Recombinant Attenuated Salmonella enterica as a Delivery System of Heterologous Molecules in Cancer Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Live-Attenuated Salmonella enterica Strains in Cancer Therapy
3. Selectivity and Permanence of Salmonella enterica in the Tumor Microenvironment
4. Intrinsic Antitumor Activity of Salmonella enterica
4.1. Salmonella enterica Activates Death Domain Pathways in Tumor Cells
4.2. Salmonella enterica Activates the Antitumor Innate Immune Response
4.3. Salmonella enterica Activates the Antitumor Adaptive Immune Response
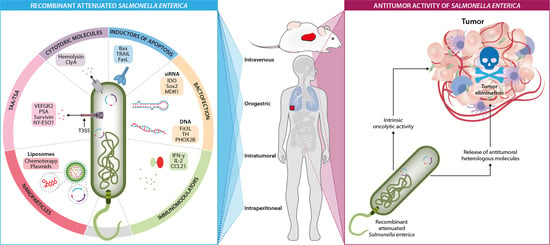
5. Salmonella enterica as a Delivery System of Heterologous Antitumor Molecules
5.1. Delivery of Tumor-Associated Antigens/Tumor-Specific Antigens
5.2. Delivery of Cytotoxic Molecules
5.3. Delivery of Immunomodulating Molecules and Apoptosis Inducers
5.4. Delivery of Nucleid Acids (Bactofection)
5.5. Delivery of Nanomolecules
6. Combination of Salmonella enterica and Conventional Antitumor Treatments
7. Clinical Trials Using Salmonella enterica as Cancer Treatment
8. Limitations
- (a)
- Biosafety. The attenuation of Salmonella enterica virulence factors was widely used to counteract its infectivity; but attenuation per se has been occasionally associated with a decrease in antitumor therapeutic efficacy [35,221]. It is imperative to establish a balance between decreased virulence and clinical efficacy. Several bacterial strains that initially yielded discouraging results have been improved in recent years, and have been shown to be much safer, as they maintain their tumor specificity, their antitumor efficacy has increased, and their toxicity in normal tissue has been minimized; this has optimized our ability to deliver antitumor therapeutic agents such as cytokines, cytotoxic drugs, tumor-associated antigens, and pro-drug enzymes [222].
- (b)
- Routes of administration. The route of administration of Salmonella enterica is pivotal to this vector’s safety and antitumor activity since the systemic administration of bacteria may be highly toxic and lead to serious adverse effects. Oral administration is considered the safest route, but at the expense of increased toxicity, possible adverse effects resulting from infection, and jeopardizing therapeutic efficacy.
- (c)
- Dose optimization. Since live bacteria proliferate in target tissues, an effective dose does not necessarily reflect the administered dose. An effective dose hinges on many factors such as the route of administration, the target tissues’ accessibility, the degree of vascularization, tumor immunogenicity, and the presence of infiltrating inflammatory cells in the tumor [222]. The administration of progressively escalating CFU doses appears to promote antitumor efficacy [126].
- (d)
- Genetic instability. Genetically modified live bacteria that carry antibiotic-resistance genes or mobile genetic elements are entirely inadequate for clinical use since these recombinant elements may be transferred horizontally from the plasmids in the treatment bacteria, thus carrying the antibiotic-resistant genes to other genes in the host or environment. Further, the plasmids may become lost or mutate upon reaching the tumor tissue and trigger an exaggerated infectious response or treatment failure. This drawback may be overcome by integrating a gene expression cassette with no antibiotic-resistance genes into the bacterial chromosome to guarantee genetic stability [223,224].
- (e)
- Control of bacterial growth in vivo. The uncontrolled growth and propagation of bacteria in the patient is of major concern, so alternatives that have been suggested include the incorporation of additional features to the modified strains, such as genetic “switches” that can guarantee bacterial containment [225,226,227]. Another approach consists in building lethal systems within the recombinant bacteria, the so-called “suicide genes”, that may specifically destroy the host bacterium without interfering with normal flora or therapeutic efficacy. Some attenuated Salmonella enterica strains are guaranteed to limit bacteria-derived infections, and should this fail, antibiotics can be used to eliminate persistent bacteria [41,228].
- (f)
- Patient selection. In clinical trials, patients that do not respond to conventional treatment or that are refractory to current standard therapies tend to be the subjects of interest for Salmonella enterica treatment; but patient selection for the administration of this treatment must be very thorough. Immunocompromised patients with underlying conditions or therapies must be excluded to prevent uncontrollable and overwhelming bacterial infection and its migration beyond the tumor site. Some patients appear to be predisposed to infections beyond the treatment’s aim. For example, certain bacteria preferentially proliferate in necrotic tissue, as is the case after radiation or due to associated comorbidities. The administered bacteria could also potentially colonize injuries or implanted medical devices such as artificial joints or valves, among others [229]. This could be prevented by correctly determining the administered dose, the route of administration, the administration intervals, and the timely elimination of bacteria post-administration. Thus, the clinician must scrupulously evaluate potential treatment candidates.
- (g)
- Pre-exposure and antibacterial immunity. One of the inconveniences of using bacteria as antitumor agents is the host’s immune response triggered when bacterial concentrations increase, and in the best case scenario, leads to the elimination of the introduced bacteria [229], leading to treatment failure. A possible solution would be the generation of optimized Salmonella strains with a greater immunostimulatory capacity and capable of overcoming the immunity resulting from bacterial pre-exposure [230]. Other proposed strategies suggest the encapsulation of Salmonella enterica with compounds that can prevent the binding of specific antibodies to the bacterium, and that do not hinder the bacteria’s ability to focus on the tumor [231]. The administration of escalating CFU doses appears to counteract antibacterial pre-immunity, thus permitting antitumor activity [126].
- (h)
- Production of biological agents. The manufacturing of live bacteria is significantly more complex than that of small molecule antitumor drugs. Unlike small molecules or other non-viable clinical agents, live therapeutic bacteria cannot be sterilized by filtration or heating, and that is the main challenge when producing biologicals following good manufacturing practices. Currently, the manufacture of bacteria-based cellular therapies, as in the case of Salmonella enterica, is a regulated process centered on product safety, consistency, and stability. The FDA recently published detailed industry guidelines on the information that should be provided when developing bacteria- or virus-based biological products. Included is a list of every component used in the manufacturing process; the generation of a seed stock; the expansion and characterization of the microbial mother/stem cell bank; the absence of any lysogenic prophage; information on the genome sequence; and all, if any, chromosomal modifications, phenotypic confirmation of attenuation, microbial purity (clonality), cell viability and stability, among others [224,232].
