Simple Summary
This systemic review and meta-analysis compared and analyzed the safety and effectiveness of transoral robotic thyroidectomy on the thyroid tumor with other thyroid approaches. Transoral robotic thyroidectomy showed similar results to other robotic-assisted thyroid surgeries. Compared to a conventional open thyroidectomy, transoral robotic thyroidectomy had longer operational times and hospitalization days, and worse postoperative pain, but a higher cosmetic satiation score. However, more randomized controlled studies need to be included and analyzed.
Abstract
Background: To assess the safety and effectiveness of transoral robotic thyroidectomy (TORT) in thyroid tumor. Methods: PubMed, Embase, Web of Science, SCOPUS, Cochrane database, and Google Scholar up to June 2022. Studies comparing outcomes and complications between TORT and control groups (robotic bilateral axillo-breast, trans-axillary, postauricular approach, conventional open thyroidectomy (OT), and transoral endoscopic approach) were analyzed. Results: Ten studies of 1420 individuals. The operative time (SMD 1.15, 95%CI [0.48; 1.89]) was significantly longer and the number of retrieved lymph nodes (LNs) (SMD −0.27, 95%CI [−0.39; −0.16]) was fewer in TORT than in the control group. The postoperative cosmetic satisfaction score (SMD 0.60, 95%CI [0.28; 0.92]) was statistically higher in TORT than in the control group. In subgroup analysis, there was no significant difference between robotic surgeries. However, TORT had significantly longer operative times (SMD 2.08, 95%CI [0.95; 3.20]) and fewer retrieved LNs (SMD −0.32, 95%CI [−0.46; −0.17]) than OT. TORT satisfied significantly more patients in cosmetic view. However, it increased hospitalization days and postoperative pain on the operation day and first day compared to OT. Conclusions: TORT is not inferior to other robotic-assisted approaches. Its operation time and hospitalization days are longer and postoperative pain is greater than OT, although its cosmetic satisfaction is high.
1. Introduction
The prevalence of thyroid tumors, including thyroid cancer, has been increasing for several decades [1]. With the development of diagnostic tools for small thyroid lesions, thyroid surgery from lobectomy to total thyroidectomy has increased [2,3]. In the past, the standard treatment for thyroid tumors was conventional open thyroidectomy (OT) [4]. Although conventional OT exposes the thyroid gland sufficiently, it can leave a large scar on the neck [5]. Most patients with thyroid nodules are women. About 5% of females have thyroid nodules [1]. Most patients with thyroid tumors, especially young female patients, are concerned about neck scars [6,7]. Various endoscopic and robotic thyroid surgeries have been studied for decades to avoid anterior neck incision [4,5].
Since 2000, surgical techniques for endoscopic thyroid surgery [8] and minimally invasive video-assisted thyroidectomy [9] have been introduced. Since then, various surgical methods such as the robotic bilateral axillo-breast approach (BABA), robotic postauricular approach (PAA), and transoral endoscopic approach via vestibular approach (TOETVA) have been developed [5,10,11,12]. Among them, TOETVA is the only technique that does not cause visible scarring on the skin [13,14,15]. In 2007, da Vinci robot-assisted thyroid surgery was introduced [16]. Transoral robotic thyroidectomy (TORT) was introduced later [17,18]. It was found to be safe and effective in achieving functional and oncological outcomes [17]. In addition, it is more useful for identifying central lymph nodes than other thyroid surgery [17,18,19,20,21,22]. Robotic thyroid surgery using a trans-axillary approach is one of the most popular robotic techniques [21]. Conventional OT has already demonstrated excellent results for a long time. However, robotic-assisted thyroid surgery is relatively controversial [23].
Thus, the objective of this study was to perform a meta-analysis to evaluate the safety and effectiveness of TORT for thyroid tumor compared to traditional OT, robotic BABA, and TOETVA for operative outcomes, postoperative outcomes, and complications. To the best of our knowledge, this is the first meta-analysis that directly compares TORT with other approaches for thyroid tumors.
2. Materials and Methods
2.1. Search Strategy
PubMed, SCOPUS, Embase, the Web of Science, Google Scholar, and the Cochrane database were searched to retrieve prospective or retrospective articles published in English prior to June 2022. Thyroidectomy, transoral thyroidectomy, remote-access thyroid surgery, minimally invasive surgery, robotic thyroidectomy, robotic thyroid surgery, surgical approaches, transoral approach, vestibular approach, transoral-vestibular robotic thyroidectomy, bilateral axillo-breast approach, open thyroidectomy, thyroid neoplasm, thyroid carcinoma, thyroid nodule, cosmesis, and comparison were used as search terms and keywords. Two independent literature reviewers reviewed and screened titles and abstracts of all searched studies, excluding studies about patients with thyroid neoplasm, not related to TORT. If the abstract alone could not determine whether or not the study should be included, the full text was then checked to determine its eligibility. Figure 1 presents a flow chart for selecting eligible studies to be analyzed. We registered the study protocol on Open Science Framework (https://osf.io/gtnck/) (accessed on 25 July 2022).
