T-Cell Density at the Invasive Margin and Immune Phenotypes Predict Outcome in Vulvar Squamous Cell Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissues
2.2. Immunohistochemistry
2.3. Definition of Analyzed Compartments and Quantification of CD3 and CD8 Immunostaining
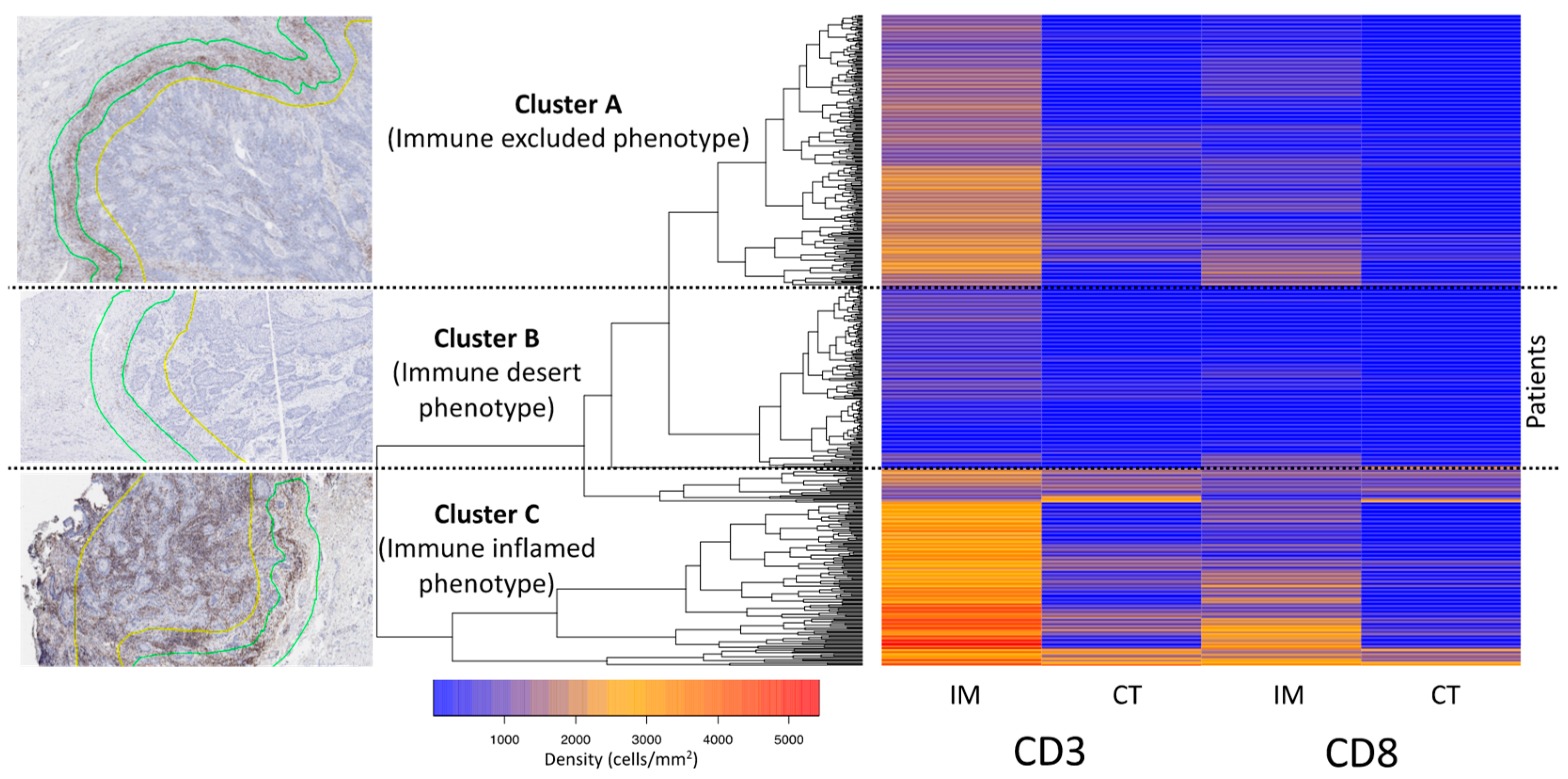
2.4. Definition of Immune Phenotypes and Cluster Analysis
2.5. Statistics
3. Results
3.1. T-Cell Densities
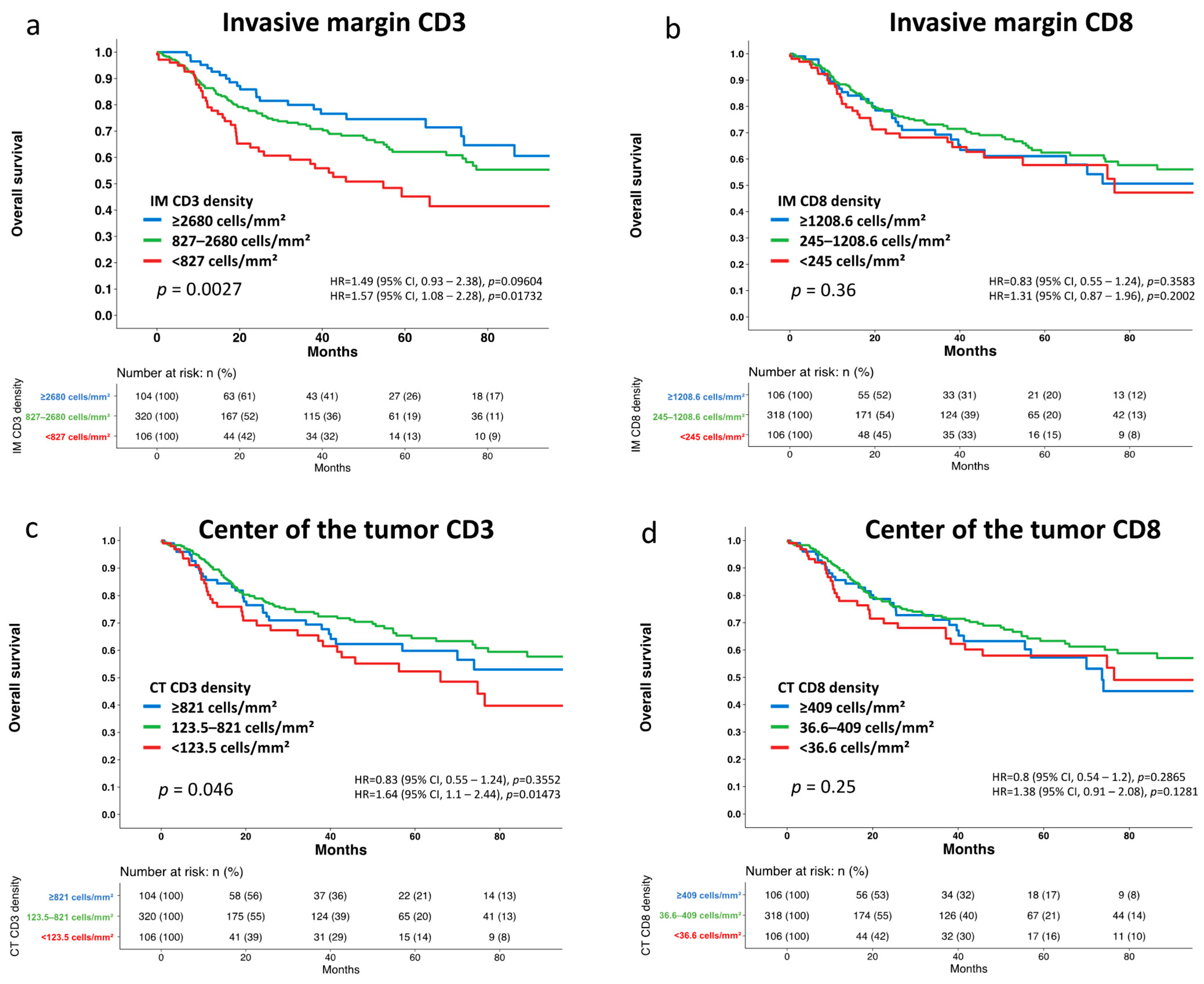
3.2. Association with Tumor Phenotype and Patient Survival
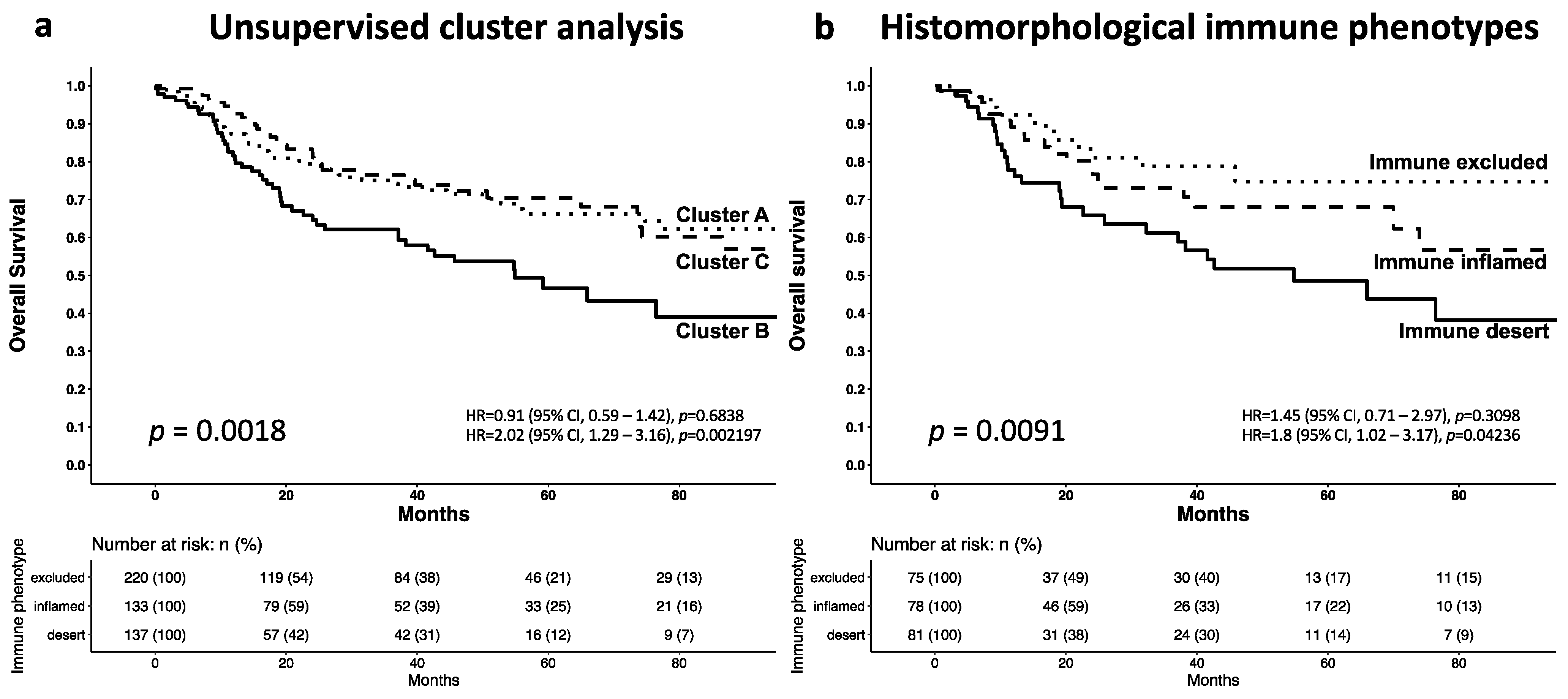
3.3. Cluster Analysis of the T-Cell Density
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brinton, L.A.; Thistle, J.E.; Liao, L.M.; Trabert, B. Epidemiology of vulvar neoplasia in the NIH-AARP Study. Gynecol. Oncol. 2017, 145, 298–304. [Google Scholar] [CrossRef]
- Mitra, S.; Sharma, M.K.; Kaur, I.; Khurana, R.; Modi, K.B.; Narang, R.; Mandal, A.; Dutta, S. Vulvar carcinoma: Dilemma, debates, and decisions. Cancer Manag. Res. 2018, 10, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, T.R.; Graybill, W.S.; Pierce, J.Y. Morbidity and mortality of vulvar and vaginal cancers: Impact of 2-, 4-, and 9-valent HPV vaccines. Hum. Vaccines Immunother. 