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coley, W.B., II. Contribution to the Knowledge of Sarcoma. Ann. Surg. 1891, 14, 199–220. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar] [PubMed]
- Saluja, M.; Gilling, P. Intravesical bacillus Calmette-Guérin instillation in non-muscle-invasive bladder cancer: A review. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2018, 25, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Barton, T. The Encyclopedia of Bacteriology Research Developments; Nova Science Publishers, Incorporated: New York, NY, USA, 2021; pp. 1281–1313. [Google Scholar]
- Hernández-Luna, M.A.; Luria-Pérez, R. Cancer Immunotherapy: Priming the Host Immune Response with Live Attenuated Salmonella enterica. J. Immunol. Res. 2018, 2018, 2984247. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Chávez, A.A.; Muñoz-López, P.; Becerra-Báez, E.I.; Flores-Martínez, L.F.; Prada-Gracia, D.; Moreno-Vargas, L.M.; Baay-Guzmán, G.J.; Juárez-Hernández, U.; Chávez-Munguía, B.; Cabrera-Muñóz, L.; et al. Live Attenuated Salmonella enterica Expressing and Releasing Cell-Permeable Bax BH3 Peptide Through the MisL Autotransporter System Elicits Antitumor Activity in a Murine Xenograft Model of Human B Non-hodgkin’s Lymphoma. Front. Immunol. 2019, 10, 2562. [Google Scholar] [CrossRef]
- Chavez-Navarro, H.H.-C.D.; Vilchis-Estrada, A.; Bermudez-Pulido, D.C.; Antonio-Andres, G.; Luria-Perez, R. Salmonella enterica: An ally in the therapy of cancer. Bol. Med. Del Hosp. Infant. De Mex. 2015, 72, 15–25. [Google Scholar]
- Duong, M.T.; Qin, Y.; You, S.H.; Min, J.J. Bacteria-cancer interactions: Bacteria-based cancer therapy. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Doleweerd, F. Salmonella enterica: Molecular Characterization, Role in Infectious Diseases and Emerging Research; Nova Science Publishers Inc.: Waltham, MA, USA, 2018. [Google Scholar]
- Forbes, N.S. Engineering the perfect (bacterial) cancer therapy. Nat. Rev. Cancer 2010, 10, 785–794. [Google Scholar] [CrossRef]
- Bacon, G.A.; Burrows, T.W.; Yates, M. The effects of biochemical mutation on the virulence of Bacterium typhosum; the loss of virulence of certain mutants. Br. J. Exp. Pathol. 1951, 32, 85–96. [Google Scholar]
- Hoiseth, S.K.; Stocker, B.A.D. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 1981, 291, 238–239. [Google Scholar] [CrossRef]
- O’Callaghan, D.; Maskell, D.; Liew, F.Y.; Easmon, C.S.; Dougan, G. Characterization of aromatic- and purine-dependent Salmonella typhimurium: Attention, persistence, and ability to induce protective immunity in BALB/c mice. Infect. Immun. 1988, 56, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, T.K.; Bushnell, B.; Meissler, J.J., Jr.; Dalal, N.; Schafer, R.; Havas, H.F. Immunotherapy of a plasmacytoma with attenuated salmonella. Med. Oncol. 1995, 12, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Guzmán, C.A.; Staendner, L.H.; Colombo, M.P.; Paglia, P. Salmonella vaccine carrier strains: Effective delivery system to trigger anti-tumor immunity by oral route. Eur. J. Immunol. 1999, 29, 693–699. [Google Scholar] [CrossRef]
- Gentschev, I.; Fensterle, J.; Schmidt, A.; Potapenko, T.; Troppmair, J.; Goebel, W.; Rapp, U.R. Use of a recombinant Salmonella enterica serovar Typhimurium strain expressing C-Raf for protection against C-Raf induced lung adenoma in mice. BMC Cancer 2005, 5, 15. [Google Scholar] [CrossRef]
- Yu, B.; Yang, M.; Shi, L.; Yao, Y.; Jiang, Q.; Li, X.; Tang, L.-H.; Zheng, B.-J.; Yuen, K.-Y.; Smith, D.K.; et al. Explicit hypoxia targeting with tumor suppression by creating an “obligate” anaerobic Salmonella typhimurium strain. Sci. Rep. 2012, 2, 436. [Google Scholar] [CrossRef]
- Chabalgoity, J.A.; Moreno, M.; Carol, H.; Dougan, G.; Hormaeche, C.E. Salmonella typhimurium as a basis for a live oral Echinococcus granulosus vaccine. Vaccine 2000, 19, 460–469. [Google Scholar] [CrossRef]
- Grille, S.; Moreno, M.; Bascuas, T.; Marqués, J.M.; Muñoz, N.; Lens, D.; Chabalgoity, J.A. Salmonella enterica serovar Typhimurium immunotherapy for B-cell lymphoma induces broad anti-tumour immunity with therapeutic effect. Immunology 2014, 143, 428–437. [Google Scholar] [CrossRef]
- al-Ramadi, B.K.; Fernandez-Cabezudo, M.J.; El-Hasasna, H.; Al-Salam, S.; Bashir, G.; Chouaib, S. Potent anti-tumor activity of systemically-administered IL2-expressing Salmonella correlates with decreased angiogenesis and enhanced tumor apoptosis. Clin. Immunol. 2009, 130, 89–97. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, M.; Li, X.M.; Jiang, P.; Baranov, E.; Li, S.; Xu, M.; Penman, S.; Hoffman, R.M. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 2005, 102, 755–760. [Google Scholar] [CrossRef]
- Zhang, Y.; Miwa, S.; Zhang, N.; Hoffman, R.M.; Zhao, M. Tumor-targeting Salmonella typhimurium A1-R arrests growth of breast-cancer brain metastasis. Oncotarget 2015, 6, 2615–2622. [Google Scholar] [CrossRef]
- Hayashi, K.; Zhao, M.; Yamauchi, K.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Hoffman, R.M. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium. J. Cell Biochem. 2009, 106, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Geller, J.; Ma, H.; Yang, M.; Penman, S.; Hoffman, R.M. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 10170–10174. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Zhao, M.; Yamauchi, K.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Kishimoto, H.; Bouvet, M.; Hoffman, R.M. Systemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhymurium. Cell Cycle 2009, 8, 870–875. [Google Scholar] [CrossRef]
- Nagakura, C.; Hayashi, K.; Zhao, M.; Yamauchi, K.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Bouvet, M.; Hoffman, R.M. Efficacy of a Genetically-modified Salmonella typhimurium in an Orthotopic Human Pancreatic Cancer in Nude Mice. Anticancer Res. 2009, 29, 1873. [Google Scholar] [PubMed]
- Kimura, H.; Zhang, L.; Zhao, M.; Hayashi, K.; Tsuchiya, H.; Tomita, K.; Bouvet, M.; Wessels, J.; Hoffman, R.M. Targeted therapy of spinal cord glioma with a genetically modified Salmonella typhimurium. Cell Prolif. 2010, 43, 41–48. [Google Scholar] [CrossRef]