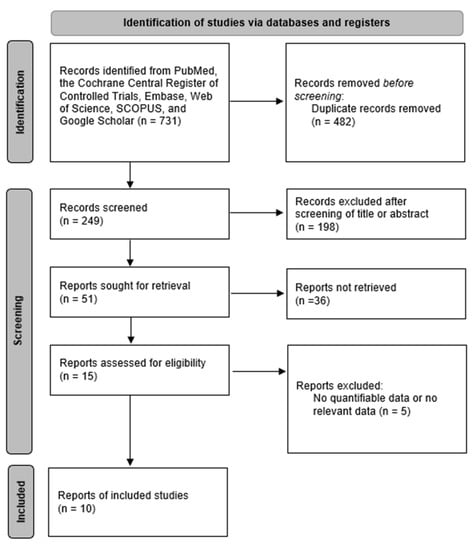
Figure 1.
Diagram of selection of studies.
2.2. Data Extraction and Risk of Bias Assessment
Data were extracted from selected eligible studies regarding the number of patients, scale and score used for cosmetic satisfaction, operative time, hospitalization day, postoperative pain score, retrieved lymph node (LN) number, incidence or percentage of postoperative bleeding, skin flap perforation, postoperative hypoparathyroidism, vocal cord palsy, incidental parathyroidectomy, seroma, and p-value in comparison between treatment (TORT) group and control group (other types of thyroidectomy, including robotic BABA, robotic PAA, conventional OT, TOETVA). Data were then organized using a standardized format [17,18,19,21,24,25,26,27,28,29,30]. The Newcastle–Ottawa Scale was used to evaluate non-randomized controlled studies [31,32].
2.3. Statistical Analysis
Meta-analyses were conducted using ‘R’ statistical software (R Foundation for Statistical Computing, Vienna, Austria). When representing the original data as a continuous variable, meta-analysis was performed using standard mean difference (SMD). For all other cases, incidence analyses were performed using odds ratio (OR). Sensitivity analyses were also conducted to assess the effect of each study on overall meta-analysis results.
3. Results
Ten studies of 1420 individuals were included. Study characteristics are summarized in Table 1. Egger’s test and Begg’s funnel plot analyses revealed no publication bias for operative time (OR 0.4171), retrieved LN number (OR 0.6468), or incidence of temporary vocal cord palsy (OR 0.19) in the included studies (Figure 2). In contrast, the number of included studies for cosmetic satisfaction, hospital day, pain score, and incidence of bleeding, flap perforation, parathyroidectomy, seroma, permanent hypoparathyroidism, and vocal cord palsy was insufficient to adequately obtain a funnel plot or perform advanced regression-based assessments. Thus, publication bias was not evaluated for them.

Table 1.
The characteristics of included studies.
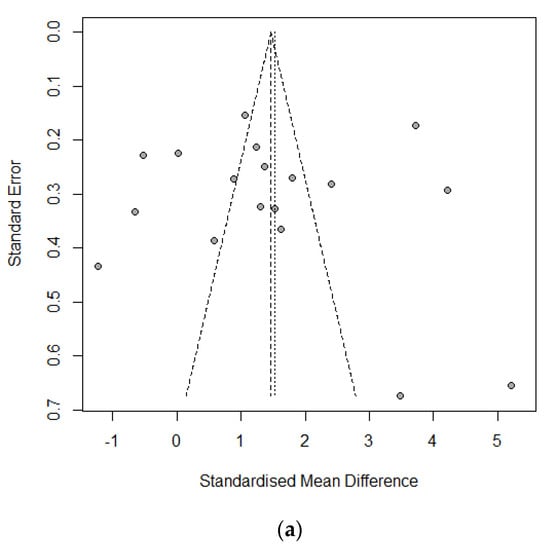
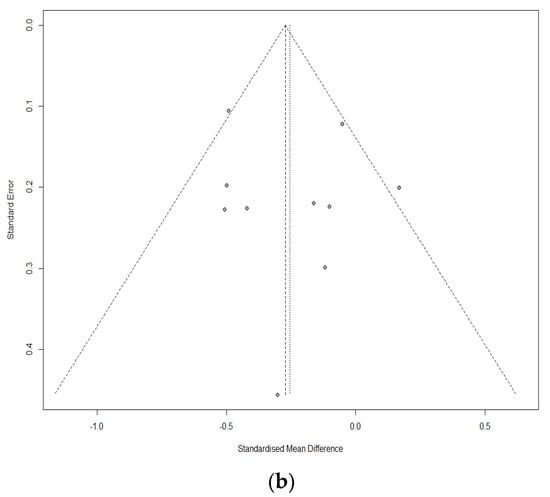
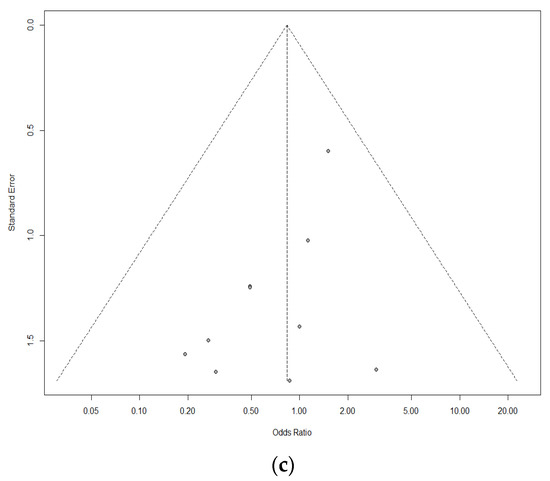
Figure 2.