2016, 12, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Mahner, S.; Jueckstock, J.; Hilpert, F.; Neuser, P.; Harter, P.; de Gregorio, N.; Hasenburg, A.; Sehouli, J.; Habermann, A.; Hillemanns, P.; et al. Adjuvant Therapy in Lymph Node–Positive Vulvar Cancer: The AGO-CaRE-1 Study. JNCI J. Natl. Cancer Inst. 2015, 107, dju426. [Google Scholar] [CrossRef] [PubMed]
- Schnürch, H.G.; Ackermann, S.; Alt, C.D.; Barinoff, J.; Böing, C.; Dannecker, C.; Gieseking, F.; Günthert, A.; Hantschmann, P.; Horn, L.C.; et al. Diagnosis, Therapy and Follow-up Care of Vulvar Cancer and its Precursors. Guideline of the DGGG and DKG (S2k-Level, AWMF Registry Number 015/059, November 2015. Geburtshilfe Frauenheilkd. 2016, 76, 1035–1049. [Google Scholar] [CrossRef]
- Liang, F.; Zhang, S.; Wang, Q.; Li, W. Clinical benefit of immune checkpoint inhibitors approved by US Food and Drug Administration. BMC Cancer 2020, 20, 823. [Google Scholar] [CrossRef]
- Hsu, F.-S.; Su, C.-H.; Huang, K.-H. A Comprehensive Review of US FDA-Approved Immune Checkpoint Inhibitors in Urothelial Carcinoma. J. Immunol. Res. 2017, 2017, 6940546. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Mehra, R.; Seiwert, T.Y.; Gupta, S.; Weiss, J.; Gluck, I.; Eder, J.P.; Burtness, B.; Tahara, M.; Keam, B.; Kang, H.; et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: Pooled analyses after long-term follow-up in KEYNOTE-012. Br. J. Cancer 2018, 119, 153–159. [Google Scholar] [CrossRef]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Bang, Y.