- Clairmont, C.; Lee, K.C.; Pike, J.; Ittensohn, M.; Low, K.B.; Pawelek, J.; Bermudes, D.; Brecher, S.M.; Margitich, D.; Turnier, J.; et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J. Infect. Dis. 2000, 181, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Toso, J.F.; Gill, V.J.; Hwu, P.; Marincola, F.M.; Restifo, N.P.; Schwartzentruber, D.J.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Stock, F.; et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Pasetti, M.F.; Noriega, F.R.; Anderson, R.J.; Wasserman, S.S.; Galen, J.E.; Sztein, M.B.; Levine, M.M. Construction, genotypic and phenotypic characterization, and immunogenicity of attenuated DeltaguaBA Salmonella enterica serovar Typhi strain CVD 915. Infect. Immun. 2001, 69, 4734–4741. [Google Scholar] [CrossRef]
- Vendrell, A.; Gravisaco, M.J.; Pasetti, M.F.; Croci, M.; Colombo, L.; Rodríguez, C.; Mongini, C.; Waldner, C.I. A novel Salmonella Typhi-based immunotherapy promotes tumor killing via an antitumor Th1-type cellular immune response and neutrophil activation in a mouse model of breast cancer. Vaccine 2011, 29, 728–736. [Google Scholar] [CrossRef]
- Lewēn, S.; Zhou, H.; Hu, H.D.; Cheng, T.; Markowitz, D.; Reisfeld, R.A.; Xiang, R.; Luo, Y. A Legumain-based minigene vaccine targets the tumor stroma and suppresses breast cancer growth and angiogenesis. Cancer Immunol. Immunother. 2008, 57, 507–515. [Google Scholar] [CrossRef]
- Roider, E.; Jellbauer, S.; Köhn, B.; Berchtold, C.; Partilla, M.; Busch, D.H.; Rüssmann, H.; Panthel, K. Invasion and destruction of a murine fibrosarcoma by Salmonella-induced effector CD8 T cells as a therapeutic intervention against cancer. Cancer Immunol. Immunother. 2011, 60, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yu, B.; Cai, C.H.; Huang, J.D. Angiogenic inhibitors delivered by the type III secretion system of tumor-targeting Salmonella typhimurium safely shrink tumors in mice. AMB Express 2016, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Frahm, M.; Felgner, S.; Kocijancic, D.; Rohde, M.; Hensel, M.; Curtiss, R., 3rd; Erhardt, M.; Weiss, S. Efficiency of conditionally attenuated Salmonella enterica serovar Typhimurium in bacterium-mediated tumor therapy. mBio 2015, 6, e00254-15. [Google Scholar] [CrossRef]
- Felgner, S.; Frahm, M.; Kocijancic, D.; Rohde, M.; Eckweiler, D.; Bielecka, A.; Bueno, E.; Cava, F.; Abraham, W.-R.; Curtiss, R.; et al. aroA-Deficient Salmonella enterica Serovar Typhimurium Is More Than a Metabolically Attenuated Mutant. mBio 2016, 7, e01220-16. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Liu, Q.; Li, P.; Han, Y.; Bian, X.; Tang, Y.; Kong, Q. Endostatin gene therapy delivered by attenuated Salmonella typhimurium in murine tumor models. Cancer Gene Ther. 2018, 25, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhang, X.; Zhou, Y.; Zhang, C.; Hua, Z.-C. A Salmonella typhimurium mutant strain capable of RNAi delivery: Higher tumor-targeting and lower toxicity. Cancer Biol. Ther. 2014, 15, 1068–1076. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, L.; Zhao, L.; Guo, B.; Ji, K.; Tian, Y.; Wang, J.; Yu, H.; Hu, J.; Kalvakolanu, D.V.; et al. Intratumoral delivery and suppression of prostate tumor growth by attenuated Salmonella enterica serovar typhimurium carrying plasmid-based small interfering RNAs. Cancer Res. 2007, 67, 5859–5864. [Google Scholar] [CrossRef]
- Song, M.; Kim, H.J.; Kim, E.Y.; Shin, M.; Lee, H.C.; Hong, Y.; Rhee, J.H.; Yoon, H.; Ryu, S.; Lim, S.; et al. ppGpp-dependent stationary phase induction of genes on Salmonella pathogenicity island 1. J. Biol. Chem. 2004, 279, 34183–34190. [Google Scholar] [CrossRef]
- Kim, J.E.; Phan, T.X.; Nguyen, V.H.; Dinh-Vu, H.V.; Zheng, J.H.; Yun, M.; Park, S.G.; Hong, Y.; Choy, H.E.; Szardenings, M.; et al. Salmonella typhimurium Suppresses Tumor Growth via the Pro-Inflammatory Cytokine Interleukin-1β. Theranostics 2015, 5, 1328–1342. [Google Scholar] [CrossRef]
- Phan, T.X.; Nguyen, V.H.; Duong, M.T.; Hong, Y.; Choy, H.E.; Min, J.J. Activation of inflammasome by attenuated Salmonella typhimurium in bacteria-mediated cancer therapy. Microbiol. Immunol. 2015, 59, 664–675. [Google Scholar] [CrossRef]
- Chirullo, B.; Ammendola, S.; Leonardi, L.; Falcini, R.; Petrucci, P.; Pistoia, C.; Vendetti, S.; Battistoni, A.; Pasquali, P. Attenuated mutant strain of Salmonella typhimurium lacking the ZnuABC transporter contrasts tumor growth promoting anti-cancer immune response. Oncotarget 2015, 6, 17648–17660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saltzman, D.A.; Heise, C.P.; Hasz, D.E.; Zebede, M.; Kelly, S.M.; Curtiss, R., 3rd; Leonard, A.S.; Anderson, P.M. Attenuated Salmonella typhimurium containing interleukin-2 decreases MC-38 hepatic metastases: A novel anti-tumor agent. Cancer Biother. Radiopharm. 1996, 11, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Pawelek, J.M.; Low, K.B.; Bermudes, D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997, 57, 4537–4544. [Google Scholar] [PubMed]
- Crull, K.; Bumann, D.; Weiss, S. Influence of infection route and virulence factors on colonization of solid tumors by Salmonella enterica serovar Typhimurium. FEMS Immunol. Med. Microbiol. 2011, 62, 75–83. [Google Scholar] [CrossRef]
- Domingos-Pereira, S.; Sathiyanadan, K.; La Rosa, S.; Polák, L.; Chevalier, M.F.; Martel, P.; Hojeij, R.; Derré, L.; Haefliger, J.A.; Jichlinski, P.; et al. Intravesical Ty21a Vaccine Promotes Dendritic Cells and T Cell-Mediated Tumor Regression in the MB49 Bladder Cancer Model. Cancer Immunol. Res. 2019, 7, 621–629. [Google Scholar] [CrossRef]
- Ganai, S.; Arenas, R.B.; Sauer, J.P.; Bentley, B.; Forbes, N.S. In tumors Salmonella migrate away from vasculature toward the transition zone and induce apoptosis. Cancer Gene Ther. 2011, 18, 457–466. [Google Scholar] [CrossRef]
- Chorobik, P.; Czaplicki, D.; Ossysek, K.; Bereta, J. Salmonella and cancer: From pathogens to therapeutics. Acta Biochim. Pol. 2013, 60, 285–297. [Google Scholar] [CrossRef]
- Wei, M.Q.; Ellem, K.A.; Dunn, P.; West, M.J.; Bai, C.X.; Vogelstein, B. Facultative or obligate anaerobic bacteria have the potential for multimodality therapy of solid tumours. Eur. J. Cancer 2007, 43, 490–496. [Google Scholar] [CrossRef]
- Kasinskas, R.W.; Forbes, N.S. Salmonella typhimurium specifically chemotax and proliferate in heterogeneous tumor tissue in vitro. Biotechnol. Bioeng. 2006, 94, 710–721. [Google Scholar] [CrossRef]
- Kasinskas, R.W.; Forbes, N.S. Salmonella typhimurium lacking ribose chemoreceptors localize in tumor quiescence and induce apoptosis. Cancer Res. 2007, 67, 3201–3209. [Google Scholar] [CrossRef]
- Anderson, C.J.; Clark, D.E.; Adli, M.; Kendall, M.M. Ethanolamine Signaling Promotes Salmonella Niche Recognition and Adaptation during Infection. PLoS Pathogens 2015, 11, e1005278. [Google Scholar]
- Cheng, M.; Bhujwalla, Z.M.; Glunde, K. Targeting Phospholipid Metabolism in Cancer. Front. Oncol. 2016, 6, 266. [Google Scholar] [CrossRef] [PubMed]