Funnel plot for publication bias of operative time (a), retrieved lymph node number (b), and temporary vocal cord palsy (c).
3.1. Operation-Related Outcomes between Treatment Group and Control Group
The operative time (SMD 1.15, 95% CI [0.48; 1.82]; I2 = 96%) was significantly longer and the retrieved LN number (SMD −0.27, 95% CI [−0.39; −0.16]; I2 = 45%) was fewer in the TORT group than in the control group (Figure 3). There was no significant difference in the positive LN number (SMD −0.0858, 95% confidence interval (CI) [−0.3441; 0.1725]; I2 = 66.7%), incidence of skin flap perforation (OR 6.0165, 95% CI [0.9318; 38.8464]; I2 = 0.0%), or incidental parathyroidectomy (SMD 1.3392, 95% [0.3981; 4.5049]; I2 = 0.0%) between the treatment group and the control group (Table 2).
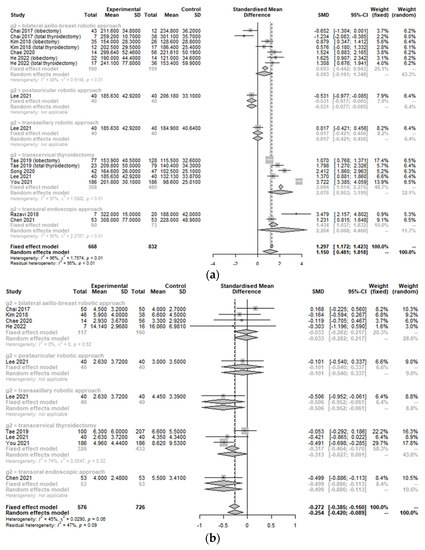
Figure 3.
Forest plot for standard mean difference in operation-related outcomes (operative time (a) and retrieved lymph node number (b)) (total: number of participants per group).

Table 2.
Subgroup analysis of operation-related measurements according to the comparison with other operative types.
There was a significant heterogeneity (I2 > 50%) in operation-related measurements because control groups involved different types of approaches. Therefore, subgroup analysis was conducted to assess the change in comparative advantage or disadvantage of TORT according to the type of operation. In subgroup analysis, there were no significant differences in some operation-related measurements between robotic surgeries (TORT, trans-axillary robotic approach, and robotic PAA). However, the TORT group had significantly longer operative times and fewer LNs retrieved than the conventional OT group (SMD 2.08, 95% CI [0.95; 3.20]/SMD −0.32, 95% CI [−0.46; −0.17]) and the TOETVA group (SMD 2.26, 95% CI [0.07; 4.46]/SMD −0.50, 95% CI [−0.89; −0.11]). The TORT group had significantly longer operative times than the robotic BABA group (SMD 0.59, 95% CI [−0.16; 1.35]).
3.2. Peri-Operative Complications between the Treatment Group and Control Group
Incidences of postoperative bleeding (hematoma) (OR 0.85, 95% CI [0.30; 2.45]; I2 = 0%), temporary and permanent hypoparathyroidism (OR 0.53, 95% CI [0.28; 1.00]; I2 = 0%/OR 0.87, 95% CI [0.15; 5.12]; I2 = 0%), temporary and permanent vocal cord palsy (OR 0.83, 95% CI [0.42; 1.66]; I2 = 0%/OR 1.06, 95% CI [0.12; 9.67]; I2 = 0%), and seroma (OR 1.06, 95% CI [0.62; 1.81]; I2 = 0%) were not significantly different between the TORT group and the control group (Figure 4). However, the postoperative infection rate (OR 10.67, 95% CI [1.24; 91.66]; I2 = 0.4%) was significantly higher in the TORT group than in the control group. There was no significant heterogeneity (I2 < 50%) in postoperative complications (Figure 4).
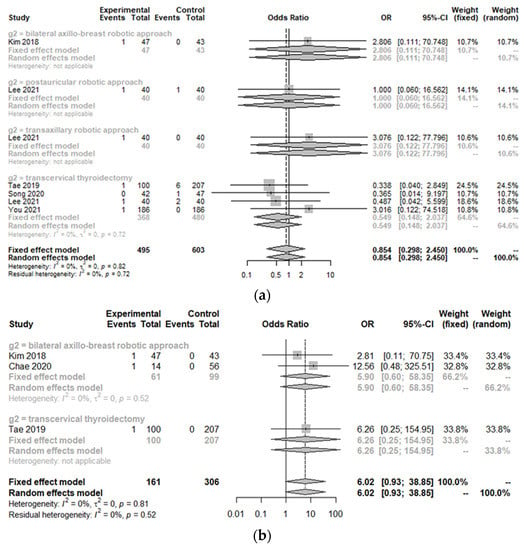
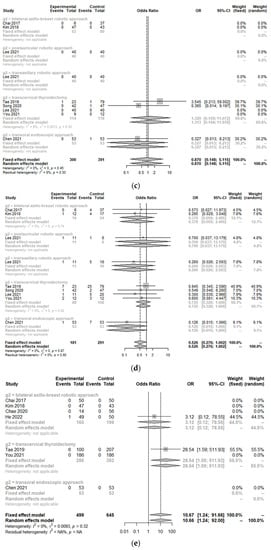
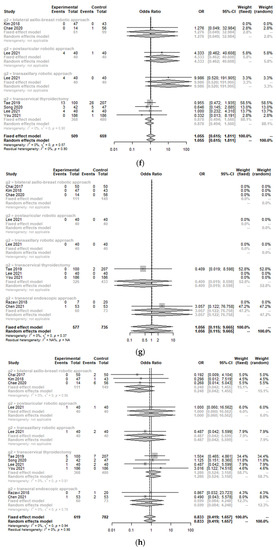
Figure 4.