-J.; Piha-Paul, S.A.; Razak, A.R.A.; Bennouna, J.; Soria, J.-C.; Rugo, H.S.; Cohen, R.B.; O’Neil, B.H.; Mehnert, J.M.; et al. T-Cell–Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated with Pembrolizumab Across 20 Cancers: KEYNOTE-028. J. Clin. Oncol. 2019, 37, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Naumann, R.W.; Hollebecque, A.; Meyer, T.; Devlin, M.-J.; Oaknin, A.; Kerger, J.; López-Picazo, J.M.; Machiels, J.-P.; Delord, J.-P.; Evans, T.R.J.; et al. Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results from the Phase I/II CheckMate 358 Trial. J. Clin. Oncol. 2019, 37, 2825–2834. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, K.E.; Santegoets, S.J.; Abdulrahman, Z.; Van Ham, V.J.; Van Der Tol, M.; Ehsan, I.; Van Doorn, H.C.; Bosse, T.; Van Poelgeest, M.I.E.; Van Der Burg, S.H. High numbers of activated helper T cells are associated with better clinical outcome in early stage vulvar cancer, irrespective of HPV or p53 status. J. Immunother. Cancer 2019, 7, 236. [Google Scholar] [CrossRef]
- Sznurkowski, J.J.; Żawrocki, A.; Biernat, W. Local immune response depends on p16INK4a status of primary tumor in vulvar squamous cell carcinoma. Oncotarget 2017, 8, 46204–46210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Jong, R.A.; Toppen, N.L.; Hoor, K.T.; Boezen, H.; Kema, I.; Hollema, H.; Nijman, H. Status of cellular immunity lacks prognostic significance in vulvar squamous carcinoma. Gynecol. Oncol. 2012, 125, 186–193. [Google Scholar] [CrossRef]
- Sznurkowski, J.J.; Żawrocki, A.; Emerich, J.; Biernat, W. Prognostic Significance of CD4+and CD8+T Cell Infiltration within Cancer Cell Nests in Vulvar Squamous Cell Carcinoma. Int. J. Gynecol. Cancer 2011, 21, 717–721. [Google Scholar] [CrossRef]
- Sznurkowski, J.J.; Żawrocki, A.; Biernat, W. Subtypes of cytotoxic lymphocytes and natural killer cells infiltrating cancer nests correlate with prognosis in patients with vulvar squamous cell carcinoma. Cancer Immunol. Immunother. 2014, 63, 297–303. [Google Scholar] [CrossRef][Green Version]
- Imoto, S.; Inamine, M.; Kudaka, W.; Nagai, Y.; Wakayama, A.; Nakamoto, T.; Ooyama, T.; Aoki, Y. Prognostic factors in patients with vulvar cancer treated with primary surgery: A single-center experience. SpringerPlus 2016, 5, 125. [Google Scholar] [CrossRef]