- Silva-Valenzuela, C.A.; Desai, P.T.; Molina-Quiroz, R.C.; Pezoa, D.; Zhang, Y.; Porwollik, S.; Zhao, M.; Hoffman, R.M.; Contreras, I.; Santiviago, C.A.; et al. Solid tumors provide niche-specific conditions that lead to preferential growth of Salmonella. Oncotarget 2016, 7, 35169–35180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Toley, B.J.; Forbes, N.S. Motility is critical for effective distribution and accumulation of bacteria in tumor tissue. Integr. Biol. Quant. Biosci. Nano Macro 2012, 4, 165–176. [Google Scholar] [CrossRef]
- Broadway, K.M.; Suh, S.; Behkam, B.; Scharf, B.E. Optimizing the restored chemotactic behavior of anticancer agent Salmonella enterica serovar Typhimurium VNP20009. J. Biotechnol. 2017, 251, 76–83. [Google Scholar] [CrossRef]
- Broadway, K.M.; Denson, E.A.; Jensen, R.V.; Scharf, B.E. Rescuing chemotaxis of the anticancer agent Salmonella enterica serovar Typhimurium VNP20009. J. Biotechnol. 2015, 211, 117–120. [Google Scholar] [CrossRef]
- Stritzker, J.; Weibel, S.; Seubert, C.; Götz, A.; Tresch, A.; van Rooijen, N.; Oelschlaeger, T.A.; Hill, P.J.; Gentschev, I.; Szalay, A.A. Enterobacterial tumor colonization in mice depends on bacterial metabolism and macrophages but is independent of chemotaxis and motility. Int. J. Med. Microbiol. 2010, 300, 449–456. [Google Scholar] [CrossRef]
- Westphal, K.; Leschner, S.; Jablonska, J.; Loessner, H.; Weiss, S. Containment of tumor-colonizing bacteria by host neutrophils. Cancer Res. 2008, 68, 2952–2960. [Google Scholar] [CrossRef]
- Kaimala, S.; Mohamed, Y.A.; Nader, N.; Issac, J.; Elkord, E.; Chouaib, S.; Fernandez-Cabezudo, M.J.; Al-Ramadi, B.K. Salmonella-mediated tumor regression involves targeting of tumor myeloid suppressor cells causing a shift to M1-like phenotype and reduction in suppressive capacity. Cancer Immunol. Immunother. 2014, 63, 587–599. [Google Scholar] [CrossRef]
- Loeffler, M.; Le’Negrate, G.; Krajewska, M.; Reed, J.C. Salmonella typhimurium engineered to produce CCL21 inhibit tumor growth. Cancer Immunol. Immunother. 2009, 58, 769–775. [Google Scholar] [CrossRef]
- Crull, K.; Rohde, M.; Westphal, K.; Loessner, H.; Wolf, K.; Felipe-López, A.; Hensel, M.; Weiss, S. Biofilm formation by Salmonella enterica serovar Typhimurium colonizing solid tumours. Cell. Microbiol. 2011, 13, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.; Pan, S.; Jiang, S.N.; Nguyen, V.H.; Park, S.H.; Jung, C.H.; Kim, H.S.; Min, J.J.; Choy, H.E.; Hong, Y. Effect of Salmonella treatment on an implanted tumor (CT26) in a mouse model. J. Microbiol. 2012, 50, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Kazmierczak, R.A.; Eisenstark, A. Phenotypic evolution of therapeutic Salmonella enterica serovar Typhimurium after invasion of TRAMP mouse prostate tumor. mBio 2014, 5, e01182-14. [Google Scholar] [CrossRef] [PubMed]
- Vendrell, A.; Gravisaco, M.J.; Goin, J.C.; Pasetti, M.F.; Herschllik, L.; De Toro, J.; Rodríguez, C.; Larotonda, G.; Mongini, C.; Waldner, C.I. Therapeutic effects of Salmonella typhi in a mouse model of T-cell lymphoma. J. Immunother. 2013, 36, 171–180. [Google Scholar] [CrossRef]
- Grille, S.; Moreno, M.; Brugnini, A.; Lens, D.; Chabalgoity, J.A. A therapeutic vaccine using Salmonella-modified tumor cells combined with interleukin-2 induces enhanced antitumor immunity in B-cell lymphoma. Leuk. Res. 2013, 37, 341–348. [Google Scholar] [CrossRef]
- Raman, V.; Van Dessel, N.; O’Connor, O.M.; Forbes, N.S. The motility regulator flhDC drives intracellular accumulation and tumor colonization of Salmonella. J. ImmunoTherapy Cancer 2019, 7, 44. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, M.; Ma, H.; Li, X.; Tan, X.; Li, S.; Yang, Z.; Hoffman, R.M. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 2006, 66, 7647–7652. [Google Scholar] [CrossRef]
- Fu, X.Y.; Besterman, J.M.; Monosov, A.; Hoffman, R.M. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc. Natl. Acad. Sci. USA 1991, 88, 9345–9349. [Google Scholar] [CrossRef]
- Hoffman, R.M. Patient-derived orthotopic xenografts: Better mimic of metastasis than subcutaneous xenografts. Nat. Rev. Cancer 2015, 15, 451–452. [Google Scholar] [CrossRef]
- Igarashi, K.; Kawaguchi, K.; Kiyuna, T.; Miyake, K.; Miyake, M.; Li, S.; Han, Q.; Tan, Y.; Zhao, M.; Li, Y.; et al. Tumor-targeting Salmonella typhimurium A1-R combined with recombinant methioninase and cisplatinum eradicates an osteosarcoma cisplatinum-resistant lung metastasis in a patient-derived orthotopic xenograft (PDOX) mouse model: Decoy, trap and kill chemotherapy moves toward the clinic. Cell Cycle 2018, 17, 801–809. [Google Scholar]
- Igarashi, K.; Kawaguchi, K.; Murakami, T.; Kiyuna, T.; Miyake, K.; Nelson, S.D.; Dry, S.M.; Li, Y.; Yanagawa, J.; Russell, T.A.; et al. Intra-arterial administration of tumor-targeting Salmonella typhimurium A1-R regresses a cisplatin-resistant relapsed osteosarcoma in a patient-derived orthotopic xenograft (PDOX) mouse model. Cell Cycle 2017, 16, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Igarashi, K.; Kawaguchi, K.; Kiyuna, T.; Zhang, Y.; Zhao, M.; Hiroshima, Y.; Nelson, S.D.; Dry, S.M.; Li, Y.; et al. Tumor-targeting Salmonella typhimurium A1-R regresses an osteosarcoma in a patient-derived xenograft model resistant to a molecular-targeting drug. Oncotarget 2017, 8, 8035–8042. [Google Scholar] [CrossRef] [PubMed]
- Kiyuna, T.; Murakami, T.; Tome, Y.; Kawaguchi, K.; Igarashi, K.; Zhang, Y.; Zhao, M.; Li, Y.; Bouvet, M.; Kanaya, F.; et al. High efficacy of tumor-targeting Salmonella typhimurium A1-R on a doxorubicin- and dactolisib-resistant follicular dendritic-cell sarcoma in a patient-derived orthotopic xenograft PDOX nude mouse model. Oncotarget 2016, 7, 33046–33054. [Google Scholar] [CrossRef]