Forest plot for standard mean difference in complications (hematoma (a), flap perforation (b), permanent hypoparathyroidism (c), temporary hypoparathyroidism (d), postoperative infection (e), seroma (f), permanent vocal cord palsy (g), and temporary vocal cord palsy (h)) (total: number of participants per group).
3.3. Postoperative Outcomes between the Treatment Group and Control Group
The postoperative cosmetic satisfaction score (SMD 0.60, 95% CI [0.28; 0.92]; I2 = 75%) was statistically higher in the TORT group than in the control group. However, there was no significant difference in postoperative pain score on op day (SMD −1.14, 95% CI [−2.34; 0.06]; I2 = 97.6%), at 1 day postoperation (SMD −0.68, 95% CI [−1.60; 0.23]; I2 = 96.9%), 2 days postoperation (SMD −0.71, 95% CI [−1.55; 0.14]; I2 = 96.4%), or 3 days postoperation (SMD 0.01, 95% CI [−0.31; 0.33]; I2 = 66.3%) or hospitalization days (SMD 0.14, 95% CI [−0.18; 0.45]; I2 = 76.8%) between the two groups (TORT and control) (Figure 5).
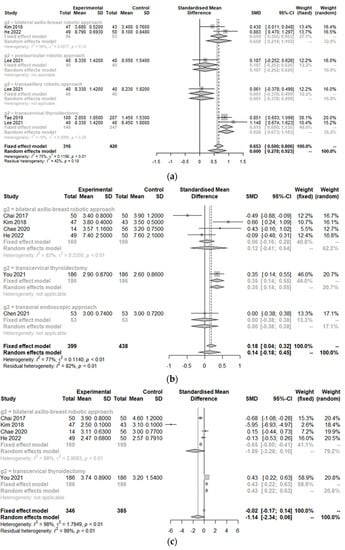
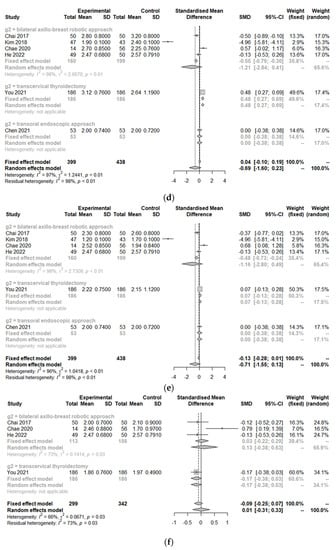
Figure 5.
Forest plot for standard mean difference in postoperative outcomes (cosmetic satisfaction (a), hospitalization day (b), postoperative pain score on the operation day (c), postoperative day 1 (d), day 2 (e), day 3 (f)) (total: number of participants per group).
Because of significant heterogeneity (I2 > 50%) in postoperative outcomes, subgroup analysis was conducted according to the type of operation. There were no significant differences in postoperative outcomes (cosmetics, pain, or hospitalization) between robotic surgeries (TORT and robotic BABA). However, the TORT group had significantly higher patient satisfaction regarding cosmetic view (SMD 0.93, 95% CI [0.67; 1.18]). The TORT group also showed significantly increased hospitalization days (SMD 0.35, 95% CI [0.18; 0.51]) and postoperative pain on the operation day (SMD 0.43, 95% CI [0.22; 0.63]) and the first postoperative day (SMD 0.48, 95% CI [0.27; 0.69]) compared to the conventional OT group.
3.4. Sensitivity Analyses
Sensitivity analyses were performed to determine differences in integrated estimates in such a way that the meta-analysis was repeated, excluding one study each time. All results were consistent with the results described above.
4. Discussion
This meta-analysis compared operative outcomes and complications of TORT with other thyroid surgeries (robotic BABA, robotic PAA, conventional OT, and TOETVA). Many systematic reviews and meta-analyses have compared robot-assisted thyroid surgery, endoscopic thyroid surgery, conventional OT, and other approaches [2,33,34,35,36,37,38,39,40,41,42,43]. However, to the best of our knowledge, this meta-analysis is the first to directly compare TORT with other approaches for thyroid tumors.