- Hegde, P.S.; Karanikas, V.; Evers, S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin. Cancer Res. 2016, 22, 1865–1874. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015, 28, 690–714. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Mandelkow, T.; Blessin, N.C.; Lueerss, E.; Pott, L.; Simon, R.; Li, W.; Wellge, B.; Debatin, N.F.; Höflmayer, D.; Izbicki, J.R.; et al. Immune Exclusion Is Frequent in Small-Cell Carcinoma of the Bladder. Dis. Markers 2019, 2019, 2532518. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non–T-Cell–Inflamed Tumor Microenvironment. Semin. Oncol. 2015, 42, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Kather, J.N.; Suarez-Carmona, M.; Charoentong, P.; Weis, C.-A.; Hirsch, D.; Bankhead, P.; Horning, M.; Ferber, D.; Kel, I.; Herpel, E.; et al. Topography of cancer-associated immune cells in human solid tumors. eLife 2018, 7, e36967. [Google Scholar] [CrossRef]
- Ribbat-Idel, J.; Perner, S.; Kuppler, P.; Klapper, L.; Krupar, R.; Watermann, C.; Paulsen, F.-O.; Offermann, A.; Bruchhage, K.-L.; Wollenberg, B.; et al. Immunologic “Cold” Squamous Cell Carcinomas of the Head and Neck Are Associated with an Unfavorable Prognosis. Front. Med. 2021, 8, 622330. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. Available online: https://www.R-project.org/ (accessed on 17 February 2022).
- JMP®, V.; SAS Institute Inc., Cary, NC, USA. 1989–2019. Available online: http://www.jmp.com/ (accessed on 28 October 2021).