- Murakami, T.; DeLong, J.; Eilber, F.C.; Zhao, M.; Zhang, Y.; Zhang, N.; Singh, A.; Russell, T.; Deng, S.; Reynoso, J.; et al. Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget 2016, 7, 12783–12790. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kawaguchi, K.; Kiyuna, T.; Miyake, K.; Miyake, M.; Singh, A.S.; Eckardt, M.A.; Nelson, S.D.; Russell, T.A.; Dry, S.M.; et al. Tumor-targeting Salmonella typhimurium A1-R is a highly effective general therapeutic for undifferentiated soft tissue sarcoma patient-derived orthotopic xenograft nude-mouse models. Biochem. Biophys. Res. Commun. 2018, 497, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.M. Patient-Derived Orthotopic Xenograft (PDOX) Models of Melanoma. Int. J. Mol. Sci. 2017, 18, 1875. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Igarashi, K.; Murakami, T.; Chmielowski, B.; Kiyuna, T.; Zhao, M.; Zhang, Y.; Singh, A.; Unno, M.; Nelson, S.D.; et al. Tumor-targeting Salmonella typhimurium A1-R combined with temozolomide regresses malignant melanoma with a BRAF-V600E mutation in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget 2016, 7, 85929–85936. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Igarashi, K.; Murakami, T.; Kiyuna, T.; Zhao, M.; Zhang, Y.; Nelson, S.D.; Russell, T.A.; Dry, S.M.; Singh, A.S.; et al. Salmonella typhimurium A1-R targeting of a chemotherapy-resistant BRAF-V600E melanoma in a patient-derived orthotopic xenograft (PDOX) model is enhanced in combination with either vemurafenib or temozolomide. Cell Cycle 2017, 16, 1288–1294. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Igarashi, K.; Murakami, T.; Zhao, M.; Zhang, Y.; Chmielowski, B.; Kiyuna, T.; Nelson, S.D.; Russell, T.A.; Dry, S.M.; et al. Tumor-Targeting Salmonella typhimurium A1-R Sensitizes Melanoma With a BRAF-V600E Mutation to Vemurafenib in a Patient-Derived Orthotopic Xenograft (PDOX) Nude Mouse Model. J. Cell Biochem. 2017, 118, 2314–2319. [Google Scholar] [CrossRef]
- Yamamoto, M.; Zhao, M.; Hiroshima, Y.; Zhang, Y.; Shurell, E.; Eilber, F.C.; Bouvet, M.; Noda, M.; Hoffman, R.M. Efficacy of Tumor-Targeting Salmonella A1-R on a Melanoma Patient-Derived Orthotopic Xenograft (PDOX) Nude-Mouse Model. PLoS ONE 2016, 11, e0160882. [Google Scholar] [CrossRef]
- Zhang, Y.; Tome, Y.; Suetsugu, A.; Zhang, L.; Zhang, N.; Hoffman, R.M.; Zhao, M. Determination of the optimal route of administration of Salmonella typhimurium A1-R to target breast cancer in nude mice. Anticancer Res. 2012, 32, 2501–2508. [Google Scholar] [PubMed]
- Eisenstark, A.; Kazmierczak, R.A.; Dino, A.; Khreis, R.; Newman, D.; Schatten, H. Development of Salmonella strains as cancer therapy agents and testing in tumor cell lines. Methods Mol. Biol. (Clifton NJ) 2007, 394, 323–354. [Google Scholar]
- Spector, M.P.; Garcia Del Portillo, F.; Bearson, S.M.D.; Mahmud, A.; Magut, M.; Finlay, B.B.; Dougan, G.; Foster, J.W.; Pallen, M.J. The rpoS-dependent starvation-stress response locus stiA encodes a nitrate reductase (narZYWV) required for carbon-starvation-inducible thermotolerance and acid tolerance in Salmonella typhimurium. Microbiology 1999, 145 Pt 11, 3035–3045. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Barak, Y.; Schreiber, F.; Thorne, S.H.; Contag, C.H.; deBeer, D.; Matin, A. Role of nitric oxide in Salmonella typhimurium-mediated cancer cell killing. BMC Cancer 2010, 10, 146. [Google Scholar] [CrossRef]
- Tu, D.G.; Chang, W.W.; Lin, S.T.; Kuo, C.Y.; Tsao, Y.T.; Lee, C.H. Salmonella inhibits tumor angiogenesis by downregulation of vascular endothelial growth factor. Oncotarget 2016, 7, 37513–37523. [Google Scholar] [CrossRef]
- Lee, C.H.; Lin, S.T.; Liu, J.J.; Chang, W.W.; Hsieh, J.L.; Wang, W.K. Salmonella induce autophagy in melanoma by the downregulation of AKT/mTOR pathway. Gene Ther. 2014, 21, 309–316. [Google Scholar] [CrossRef]
- Kuan, Y.D.; Lee, C.H. Salmonella overcomes tumor immune tolerance by inhibition of tumor indoleamine 2, 3-dioxygenase 1 expression. Oncotarget 2016, 7, 374–385. [Google Scholar] [CrossRef]
- Stoll, G.; Iribarren, K.; Michels, J.; Leary, A.; Zitvogel, L.; Cremer, I.; Kroemer, G. Calreticulin expression: Interaction with the immune infiltrate and impact on survival in patients with ovarian and non-small cell lung cancer. Oncoimmunology 2016, 5, e1177692. [Google Scholar] [CrossRef]
- Tsao, Y.T.; Kuo, C.Y.; Cheng, S.P.; Lee, C.H. Downregulations of AKT/mTOR Signaling Pathway for Salmonella-Mediated Suppression of Matrix Metalloproteinases-9 Expression in Mouse Tumor Models. Int. J. Mol. Sci. 2018, 19, 1630. [Google Scholar] [CrossRef]
- Siccardi, D.; Mumy, K.L.; Wall, D.M.; Bien, J.D.; McCormick, B.A. Salmonella enterica serovar Typhimurium modulates P-glycoprotein in the intestinal epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1392–G1400. [Google Scholar] [CrossRef] [PubMed]
- Takara, K.; Sakaeda, T.; Okumura, K. An update on overcoming MDR1-mediated multidrug resistance in cancer chemotherapy. Curr. Pharm. Des. 2006, 12, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Lubo, R.; Zhang, Y.; Zhao, L.; Rossi, K.; Wu, X.; Zou, Y.; Castillo, A.; Leonard, J.; Bortell, R.; Greiner, D.L.; et al. A Salmonella nanoparticle mimic overcomes multidrug resistance in tumours. Nat. Commun. 2016, 7, 12225. [Google Scholar] [CrossRef] [PubMed]
- Mónaco, A.; Chilibroste, S.; Yim, L.; Chabalgoity, J.A.; Moreno, M. Inflammasome activation, NLRP3 engagement and macrophage recruitment to tumor microenvironment are all required for Salmonella antitumor effect. Cancer Immunol. Immunother. 2022, 71, 2141–2150. [Google Scholar] [CrossRef] [PubMed]
- Avogadri, F.; Martinoli, C.; Petrovska, L.; Chiodoni, C.; Transidico, P.; Bronte, V.; Longhi, R.; Colombo, M.P.; Dougan, G.; Rescigno, M. Cancer immunotherapy based on killing of Salmonella-infected tumor cells. Cancer Res. 2005, 65, 3920–3927. [Google Scholar] [CrossRef]
- Zhong, Z.; Kazmierczak, R.A.; Dino, A.; Khreis, R.; Eisenstark, A.; Schatten, H. Salmonella-host cell interactions, changes in host cell architecture, and destruction of prostate tumor cells with genetically altered Salmonella. Microsc. Microanal. 2007, 13, 372–383. [Google Scholar] [CrossRef]
- Kurashige, S.; Mitsuhashi, S. Enhancing effects of mini-cells prepared from Salmonella typhimurium on anti-tumor immunity in sarcoma 180-bearing mice. Cancer Immunol. Immunother. 1982, 14, 1–3. [Google Scholar] [CrossRef]
- Kurashige, S.; Akuzawa, Y.; Mitsuhashi, S. Synergistic anti-suppressor effect of mini cells prepared from Salmonella typhimurium and mitomycin C in EL 4-bearing mice. Cancer Immunol. Immunother. 1985, 19, 127–129. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Vogel, S.N. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002, 4, 903–914. [Google Scholar] [CrossRef]
- Leschner, S.; Westphal, K.; Dietrich, N.; Viegas, N.; Jablonska, J.; Lyszkiewicz, M.; Lienenklaus, S.; Falk, W.; Gekara, N.; Loessner, H.; et al. Tumor invasion of Salmonella enterica serovar Typhimurium is accompanied by strong hemorrhage promoted by TNF-alpha. PLoS ONE 2009, 4, e6692. [Google Scholar] [CrossRef]