In our study, the operation time and retrieved LN number were significantly different between the TORT and the control group. The TORT group also showed significantly longer operation times and fewer retrieved LNs than the other approaches in the subgroup analysis. In general, in other surgeries as well as thyroid surgery, the surgical time of a robotic surgery is longer than that of a conventional OT [44,45]. This is because a robotic-assisted surgery requires additional operation time to make flaps and dock the robotic arm and system to approach the thyroid tumor [29]. The fact that there were fewer retrieved LNs in the TORT group from our study might be because the clinician’s accumulated experiences for TORT and conventional OT differed during the same period, resulting in a selection bias [46]. Because TORT is more newly introduced than conventional OT, the overall proficiency of TORT might be lower than that of OT. Robotic lateral neck dissection by a gasless unilateral axillo-breast approach is possible. The retrieved LN number can be changed if the clinician has more experience with the robotic-assisted approach and becomes skilled [47]. In addition, the reason why TORT has fewer retrieved LNs than conventional OT is probably that the insertion site in the oral cavity is narrow, or the carbon dioxide gas expansion makes the workspace unstable. This working environment can limit the motion range of the robotic arm for LN dissection. The type of LN dissection can also affect the retrieved LN numbers. Kim et al. have reported that the unilateral or bilateral central neck dissection type can affect retrieved LN numbers in thyroid cancer [46]. The robot-assisted approach does not have an incision at the lower central part of the neck. Thus, the central neck dissection range and retrieved LN number might be lower than those of OT [46]. For example, in robotic BABA, the central LN may not be sufficiently exposed because it is blocked by the clavicle [48]. In TORT, central neck dissection exposure could vary depending on the inflexible robotic arm and the camera. However, even though the retrieved LN number has been reported as a good prognostic factor in gastric or colon cancer surgery [49,50], whether the retrieved LN number affects survival and recurrence in thyroid cancer surgery is currently unclear [46].
According to our meta-analysis, the TORT group had a significantly higher postoperative cosmetic satisfaction score than the control group. Another study has also confirmed that cosmetic satisfaction with the robot-assisted approach is significantly higher than with conventional OT [34]. In particular, transoral thyroidectomy is a natural orifice transluminal endoscopic surgery technique that leaves no scar on the neck or body. In addition, it is less invasive than other minimally invasive thyroid surgery [51]. Although many extra-cervical approaches to the thyroid have been described to avoid anterior neck scarring, such as trans-axillary, robotic face lift, and so on, these incisions are not truly scar-free. Therefore, TORT tends to be more cosmetically effective than robotic BABA, almost reaching statistical significance (SMD 0.66, 95% CI [0.21; 1.10], p = 0.13).
However, this approach also has its own demerits beside the cosmetic aspect. Because the craniocaudal view and the workspace between both mental nerves are narrow, flap elevation might be technically challenging [51]. This difficulty causes this approach to exclude patients with a history of neck surgery or over-prominent mandible [30]. Because of this limited view and approach, patients with a highly placed upper pole of the thyroid, prominent Zuckerkandl tubercle, advanced thyroid cancer with tracheal or esophageal invasion, and posterior extrathyroid extension should be avoided. Wound changes from the external body surface to the oral cavity can also lead to numbness or paresthesia of the lower lip and chin skin (due to mental nerve injury) and postoperative infection (due to oral flora) [30]. Based on these, the clinician needs to decide which approach is suitable for patients accordingly.
From our results, the TORT group had significantly longer hospital stays than the OT group. The pain was also significantly more severe on the day of surgery and the first postoperative day in the TORT group than in the OT group. Compared to OT, TORT has a wider flap elevation with a working space from the intraoral incision and unilateral axilla to thyroid tumor [5], resulting in increased pain and a longer hospital stay. In another meta-analysis, the anterior chest pain score of a robot-assisted approach in the first week after surgery was also significantly higher than that of OT [52].
This meta-analysis has some limitations. First, since not many studies have directly analyzed TORT and other approaches for thyroid tumor, only a few studies were included in this meta-analysis. Second, most of the included studies were retrospective in nature. There were no randomized controlled trials. Thus, there might be a selection bias or reporting bias. Third, the incidence of complications was low in most groups, making it difficult to identify differences between TORT and control groups. Fourth, there might be heterogeneity of postoperative outcomes because postoperative management, such as surgical site compression, postoperative diet, and analgesic for pain control, differed depending on the institute. Lastly, postoperative pain was not evaluated by subgroup analysis depending on the location, such as the neck or anterior chest.
5. Conclusions
This meta-analysis found that TORT improved patients’ cosmetic satisfaction but increased operative time, hospitalization days, and postoperative pain compared to conventional OT. However, since the number of studies included in this meta-analysis was small, conclusive confirmation of the clinical benefit of TORT needs to be studied further.
