 0.90 |
| Count Ratio (95% Confidence Interval) | ||||||
|---|---|---|---|---|---|---|
| Prediagnosis Period | Year of Diagnosis | |||||
| Year-5 | Year-4 | Year-3 | Year-2 | Year-1 | Year 1 | |
| yCRC (ref: no yCRC) | 0.86 (0.82 to 0.90) | 0.84 (0.80 to 0.88) | 0.83 (0.80 to 0.87) | 0.81 (0.77 to 0.84) | 1.11 (1.07 to 1.15) | 2.42 (2.35 to 2.49) |
| Age A | 0.99 (0.98 to 0.99) | 0.98 (0.98 to 0.99) | 0.98 (0.98 to 0.99) | 0.98 (0.98 to 0.98) | 0.98 (0.98 to 0.98) | 0.99 (0.99 to 0.99) |
| Female (ref: male) | 1.35 (1.31 to 1.38) | 1.32 (1.29 to 1.35) | 1.27 (1.24 to 1.30) | 1.20 (1.18 to 1.24) | 1.11 (1.08 to 1.13) | 1.04 (1.02 to 1.13) |
| Neighbourhood income | ||||||
| Quintile 1 (ref: Quintile 5) | 1.28 (1.23 to 1.34) | 1.25 (1.20 to 1.30) | 1.30 (1.25 to 1.35) | 1.36 (1.30 to 1.41) | 1.31 (1.27 to 1.36) | 1.31 (1.27 to 1.35) |
| Quintile 2 (ref: Quintile 5) | 1.13 (1.08 to 1.18) | 1.14 (1.09 to 1.19) | 1.11 (1.07 to 1.16) | 1.20 (1.15 to 1.25) | 1.16 (1.11 to 1.20) | 1.13 (1.09 to 1.17) |
| Quintile 3 (ref: Quintile 5) | 1.13 (1.08 to 1.17) | 1.10 (1.06 to 1.15) | 1.06 (1.02 to 1.11) | 1.11 (1.07 to 1.16) | 1.11 (1.07 to 1.15) | 1.10 (1.06 to 1.14) |
| Quintile 4 (ref: Quintile 5) | 1.07 (1.02 to 1.12) | 1.02 (0.98 to 1.07) | 1.03 (0.99 to 1.08) | 1.06 (1.01 to 1.10) | 1.03 (0.99 to 1.07) | 1.02 (0.98 to 1.05) |
| Rural residence (ref: urban) | 0.84 (0.80 to 0.87) | 0.85 (0.82 to 0.89) | 0.87 (0.83 to 0.90) | 0.86 (0.82 to 0.89) | 0.88 (0.85 to 0.91) | 0.89 (0.86 to 0.92) |
| Comorbidities | ||||||
| Hypertension (ref: no) | 1.48 (1.40 to 1.56) | 1.51 (1.44 to 1.58) | 1.47 (1.41 to 1.54) | 1.39 (1.33 to 1.45) | 1.32 (1.27 to 1.37) | 1.24 (1.20 to 1.28) |
| Anxiety (ref: no) | 1.48 (1.37 to 1.60) | 1.57 (1.47 to 1.68) | 1.64 (1.52 to 1.75) | 1.55 (1.45 to 1.66) | 1.45 (1.37 to 1.54) | 1.42 (1.35 to 1.50) |
| Depression (ref: no) | 2.08 (2.01 to 2.15) | 1.98 (1.92 to 2.04) | 1.99 (1.93 to 2.05) | 2.05 (1.99 to 2.11) | 1.83 (1.78 to 1.88) | 1.72 (1.67 to 1.76) |
| Diabetes (ref: no) | 1.60 (1.50 to 1.71) | 1.55 (1.46 to 1.65) | 1.55 (1.47 to 1.64) | 1.45 (1.37 to 1.53) | 1.45 (1.39 to 1.52) | 1.39 (1.33 to 1.44) |
| Dyslipidemia (ref: no) | 1.32 (1.20 to 1.45) | 1.46 (1.35 to 1.58) | 1.42 (1.32 to 1.53) | 1.39 (1.30 to 1.49) | 1.16 (1.09 to 1.24) | 1.12 (1.06 to 1.18) |
| IBD (ref: no) | 1.83 (1.64 to 2.03) | 1.86 (1.68 to 2.06) | 1.98 (1.79 to 2.19) | 1.84 (1.66 to 2.04) | 1.74 (1.59 to 1.90) | 1.32 (1.24 to 1.41) |
| Charlson comorbidity score A,B | 1.24 (1.21 to 1.28) | 1.29 (1.26 to 1.32) | 1.32 (1.29 to 1.35) | 1.30 (1.28 to 1.33) | 1.28 (1.26 to 1.30) | 1.06 (1.05 to 1.07) |
| yCRC | aCRC | p-Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Unrelated visits (1) | 6536 | 48.1 | 147,682 | 61.4 | <0.001 |
| Potentially related visits (2) | 4769 | 35.1 | 70,119 | 29.2 | <0.001 |
| Potentially red flag visits (3) | 2293 | 16.9 | 22,587 | 9.4 | <0.001 |
| Specific complaints | |||||
| Nausea & vomiting | 2029 | 14.9 | 24,177 | 10.1 | <0.001 |
| Abdominal pain | 914 | 6.7 | 7138 | 3.0 | <0.001 |
| “Other disorders of intestine” OR “Other symptoms including abdomen and pelvis” | 742 | 5.5 | 15,946 | 6.6 | <0.001 |
| Hemorrhoid | 438 | 3.2 | 3397 | 1.4 | <0.001 |
| Anemia | 304 | 2.2 | 4977 | 2.1 | 0.19 |
| Rectal bleeding | 241 | 1.8 | <0.001 | ||
| Total visits | 13,598 | 240,388 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, A.; Brown, C.J.; Sayre, E.C.; Raval, M.J.; Loree, J.M.; Garg, R.; De Vera, M.A. Patterns of Healthcare Utilization Leading to Diagnosis of Young-Onset Colorectal Cancer (yCRC): Population-Based Case-Control Study. Cancers 2022, 14, 4263. https://doi.org/10.3390/cancers14174263
Farooq A, Brown CJ, Sayre EC, Raval MJ, Loree JM, Garg R, De Vera MA. Patterns of Healthcare Utilization Leading to Diagnosis of Young-Onset Colorectal Cancer (yCRC): Population-Based Case-Control Study. Cancers. 2022; 14(17):4263. https://doi.org/10.3390/cancers14174263
Chicago/Turabian StyleFarooq, Ameer, Carl J. Brown, Eric C. Sayre, Manoj J. Raval, Jonathan M. Loree, Ria Garg, and Mary A. De Vera. 2022. "Patterns of Healthcare Utilization Leading to Diagnosis of Young-Onset Colorectal Cancer (yCRC): Population-Based Case-Control Study" Cancers 14, no. 17: 4263. https://doi.org/10.3390/cancers14174263
APA StyleFarooq, A., Brown, C. J., Sayre, E. C., Raval, M. J., Loree, J. M., Garg, R., & De Vera, M. A. (2022). Patterns of Healthcare Utilization Leading to Diagnosis of Young-Onset Colorectal Cancer (yCRC): Population-Based Case-Control Study. Cancers, 14(17), 4263. https://doi.org/10.3390/cancers14174263