- Kocijancic, D.; Leschner, S.; Felgner, S.; Komoll, R.M.; Frahm, M.; Pawar, V.; Weiss, S. Therapeutic benefit of Salmonella attributed to LPS and TNF-α is exhaustible and dictated by tumor susceptibility. Oncotarget 2017, 8, 36492–36508. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wu, C.L.; Shiau, A.L. Toll-like receptor 4 mediates an antitumor host response induced by Salmonella choleraesuis. Clin. Cancer Res. 2008, 14, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Hsieh, J.L.; Wu, C.L.; Hsu, P.Y.; Shiau, A.L. T cell augments the antitumor activity of tumor-targeting Salmonella. Appl. Microbiol. Biotechnol. 2011, 90, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Hsieh, J.L.; Wu, C.L.; Hsu, H.C.; Shiau, A.L. B cells are required for tumor-targeting Salmonella in host. Appl. Microbiol. Biotechnol. 2011, 92, 1251–1260. [Google Scholar] [CrossRef]
- Leigh, N.D.; Bian, G.; Ding, X.; Liu, H.; Aygun-Sunar, S.; Burdelya, L.G.; Gudkov, A.V.; Cao, X. A flagellin-derived toll-like receptor 5 agonist stimulates cytotoxic lymphocyte-mediated tumor immunity. PLoS ONE 2014, 9, e85587. [Google Scholar]
- Cai, Z.; Sanchez, A.; Shi, Z.; Zhang, T.; Liu, M.; Zhang, D. Activation of Toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growth. Cancer Res. 2011, 71, 2466–2475. [Google Scholar] [CrossRef]
- Flentie, K.; Gonzalez, C.; Kocher, B.; Wang, Y.; Zhu, H.; Marasa, J.; Piwnica-Worms, D. Nucleoside Diphosphate Kinase-3 (NME3) Enhances TLR5-Induced NFκB Activation. Mol. Cancer Res. 2018, 16, 986. [Google Scholar] [CrossRef]
- Hancz, D.; Szabo, A.; Molnar, T.; Varga, Z.; Hancz, A.; Gregus, A.; Hueber, A.O.; Rajnavolgyi, E.; Koncz, G. Flagellin increases death receptor-mediated cell death in a RIP1-dependent manner. Immunol. Lett. 2018, 193, 42–50. [Google Scholar] [CrossRef]
- Sfondrini, L.; Rossini, A.; Besusso, D.; Merlo, A.; Tagliabue, E.; Mènard, S.; Balsari, A. Antitumor activity of the TLR-5 ligand flagellin in mouse models of cancer. J. Immunol. 2006, 176, 6624–6630. [Google Scholar] [CrossRef]
- Braga, C.J.M.; Rittner, G.M.G.; Muñoz Henao, J.E.; Teixeira, A.F.; Massis, L.M.; Sbrogio-Almeida, M.E.; Taborda, C.P.; Travassos, L.R.; Ferreira, L.C.S. Paracoccidioides brasiliensis vaccine formulations based on the gp43-derived P10 sequence and the Salmonella enterica FliC flagellin. Infect. Immun. 2009, 77, 1700–1707. [Google Scholar] [CrossRef]
- Zheng, J.H.; Nguyen, V.H.; Jiang, S.N.; Park, S.H.; Tan, W.; Hong, S.H.; Shin, M.G.; Chung, I.J.; Hong, Y.; Bom, H.S.; et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci. Transl. Med. 2017, 9, eaak9537. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-Y.; Kim, Y.-J.; Ko, H.-J. Potential therapeutic anti-tumor effect of a Salmonella-based vaccine. Hum. Vaccin Immunother. 2013, 9, 1654–1660. [Google Scholar] [CrossRef][Green Version]
- Liu, T.; Chopra, A.K. An enteric pathogen Salmonella enterica serovar Typhimurium suppresses tumor growth by downregulating CD44high and CD4T regulatory (Treg) cell expression in mice: The critical role of lipopolysaccharide and Braun lipoprotein in modulating tumor growth. Cancer Gene Ther. 2010, 17, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Fallarino, F.; Grohmann, U.; Puccetti, P. Indoleamine 2,3-dioxygenase: From catalyst to signaling function. Eur. J. Immunol. 2012, 42, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H. Indoleamine 2,3-dioxygenase, tumor-induced tolerance and counter-regulation. Curr. Opin. Immunol. 2006, 18, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Hurley, D.; McCusker, M.P.; Fanning, S.; Martins, M. Salmonella–Host Interactions—Modulation of the Host Innate Immune System. Front. Immunol. 2014, 5, 481. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.A.; Shabahang, S.; Timiryasova, T.M.; Zhang, Q.; Beltz, R.; Gentschev, I.; Goebel, W.; Szalay, A.A. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat. Biotechnol. 2004, 22, 313–320. [Google Scholar] [CrossRef]
- O’Donnell, H.; McSorley, S.J. Salmonella as a model for non-cognate Th1 cell stimulation. Front. Immunol. 2014, 5, 621. [Google Scholar]
- Shilling, D.A.; Smith, M.J.; Tyther, R.; Sheehan, D.; England, K.; Kavanagh, E.G.; Redmond, H.P.; Shanahan, F.; O’Mahony, L. Salmonella typhimurium stimulation combined with tumour-derived heat shock proteins induces potent dendritic cell anti-tumour responses in a murine model. Clin. Exp. Immunol. 2007, 149, 109–116. [Google Scholar] [CrossRef]
- Saccheri, F.; Pozzi, C.; Avogadri, F.; Barozzi, S.; Faretta, M.; Fusi, P.; Rescigno, M. Bacteria-induced gap junctions in tumors favor antigen cross-presentation and antitumor immunity. Sci. Transl. Med. 2010, 2, 44ra57. [Google Scholar] [CrossRef]
- Oviedo-Orta, E.; Howard Evans, W. Gap junctions and connexin-mediated communication in the immune system. Biochim. Et Biophys. Acta 2004, 1662, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Matsue, H.; Yao, J.; Matsue, K.; Nagasaka, A.; Sugiyama, H.; Aoki, R.; Kitamura, M.; Shimada, S. Gap junction-mediated intercellular communication between dendritic cells (DCs) is required for effective activation of DCs. J. Immunol. 2006, 176, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Guo, B.; Jia, H.; Ji, K.; Sun, Y.; Li, Y.; Zhao, T.; Gao, L.; Meng, Y.; Kalvakolanu, D.V.; et al. Targeted therapy via oral administration of attenuated Salmonella expression plasmid-vectored Stat3-shRNA cures orthotopically transplanted mouse HCC. Cancer Gene Ther. 2012, 19, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, A.G.; Lubenau, H.; Mikus, G.; Knebel, P.; Hohmann, N.; Leowardi, C.; Beckhove, P.; Akhisaroglu, M.; Ge, Y.; Springer, M.; et al. Double-blind, placebo-controlled first in human study to investigate an oral vaccine aimed to elicit an immune reaction against the VEGF-Receptor 2 in patients with stage IV and locally advanced pancreatic cancer. BMC Cancer 2012, 12, 361. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wei, X.; Kalvakolanu, D.V.; Guo, B.; Zhang, L. Perspectives on Oncolytic Salmonella in Cancer Immunotherapy—A Promising Strategy. Front. Immunol. 2021, 12, 615930. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Liu, Q.; Li, P.; Luo, H.; Wang, H.; Kong, Q. Genetically engineered Salmonella typhimurium: Recent advances in cancer therapy. Cancer Lett. 2019, 448, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.H.; Min, J.-J. Targeted Cancer Therapy Using Engineered Salmonella typhimurium. Chonnam Med. J. 2016, 52, 173–184. [Google Scholar] [CrossRef]