Author Contributions
Conceptualization, Y.J.K. and S.H.H.; methodology, S.H.H., G.S. and J.-H.C.; software, J.-H.C.; validation, Y.J.K. and S.H.H.; formal analysis, S.H.H.; data curation, G.S., Y.J.K.; writing—original draft preparation, Y.J.K.; writing—review and editing, Y.J.K., J.-H.C. and S.H.H.; visualization, S.H.H.; supervision, J.-H.C. and S.H.H.; project administration, G.S., S.H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- De Vries, L.H.; Aykan, D.; Lodewijk, L.; Damen, J.A.A.; Borel Rinkes, I.H.M.; Vriens, M.R. Outcomes of Minimally Invasive Thyroid Surgery—A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 719397. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, S.; Franceschi, S.; Bray, F.; Wild, C.P.; Plummer, M.; Dal Maso, L. Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. N. Engl. J. Med. 2016, 375, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Jantharapattana, K.; Maethasith, J. Transaxillary gasless endoscopic thyroidectomy versus conventional open thyroidectomy: A randomized study. Eur. Arch. Otorhinolaryngol. 2017, 274, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Tae, K.; Ji, Y.B.; Song, C.M.; Ryu, J. Robotic and Endoscopic Thyroid Surgery: Evolution and Advances. Clin. Exp. Otorhinolaryngol. 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, J.H.; Kim, Y.H.; Lee, Y.S.; Chang, H.S.; Park, C.S.; Roh, M.R. Impact of postthyroidectomy scar on the quality of life of thyroid cancer patients. Ann. Dermatol. 2014, 26, 693–699. [Google Scholar] [CrossRef]
- Juarez, M.C.; Ishii, L.; Nellis, J.C.; Bater, K.; Huynh, P.P.; Fung, N.; Darrach, H.; Russell, J.O.; Ishii, M. Objectively measuring social attention of thyroid neck scars and transoral surgery using eye tracking. Laryngoscope 2019, 129, 2789–2794. [Google Scholar] [CrossRef]
- Hüscher, C.S.; Chiodini, S.; Napolitano, C.; Recher, A. Endoscopic right thyroid lobectomy. Surg. Endosc. 1997, 11, 877. [Google Scholar] [CrossRef]
- Miccoli, P.; Berti, P.; Conte, M.; Bendinelli, C.; Marcocci, C. Minimally invasive surgery for thyroid small nodules: Preliminary report. J. Endocrinol. Investig. 1999, 22, 849–851. [Google Scholar] [CrossRef]
- Ikeda, Y.; Takami, H.; Sasaki, Y.; Kan, S.; Niimi, M. Endoscopic neck surgery by the axillary approach. J. Am. Coll. Surg. 2000, 191, 336–340. [Google Scholar] [CrossRef]
- Lee, K.E.; Rao, J.; Youn, Y.K. Endoscopic thyroidectomy with the da Vinci robot system using the bilateral axillary breast approach (BABA) technique: Our initial experience. Surg. Laparosc. Endosc. Percutan. Tech. 2009, 19, e71–e75. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.W.; Kim, J.S.; Hur, S.M.; Kim, S.H.; Lee, S.K.; Choi, J.H.; Kim, S.; Lee, J.E.; Kim, J.H.; Nam, S.J.; et al. Is robotic surgery superior to endoscopic and open surgeries in thyroid cancer? World J. Surg. 2011, 35, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Luna-Ortiz, K.; Gómez-Pedraza, A.; Anuwong, A. Lessons Learned from the Transoral Endoscopic Thyroidectomy with Vestibular Approach (TOETVA) for the Treatment of Thyroid Carcinoma. Ann. Surg. Oncol. 2020, 27, 1356–1360. [Google Scholar] [CrossRef] [PubMed]
- Anuwong, A. Transoral Endoscopic Thyroidectomy Vestibular Approach: A Series of the First 60 Human Cases. World J. Surg. 2016, 40, 491–497. [Google Scholar] [CrossRef]
- Dionigi, G.; Bacuzzi, A.; Lavazza, M.; Inversini, D.; Pappalardo, V.; Boni, L.; Rausei, S.; Barczynski, M.; Tufano, R.P.; Kim, H.Y.; et al. Transoral endoscopic thyroidectomy via vestibular approach: Operative steps and video. Gland Surg. 2016, 5, 625–627. [Google Scholar] [CrossRef]
- Chang, E.H.E.; Kim, H.Y.; Koh, Y.W.; Chung, W.Y. Overview of robotic thyroidectomy. Gland Surg. 2017, 6, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.J.; Kim, H.Y.; Kim, H.K.; Jun, S.H.; Dionigi, G.; Anuwong, A.; Richmon, J.D.; Tufano, R.P. Comparative analysis of 2 robotic thyroidectomy procedures: Transoral versus bilateral axillo-breast approach. Head Neck 2018, 40, 886–892. [Google Scholar] [CrossRef]
- Kim, W.W.; Lee, J.; Jung, J.H.; Park, H.Y.; Tufano, R.P.; Kim, H.Y. A comparison study of the transoral and bilateral axillo-breast approaches in robotic thyroidectomy. J. Surg. Oncol. 2018, 118, 381–387. [Google Scholar] [CrossRef]