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000; ISBN 0-387-98784-3. [Google Scholar]
- Zhang, N.; Bevan, M.J. CD8+ T Cells: Foot Soldiers of the Immune System. Immunity 2011, 35, 161–168. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell. Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef]
- Cocks, M.; Chaux, A.; Jenson, E.G.; Miller, J.A.; Pena, M.D.C.R.; Tregnago, A.C.; Taheri, D.; Eich, M.-L.; Sharma, R.; Vang, R.; et al. Immune checkpoint status and tumor microenvironment in vulvar squamous cell carcinoma. Virchows Arch. 2020, 477, 93–102. [Google Scholar] [CrossRef]
- Schmidt, M.; Weyer-Elberich, V.; Hengstler, J.G.; Heimes, A.-S.; Almstedt, K.; Gerhold-Ay, A.; Lebrecht, A.; Battista, M.J.; Hasenburg, A.; Sahin, U.; et al. Prognostic impact of CD4-positive T cell subsets in early breast cancer: A study based on the FinHer trial patient population. Breast Cancer Res. 2018, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Gu-Trantien, C.; Loi, S.; Garaud, S.; Equeter, C.; Libin, M.; De Wind, A.; Ravoet, M.; Le Buanec, H.; Sibille, C.; Manfouo-Foutsop, G.; et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Investig. 2013, 123, 2873–2892. [Google Scholar] [CrossRef] [PubMed]
- Diederichsen, A.C.P.; Hjelmborg, J.; Christensen, P.B.; Zeuthen, J.; Fenger, C. Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol. Immunother. 2003, 52, 423–428. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, H.; Zhao, J.; Sileng, A.; Liu, Z.; Han, T.; Zhang, X.; Du, J. Prognostic significance of CD4 and interleukin-22 expression in pancreatic cancer. Int. J. Clin. Exp. Pathol. 2017, 10, 9846–9852. [Google Scholar] [PubMed]
- Ito, N.; Suzuki, Y.; Taniguchi, Y.; Ishiguro, K.; Nakamura, H.; Ohgi, S. Prognostic significance of T helper 1 and 2 and T cytotoxic 1 and 2 cells in patients with non-small cell lung cancer. Anticancer Res. 2005, 25, 2027–2031. [Google Scholar]
- van Esch, E.M.; van Poelgeest, M.I.; Kouwenberg, S.; Osse, E.M.; Trimbos, J.B.M.; Fleuren, G.J.; Jordanova, E.S.; van der Burg, S.H. Expression of coinhibitory receptors on T cells in the microenvironment of usual vulvar intraepithelial neoplasia is related to proinflammatory effector T cells and an increased recurrence-free survival. Int. J. Cancer 2015, 136, E95–E106. [Google Scholar] [CrossRef]
- van Esch, E.M.G.; van Poelgeest, M.I.E.; Trimbos, J.B.M.Z.; Fleuren, G.J.; Jordanova, E.S.; van der Burg, S.H. Intraepithelial macrophage infiltration is related to a high number of regulatory T cells and promotes a progressive course of HPV-induced vulvar neoplasia. Int. J. Cancer 2015, 136, E85–E94. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, Density, and Location of Immune Cells within Human Colorectal Tumors Predict Clinical Outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Yoon, H.H.; Shi, Q.; Heying, E.N.; Muranyi, A.; Bredno, J.; Ough, F.; Djalilvand, A.; Clements, J.; Bowermaster, R.; Liu, W.-W.; et al. Intertumoral Heterogeneity of CD3+ and CD8+ T-Cell Densities in the Microenvironment of DNA Mismatch-Repair–Deficient Colon Cancers: Implications for Prognosis. Clin. Cancer Res. 2019, 25, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Ericsson, P.G.; et al. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Zlobec, I.; Lugli, A. Invasive front of colorectal cancer: Dynamic interface of pro-/anti-tumor factors. World J. Gastroenterol. 2009, 15, 5898–5906. [Google Scholar] [CrossRef]