- Fensterle, J.; Bergmann, B.; Yone, C.L.; Hotz, C.; Meyer, S.R.; Spreng, S.; Goebel, W.; Rapp, U.R.; Gentschev, I. Cancer immunotherapy based on recombinant Salmonella enterica serovar Typhimurium aroA strains secreting prostate-specific antigen and cholera toxin subunit B. Cancer Gene Ther. 2008, 15, 85–93. [Google Scholar] [CrossRef]
- Jellbauer, S.; Panthel, K.; Hetrodt, J.H.; Rüssmann, H. CD8 T-cell induction against vascular endothelial growth factor receptor 2 by Salmonella for vaccination purposes against a murine melanoma. PLoS ONE 2012, 7, e34214. [Google Scholar]
- Panthel, K.; Meinel, K.M.; Sevil Domènech, V.E.; Geginat, G.; Linkemann, K.; Busch, D.H.; Rüssmann, H. Prophylactic anti-tumor immunity against a murine fibrosarcoma triggered by the Salmonella type III secretion system. Microbes Infect. 2006, 8, 2539–2546. [Google Scholar] [CrossRef]
- Nishikawa, H.; Sato, E.; Briones, G.; Chen, L.-M.; Matsuo, M.; Nagata, Y.; Ritter, G.; Jäger, E.; Nomura, H.; Kondo, S.; et al. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J. Clin. Investig. 2006, 116, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.; Choi, J.H.; Kim, S.; Park, Y.K. Engineered Salmonella typhimurium expressing E7 fusion protein, derived from human papillomavirus, inhibits tumor growth in cervical tumor-bearing mice. Biotechnol. Lett. 2014, 36, 349–356. [Google Scholar] [CrossRef]
- Mei, Y.; Zhao, L.; Liu, Y.; Gong, H.; Song, Y.; Lei, L.; Zhu, Y.; Jin, Z.; Ma, S.; Hu, B.; et al. Combining DNA Vaccine and AIDA-1 in Attenuated Salmonella Activates Tumor-Specific CD4(+) and CD8(+) T-cell Responses. Cancer Immunol. Res. 2017, 5, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; He, H.; Zhang, S.; Zhao, W.; Li, N.; Shao, R. Salmonella typhimurium strain SL7207 induces apoptosis and inhibits the growth of HepG2 hepatoma cells in vitro and in vivo. Acta Pharm. Sin. B 2012, 2, 562–568. [Google Scholar] [CrossRef][Green Version]
- Xu, X.; Hegazy, W.A.H.; Guo, L.; Gao, X.; Courtney, A.N.; Kurbanov, S.; Liu, D.; Tian, G.; Manuel, E.R.; Diamond, D.J.; et al. Effective Cancer Vaccine Platform Based on Attenuated Salmonella and a Type III Secretion System. Cancer Res. 2014, 74, 6260. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.M.; Green, J.; Williams, P.J.; Tazzyman, S.; Hunt, S.; Harmey, J.H.; Kehoe, S.C.; Lewis, C.E. Bacterial delivery of a novel cytolysin to hypoxic areas of solid tumors. Gene Ther. 2009, 16, 329–339. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Kim, H.S.; Ha, J.M.; Hong, Y.; Choy, H.E.; Min, J.J. Genetically engineered Salmonella typhimurium as an imageable therapeutic probe for cancer. Cancer Res. 2010, 70, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.N.; Park, S.H.; Lee, H.J.; Zheng, J.H.; Kim, H.S.; Bom, H.S.; Hong, Y.; Szardenings, M.; Shin, M.G.; Kim, S.C.; et al. Engineering of bacteria for the visualization of targeted delivery of a cytolytic anticancer agent. Mol. Ther. 2013, 21, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Lan, H.; Liang, S.; Gao, T.; Ren, D. Suicide gene/prodrug therapy using salmonella-mediated delivery of Escherichia coli purine nucleoside phosphorylase gene and 6-methoxypurine 2’-deoxyriboside in murine mammary carcinoma 4T1 model. Cancer Sci. 2008, 99, 1172–1179. [Google Scholar] [CrossRef]
- Chen, G.; Tang, B.; Yang, B.Y.; Chen, J.X.; Zhou, J.H.; Li, J.H.; Hua, Z.C. Tumor-targeting Salmonella typhimurium, a natural tool for activation of prodrug 6MePdR and their combination therapy in murine melanoma model. Appl. Microbiol. Biotechnol. 2013, 97, 4393–4401. [Google Scholar] [CrossRef]
- Massa, P.E.; Paniccia, A.; Monegal, A.; de Marco, A.; Rescigno, M. Salmonella engineered to express CD20-targeting antibodies and a drug-converting enzyme can eradicate human lymphomas. Blood 2013, 122, 705–714. [Google Scholar] [CrossRef]
- Friedlos, F.; Lehouritis, P.; Ogilvie, L.; Hedley, D.; Davies, L.; Bermudes, D.; King, I.; Martin, J.; Marais, R.; Springer, C.J. Attenuated Salmonella; Targets Prodrug Activating Enzyme Carboxypeptidase G2 to Mouse Melanoma and Human Breast and Colon Carcinomas for Effective Suicide Gene Therapy. Clin. Cancer Res. 2008, 14, 4259. [Google Scholar] [CrossRef]
- Lim, D.; Kim, K.S.; Kim, H.; Ko, K.C.; Song, J.J.; Choi, J.H.; Shin, M.; Min, J.J.; Jeong, J.H.; Choy, H.E. Anti-tumor activity of an immunotoxin (TGFα-PE38) delivered by attenuated Salmonella typhimurium. Oncotarget 2017, 8, 37550–37560. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, D.A.; Katsanis, E.; Heise, C.P.; Hasz, D.E.; Vigdorovich, V.; Kelly, S.M.; Curtiss, R., 3rd; Leonard, A.S.; Anderson, P.M. Antitumor mechanisms of attenuated Salmonella typhimurium containing the gene for human interleukin-2: A novel antitumor agent? J. Pediatric Surg. 1997, 32, 301–306. [Google Scholar] [CrossRef]
- Agorio, C.; Schreiber, F.; Sheppard, M.; Mastroeni, P.; Fernandez, M.; Martinez, M.A.; Chabalgoity, J.A. Live attenuated Salmonella as a vector for oral cytokine gene therapy in melanoma. J. Gene Med. 2007, 9, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, M.; Le’Negrate, G.; Krajewska, M.; Reed, J.C. IL-18-producing Salmonella inhibit tumor growth. Cancer Gene Ther. 2008, 15, 787–794. [Google Scholar] [CrossRef]
- Cao, H.; Xiang, T.; Zhang, C.; Yang, H.; Jiang, L.; Liu, S.; Huang, X. MDA7 combined with targeted attenuated Salmonella vector SL7207/pBud-VP3 inhibited growth of gastric cancer cells. Biomed. Pharmacother. 2016, 83, 809–815. [Google Scholar] [CrossRef]
- Cao, H.D.; Yang, Y.X.; Lü, L.; Liu, S.N.; Wang, P.L.; Tao, X.H.; Wang, L.J.; Xiang, T.X. Attenuated Salmonella typhimurium carrying TRAIL and VP3 genes inhibits the growth of gastric cancer cells in vitro and in vivo. Tumori 2010, 96, 296–303. [Google Scholar] [CrossRef]
- Yoon, W.; Park, Y.C.; Kim, J.; Chae, Y.S.; Byeon, J.H.; Min, S.H.; Park, S.; Yoo, Y.; Park, Y.K.; Kim, B.M. Application of genetically engineered Salmonella typhimurium for interferon-gamma-induced therapy against melanoma. Eur. J. Cancer 2017, 70, 48–61. [Google Scholar] [CrossRef]
- Loeffler, M.; Le’Negrate, G.; Krajewska, M.; Reed, J.C. Attenuated Salmonella engineered to produce human cytokine LIGHT inhibit tumor growth. Proc. Natl. Acad. Sci. USA 2007, 104, 12879–12883. [Google Scholar] [CrossRef]