- You, J.Y.; Kim, H.Y.; Chai, Y.J.; Kim, H.K.; Anuwong, A.; Tufano, R.P.; Dionigi, G. Transoral Robotic Thyroidectomy Versus Conventional Open Thyroidectomy: Comparative Analysis of Surgical Outcomes in Thyroid Malignancies. J. Laparoendosc. Adv. Surg. Tech. A 2019, 29, 796–800. [Google Scholar] [CrossRef]
- Sun, H.; Dionigi, G. Applicability of transoral robotic thyroidectomy: Is it the final solution? J. Surg. Oncol. 2019, 119, 541–542. [Google Scholar] [CrossRef]
- Chen, Y.H.; Kim, H.Y.; Anuwong, A.; Huang, T.S.; Duh, Q.Y. Transoral robotic thyroidectomy versus transoral endoscopic thyroidectomy: A propensity-score-matched analysis of surgical outcomes. Surg. Endosc. 2021, 35, 6179–6189. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Kim, H.Y.; Chai, Y.J.; Dionigi, G.; Berber, E.; Tufano, R.P. Transoral Robotic Thyroidectomy: Comparison of Surgical Outcomes Between the da Vinci Xi and Si. Surg. Laparosc. Endosc. Percutan. Tech. 2018, 28, 404–409. [Google Scholar] [CrossRef]
- Paek, S.H.; Kang, K.H. Robotic thyroidectomy and cervical neck dissection for thyroid cancer. Gland Surg. 2016, 5, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Razavi, C.R.; Khadem, M.G.A.; Fondong, A.; Clark, J.H.; Richmon, J.D.; Tufano, R.P.; Russell, J.O. Early outcomes in transoral vestibular thyroidectomy: Robotic versus endoscopic techniques. Head Neck 2018, 40, 2246–2253. [Google Scholar] [CrossRef] [PubMed]
- Tae, K.; Ji, Y.B.; Song, C.M.; Park, J.S.; Park, J.H.; Kim, D.S. Safety and efficacy of transoral robotic and endoscopic thyroidectomy: The first 100 cases. Head Neck 2020, 42, 321–329. [Google Scholar] [CrossRef]
- Chae, S.; Min, S.Y.; Park, W.S. Comparison Study of Robotic Thyroidectomies Through a Bilateral Axillo-Breast Approach and a Transoral Approach. J. Laparoendosc. Adv. Surg. Tech. A 2020, 30, 175–182. [Google Scholar] [CrossRef]
- Song, C.M.; Park, J.S.; Park, H.J.; Tae, K. Voice outcomes of transoral robotic thyroidectomy: Comparison with conventional trans-cervical thyroidectomy. Oral Oncol. 2020, 107, 104748. [Google Scholar] [CrossRef]
- Lee, D.W.; Bang, H.S.; Jeong, J.H.; Kwak, S.G.; Choi, Y.Y.; Tae, K. Cosmetic outcomes after transoral robotic thyroidectomy: Comparison with transaxillary, postauricular, and conventional approaches. Oral Oncol. 2021, 114, 105139. [Google Scholar] [CrossRef]
- You, J.Y.; Kim, H.Y.; Park, D.W.; Yang, H.W.; Kim, H.K.; Dionigi, G.; Tufano, R.P. Transoral robotic thyroidectomy versus conventional open thyroidectomy: Comparative analysis of surgical outcomes using propensity score matching. Surg. Endosc. 2021, 35, 124–129. [Google Scholar] [CrossRef]
- He, Q.; Zhu, J.; Li, X.; Wang, M.; Wang, G.; Zhou, P.; Wang, D.; Liu, C.; Zheng, L.; Zhuang, D.; et al. A comparative study of two robotic thyroidectomy procedures: Transoral vestibular versus bilateral axillary-breast approach. BMC Surg. 2022, 22, 173. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.W.; Basurrah, M.A.; Hwang, S.H. Clinical and laboratory features for various criteria of eosinophilic chronic rhinosinusitis: A systematic review and meta-analysis. Clin. Exp. Otorhinolaryngol. 2022, 15, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Liu, K.; Xiong, J.J.; Zhu, J.Q. Robotic thyroidectomy versus conventional open thyroidectomy for differentiated thyroid cancer: Meta-analysis. J. Laryngol. Otol. 2015, 129, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.H.; Zhou, H.; Zhao, X.X.; Ding, H.; Wei, L.; Qin, L.; Pan, Y.L. Robotic thyroidectomy versus conventional open thyroidectomy for thyroid cancer: A systematic review and meta-analysis. Surg. Endosc. 2017, 31, 3985–4001. [Google Scholar] [CrossRef]
- Son, S.K.; Kim, J.H.; Bae, J.S.; Lee, S.H. Surgical safety and oncologic effectiveness in robotic versus conventional open thyroidectomy in thyroid cancer: A systematic review and meta-analysis. Ann. Surg. Oncol. 2015, 22, 3022–3032. [Google Scholar] [CrossRef]
- Jackson, N.R.; Yao, L.; Tufano, R.P.; Kandil, E.H. Safety of robotic thyroidectomy approaches: Meta-analysis and systematic review. Head Neck 2014, 36, 137–143. [Google Scholar] [CrossRef]
- Liu, J.; Song, T.; Xu, M. Minimally invasive video-assisted versus conventional open thyroidectomy: A systematic review of available data. Surg. Today 2012, 42, 848–856. [Google Scholar] [CrossRef]