- Mlynska, A.; Vaišnorė, R.; Rafanavičius, V.; Jocys, S.; Janeiko, J.; Petrauskytė, M.; Bijeikis, S.; Cimmperman, P.; Intaitė, B.; Žilionytė, K.; et al. A gene signature for immune subtyping of desert, excluded, and inflamed ovarian tumors. Am. J. Reprod. Immunol. 2020, 84, e13244. [Google Scholar] [CrossRef]
- Galli, F.; Aguilera, J.V.; Palermo, B.; Markovic, S.N.; Nisticò, P.; Signore, A. Relevance of immune cell and tumor microenvironment imaging in the new era of immunotherapy. J. Exp. Clin. Cancer Res. 2020, 39, 89. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.I.; Cesano, A.; Marincola, F.M. The Paradox of Cancer Immune Exclusion: Immune Oncology Next Frontier. Cancer Treat. Res. 2020, 180, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Moreau, H.; Piel, M.; Voituriez, R.; Lennon-Duménil, A.-M. Integrating Physical and Molecular Insights on Immune Cell Migration. Trends Immunol. 2018, 39, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, J.; Liu, B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front. Immunol. 2018, 9, 978. [Google Scholar] [CrossRef] [PubMed]
- Garris, C.S.; Arlauckas, S.P.; Kohler, R.H.; Trefny, M.P.; Garren, S.; Piot, C.; Engblom, C.; Pfirschke, C.; Siwicki, M.; Gungabeesoon, J.; et al. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-γ and IL-12. Immunity 2018, 49, 1148–1161.e7. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Matlung, H.L.; Szilagyi, K.; Barclay, N.A.; Berg, T.K.V.D. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol. Rev. 2017, 276, 145–164. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Corrales, L.; Williams, J.; Horton, B.; Sivan, A.; Spranger, S. Cancer Immunotherapy Targets Based on Understanding the T Cell-Inflamed Versus Non-T Cell-Inflamed Tumor Microenvironment. Adv. Exp. Med. Biol. 2017, 1036, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Asaka, S.; Yen, T.-T.; Wang, T.-L.; Shih, I.-M.; Gaillard, S. T cell-inflamed phenotype and increased Foxp3 expression in infiltrating T-cells of mismatch-repair deficient endometrial cancers. Mod. Pathol. 2019, 32, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Monteran, L.; Erez, N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front. Immunol. 2019, 10, 1835. [Google Scholar] [CrossRef]
- Troiano, G.; Rubini, C.; Togni, L.; Caponio, V.C.A.; Zhurakivska, K.; Santarelli, A.; Cirillo, N.; Muzio, L.L.; Mascitti, M. The immune phenotype of tongue squamous cell carcinoma predicts early relapse and poor prognosis. Cancer Med. 2020, 9, 8333–8344. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tray, N.; Weber, J.S.; Adams, S. Predictive Biomarkers for Checkpoint Immunotherapy: Current Status and Challenges for Clinical Application. Cancer Immunol. Res. 2018, 6, 1122–1128. [Google Scholar] [CrossRef]
- Goltsev, Y.; Samusik, N.; Kennedy-Darling, J.; Bhate, S.; Hale, M.; Vazquez, G.; Black, S.; Nolan, G.P. Deep Profiling of Mouse Splenic Architecture with CODEX Multiplexed Imaging. Cell 2018, 174, 968–981.e15. [Google Scholar] [CrossRef]
- Bady, E.; Möller, K.; Mandelkow, T.; Simon, R.; Lennartz, M.; Hube-Magg, C.; Sauter, G.; Blessin, N.C. Abstract 597: BLEACH&STAIN 15 marker multiplexed imaging in 3098 human carcinomas revealed six major PD-L1 driven immune phenotypes with distinct spatial orchestration. Cancer Res. 2022, 82 (Suppl. 12), 597. [Google Scholar] [CrossRef]