- Sun, G.H.; Peress, L.; Pynnonen, M.A. Systematic review and meta-analysis of robotic vs conventional thyroidectomy approaches for thyroid disease. Otolaryngol. Head Neck Surg. 2014, 150, 520–532. [Google Scholar] [CrossRef]
- Kandil, E.; Hammad, A.Y.; Walvekar, R.R.; Hu, T.; Masoodi, H.; Mohamed, S.E.; Deniwar, A.; Stack, B.C., Jr. Robotic Thyroidectomy Versus Nonrobotic Approaches: A Meta-Analysis Examining Surgical Outcomes. Surg. Innov. 2016, 23, 317–325. [Google Scholar] [CrossRef]
- Jiang, W.J.; Yan, P.J.; Zhao, C.L.; Si, M.B.; Tian, W.; Zhang, Y.J.; Tian, H.W.; Feng, S.W.; Han, C.W.; Yang, J.; et al. Comparison of total endoscopic thyroidectomy with conventional open thyroidectomy for treatment of papillary thyroid cancer: A systematic review and meta-analysis. Surg. Endosc. 2020, 34, 1891–1903. [Google Scholar] [CrossRef]
- Chen, C.; Huang, S.; Huang, A.; Jia, Y.; Wang, J.; Mao, M.; Zhou, J.; Wang, L. Total endoscopic thyroidectomy versus conventional open thyroidectomy in thyroid cancer: A systematic review and meta-analysis. Ther. Clin. Risk Manag. 2018, 14, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.H.; Wong, C.K.; Tsang, J.S.; Wong, K.P. A systematic review and meta-analysis comparing outcomes between robotic-assisted thyroidectomy and non-robotic endoscopic thyroidectomy. J. Surg. Res. 2014, 191, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Qiu, Y.; Abuduwaili, M.; Xia, B.; Fei, Y.; Zhu, J.; Su, A. Surgical outcomes of different approaches in robotic assisted thyroidectomy for thyroid cancer: A systematic review and Bayesian network meta-analysis. Int. J. Surg. 2021, 89, 105941. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Yi, J.W.; Seong, C.Y.; Kim, J.K.; Bae, I.E.; Kwon, H.; Chai, Y.J.; Kim, S.J.; Choi, J.Y.; Lee, K.E. Development of a surgical training model for bilateral axillo-breast approach robotic thyroidectomy. Surg. Endosc. 2018, 32, 1360–1367. [Google Scholar] [CrossRef]
- Ohgami, M.; Ishii, S.; Arisawa, Y.; Ohmori, T.; Noga, K.; Furukawa, T.; Kitajima, M. Scarless endoscopic thyroidectomy: Breast approach for better cosmesis. Surg. Laparosc. Endosc. Percutan. Tech. 2000, 10, 1–4. [Google Scholar] [CrossRef]
- Kim, B.S.; Kang, K.H.; Kang, H.; Park, S.J. Central neck dissection using a bilateral axillo-breast approach for robotic thyroidectomy: Comparison with conventional open procedure after propensity score matching. Surg. Laparosc. Endosc. Percutan. Tech. 2014, 24, 67–72. [Google Scholar] [CrossRef]
- Tae, K.; Ji, Y.B.; Song, C.M.; Min, H.J.; Lee, S.H.; Kim, D.S. Robotic lateral neck dissection by a gasless unilateral axillobreast approach for differentiated thyroid carcinoma: Our early experience. Surg Laparosc. Endosc. Percutan. Tech. 2014, 24, e128-132. [Google Scholar] [CrossRef]
- Kang, S.W.; Lee, S.C.; Lee, S.H.; Lee, K.Y.; Jeong, J.J.; Lee, Y.S.; Nam, K.H.; Chang, H.S.; Chung, W.Y.; Park, C.S. Robotic thyroid surgery using a gasless, transaxillary approach and the da Vinci S system: The operative outcomes of 338 consecutive patients. Surgery 2009, 146, 1048–1055. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, Y.; Li, D.M.; Wang, Z.N.; Zhu, G.L.; Huang, B.J.; Li, K.; Xu, H.M. Log odds of positive lymph nodes: A novel prognostic indicator superior to the number-based and the ratio-based N category for gastric cancer patients with R0 resection. Cancer 2010, 116, 2571–2580. [Google Scholar] [CrossRef]
- Dimofte, G.; Târcoveanu, E.; Taraşi, M.; Panait, C.; Lozneanu, G.; Nicolescu, S.; Porumb, V.; Grigoraş, O. Mean number of lymph nodes in colonic cancer specimen: Possible quality control index for surgical performance. Chirurgia 2011, 106, 759–764. [Google Scholar]
- Anuwong, A.; Ketwong, K.; Jitpratoom, P.; Sasanakietkul, T.; Duh, Q.Y. Safety and Outcomes of the Transoral Endoscopic Thyroidectomy Vestibular Approach. JAMA Surg. 2018, 153, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Tae, K.; Ji, Y.B.; Jeong, J.H.; Lee, S.H.; Jeong, M.A.; Park, C.W. Robotic thyroidectomy by a gasless unilateral axillo-breast or axillary approach: Our early experiences. Surg. Endosc. 2011, 25, 221–228. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).