| Clinical Parameter | n | Density of CD3+ Cells (IM *) [cells/mm2] | p-Value | Density of CD3+ Cells (CT **) [cells/mm2] | p-Value | Density of CD8+ Cells (IM *) [cells/mm2] | p-Value | Density of CD8+ Cells (CT **) [cells/mm2] | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| pT1 | 174 | 1995 (±1239) | 0.0001 | 583 (±620) | 0.0518 | 800 (±668) | 0.0259 | 311 (±448) | 0.1573 |
| pT2 | 286 | 1738 (±1042) | 503 (±551) | 791 (±645) | 312 (±461) | ||||
| pT3-4 | 64 | 1351 (±856) | 388 (±445) | 565 (±475) | 199 (±276) | ||||
| pN- | 285 | 1863 (±1150) | 0.0638 | 523 (±584) | 0.5712 | 798 (±685) | 0.3459 | 311 (±485) | 0.3150 |
| pN+ | 180 | 1665 (±1076) | 493 (±520) | 741 (±571) | 270 (±330) | ||||
| Grade 1 | 60 | 2068 (±1240) | 0.0664 | 500 (±531) | 0.6560 | 878 (±730) | 0.2047 | 256 (±424) | 0.6782 |
| Grade 2 | 317 | 1768 (±1107) | 502 (±535) | 777 (±660) | 298 (±417) | ||||
| Grade 3 | 145 | 1674 (±1032) | 553 (±645) | 707 (±534) | 316 (±493) | ||||
| HPV- | 244 | 1818 (±1104) | 0.5099 | 520 (±586) | 0.9229 | 819 (±667) | 0.1258 | 296 (±399) | 0.7400 |
| HPV+ | 259 | 1753 (±1113) | 525 (±572) | 731 (±630) | 310 (±492) |
| Prognostic Factor | Overall Survival | Progression Free Survival | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) p-Value | p-Value | Hazard Ratio (95% CI) p-Value | p-Value | |
| IM CD3 density (cells/mm2) | ||||
| <827 vs. 827–2680 | 1.66 (1.08–2.55) * | 0.017 | 1.47 (1.04–2.07) * | 0.044 |
| 827–2680 vs. ≥2680 | 1.30 (0.77–2.19) n.s. | 1.13 (0.78–1.64) n.s. | ||
| CT CD3 density (cells/mm2) | ||||
| <123.5 vs. 123.5–821 | 2.15 (1.37–3.38) *** | 0.004 | 1.79 (1.25–2.56) * | 0.006 |
| 123.5–821 vs. ≥821 | 0.80 (0.50–1.28) n.s. | 0.88 (0.62–1.27) n.s. | ||
| IM CD8 density (cells/mm2) | ||||
| <245 vs. 245–1208.6 | 1.44 (0.88–2.36) n.s. | 0.29 | 1.30 (0.89–1.89) n.s. | 0.26 |
| 245–1208.6 vs. ≥1208.6 | 0.80 (0.33–0.51) n.s. | 0.80 (0.57–1.14) n.s. | ||
| CT CD8 density (cells/mm2) | ||||
| <36.6 vs. 36.6–409 | 1.36 (0.84–2.20) n.s. | 0.4 | 1.14 (0.77–1.68) n.s. | 0.5 |
| 36.6–409 vs. ≥409 | 0.82 (0.52–1.30) n.s. | 0.82 (0.58–1.15) n.s. | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burandt, E.; Blessin, N.C.; Rolschewski, A.-C.; Lutz, F.; Mandelkow, T.; Yang, C.; Bady, E.; Reiswich, V.; Simon, R.; Sauter, G.; et al. T-Cell Density at the Invasive Margin and Immune Phenotypes Predict Outcome in Vulvar Squamous Cell Cancer. Cancers 2022, 14, 4246. https://doi.org/10.3390/cancers14174246
Burandt E, Blessin NC, Rolschewski A-C, Lutz F, Mandelkow T, Yang C, Bady E, Reiswich V, Simon R, Sauter G, et al. T-Cell Density at the Invasive Margin and Immune Phenotypes Predict Outcome in Vulvar Squamous Cell Cancer. Cancers. 2022; 14(17):4246. https://doi.org/10.3390/cancers14174246
Chicago/Turabian StyleBurandt, Eike, Niclas C. Blessin, Ann-Christin Rolschewski, Florian Lutz, Tim Mandelkow, Cheng Yang, Elena Bady, Viktor Reiswich, Ronald Simon, Guido Sauter, and et al. 2022. "T-Cell Density at the Invasive Margin and Immune Phenotypes Predict Outcome in Vulvar Squamous Cell Cancer" Cancers 14, no. 17: 4246. https://doi.org/10.3390/cancers14174246
APA StyleBurandt, E., Blessin, N. C., Rolschewski, A.-C., Lutz, F., Mandelkow, T., Yang, C., Bady, E., Reiswich, V., Simon, R., Sauter, G., Mahner, S., Gregorio, N. d., Klapdor, R., Kalder, M., Braicu, E. I., Fürst, S., Klar, M., Strauß, H.-G., Prieske, K., & Wölber, L. (2022). T-Cell Density at the Invasive Margin and Immune Phenotypes Predict Outcome in Vulvar Squamous Cell Cancer. Cancers, 14(17), 4246. https://doi.org/10.3390/cancers14174246