- Kim, K.; Jeong, J.H.; Lim, D.; Hong, Y.; Lim, H.-J.; Kim, G.-J.; Shin, S.-R.; Lee, J.-J.; Yun, M.; Harris, R.A.; et al. L-Asparaginase delivered by Salmonella typhimurium suppresses solid tumors. Mol. Ther. Oncolytics 2015, 2, 15007. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, B.S.; Banton, K.L.; Frykman, N.L.; Leonard, A.S.; Saltzman, D.A. Attenuated Salmonella typhimurium with IL-2 gene reduces pulmonary metastases in murine osteosarcoma. Clin. Orthop. Relat. Res. 2008, 466, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.S.; Chae, Y.S.; Hong, J.; Park, Y.K. Antitumor therapeutic effects of a genetically engineered Salmonella typhimurium harboring TNF-α in mice. Appl. Microbiol. Biotechnol. 2011, 89, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.; Ismail, S.; Abou-Aisha, K. Bacterial delivery of the anti-tumor azurin-like protein Laz to glioblastoma cells. AMB Express 2020, 10, 59. [Google Scholar] [CrossRef]
- Loeffler, M.; Le’Negrate, G.; Krajewska, M.; Reed, J.C. Inhibition of tumor growth using salmonella expressing Fas ligand. J. Natl. Cancer Inst. 2008, 100, 1113–1116. [Google Scholar] [CrossRef]
- Yang, Y.W.; Zhang, C.M.; Huang, X.J.; Zhang, X.X.; Zhang, L.K.; Li, J.H.; Hua, Z.C. Tumor-targeted delivery of a C-terminally truncated FADD (N-FADD) significantly suppresses the B16F10 melanoma via enhancing apoptosis. Sci. Rep. 2016, 6, 34178. [Google Scholar] [CrossRef]
- Yoon, W.S.; Choi, W.C.; Sin, J.I.; Park, Y.K. Antitumor therapeutic effects of Salmonella typhimurium containing Flt3 Ligand expression plasmids in melanoma-bearing mouse. Biotechnol. Lett. 2007, 29, 511–516. [Google Scholar] [CrossRef]
- Guan, G.F.; Zhao, M.; Liu, L.M.; Jin, C.S.; Sun, K.; Zhang, D.J.; Yu, D.J.; Cao, H.W.; Lu, Y.Q.; Wen, L.J. Salmonella typhimurium mediated delivery of Apoptin in human laryngeal cancer. Int. J. Med. Sci. 2013, 10, 1639–1648. [Google Scholar] [CrossRef]
- Lode, H.N.; Pertl, U.; Xiang, R.; Gaedicke, G.; Reisfeld, R.A. Tyrosine hydroxylase-based DNA-vaccination is effective against murine neuroblastoma. Med. Pediatric Oncol. 2000, 35, 641–646. [Google Scholar] [CrossRef]
- Marx, M.; Zumpe, M.; Troschke-Meurer, S.; Shah, D.; Lode, H.N.; Siebert, N. Co-expression of IL-15 enhances anti-neuroblastoma effectivity of a tyrosine hydroxylase-directed DNA vaccination in mice. PLoS ONE 2018, 13, e0207320. [Google Scholar]
- Zhu, X.; Cai, J.; Huang, J.; Jiang, X.; Ren, D. The Treatment and Prevention of Mouse Melanoma With an Oral DNA Vaccine Carried by Attenuated Salmonella typhimurium. J. Immunother. 2010, 33, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Yang, B.; Zhao, L.; Wang, S.; Zhang, J.; Wang, K. Tumor suppressor gene RBM5 delivered by attenuated Salmonella inhibits lung adenocarcinoma through diverse apoptotic signaling pathways. World J. Surg. Oncol. 2013, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Blache, C.A.; Manuel, E.R.; Kaltcheva, T.I.; Wong, A.N.; Ellenhorn, J.D.; Blazar, B.R.; Diamond, D.J. Systemic delivery of Salmonella typhimurium transformed with IDO shRNA enhances intratumoral vector colonization and suppresses tumor growth. Cancer Res. 2012, 72, 6447–6456. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; He, J.; Cheng, H.; Zhu, Z.; Xu, H. Enhanced therapeutic effect of an antiangiogenesis peptide on lung cancer in vivo combined with salmonella VNP20009 carrying a Sox2 shRNA construct. J. Exp. Clin. Cancer Res. 2016, 35, 107. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhao, P.; Zhou, Z.; Liu, J.; Qin, L.; Wang, H. Using attenuated Salmonella typhi as tumor targeting vector for MDR1 siRNA delivery. Cancer Biol. Ther. 2007, 6, 555–560. [Google Scholar] [CrossRef]
- Deng, J.; Guo, Y.; Jiang, Z.; Yang, M.; Li, H.; Wang, J. Enhancement of ovarian cancer chemotherapy by delivery of multidrug-resistance gene small interfering RNA using tumor targeting Salmonella. J. Obstet. Gynaecol. Res. 2015, 41, 615–622. [Google Scholar] [CrossRef]
- Wen, L.J.; Gao, L.F.; Jin, C.S.; Zhang, H.J.; Ji, K.; Yang, J.P.; Zhao, X.J.; Wen, M.J.; Guan, G.F. Small interfering RNA survivin and GRIM-19 co-expression salmonella plasmid inhibited the growth of laryngeal cancer cells in vitro and in vivo. Int. J. Clin. Exp. Pathol. 2013, 6, 2071–2081. [Google Scholar]
- Yang, N.; Zhu, X.; Chen, L.; Li, S.; Ren, D. Oral administration of attenuated S. typhimurium carrying shRNA-expressing vectors as a cancer therapeutic. Cancer Biol. Ther. 2008, 7, 145–151. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Wang, B.; Ji, K.; Liang, Z.; Guo, B.; Hu, J.; Yin, D.; Du, Y.; Kopecko, D.J.; et al. Delivery of the co-expression plasmid pEndo-Si-Stat3 by attenuated Salmonella serovar typhimurium for prostate cancer treatment. J. Cancer Res. Clin. Oncol. 2013, 139, 971–980. [Google Scholar] [CrossRef]
- Zoaby, N.; Shainsky-Roitman, J.; Badarneh, S.; Abumanhal, H.; Leshansky, A.; Yaron, S.; Schroeder, A. Autonomous bacterial nanoswimmers target cancer. J. Control. Release 2017, 257, 68–75. [Google Scholar] [CrossRef]
- Suh, S.; Jo, A.; Traore, M.A.; Zhan, Y.; Coutermarsh-Ott, S.L.; Ringel-Scaia, V.M.; Allen, I.C.; Davis, R.M.; Behkam, B. Nanoscale Bacteria-Enabled Autonomous Drug Delivery System (NanoBEADS) Enhances Intratumoral Transport of Nanomedicine. Adv. Sci. 2019, 6, 1801309. [Google Scholar] [CrossRef] [PubMed]
- Ektate, K.; Munteanu, M.C.; Ashar, H.; Malayer, J.; Ranjan, A. Chemo-immunotherapy of colon cancer with focused ultrasound and Salmonella-laden temperature sensitive liposomes (thermobots). Sci. Rep. 2018, 8, 13062. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zang, Z.; Chen, Z.; Cui, L.; Chang, Z.; Ma, A.; Yin, T.; Liang, R.; Han, Y.; Wu, Z.; et al. Nanophotosensitizer-engineered Salmonella bacteria with hypoxia targeting and photothermal-assisted mutual bioaccumulation for solid tumor therapy. Biomaterials 2019, 214, 119226. [Google Scholar] [CrossRef]
- Kefayat, A.; Ghahremani, F.; Motaghi, H.; Rostami, S.; Mehrgardi, M.A. Alive attenuated Salmonella as a cargo shuttle for smart carrying of gold nanoparticles to tumour hypoxic regions. J. Drug Target. 2019, 27, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, S.; Piao, L.; Yuan, F. Radiotherapy combined with an engineered Salmonella typhimurium inhibits tumor growth in a mouse model of colon cancer. Exp. Anim. 2016, 65, 413–418. [Google Scholar] [CrossRef]
- Bascuas, T.; Moreno, M.; Grille, S.; Chabalgoity, J.A. Salmonella Immunotherapy Improves the Outcome of CHOP Chemotherapy in Non-Hodgkin Lymphoma-Bearing Mice. Front. Immunol. 2018, 9, 7. [Google Scholar] [CrossRef]
- Hiroshima, Y.; Zhang, Y.; Zhao, M.; Zhang, N.; Murakami, T.; Maawy, A.; Mii, S.; Uehara, F.; Yamamoto, M.; Miwa, S.; et al. Tumor-Targeting Salmonella typhimurium A1-R in Combination with Trastuzumab Eradicates HER-2-Positive Cervical Cancer Cells in Patient-Derived Mouse Models. PLoS ONE 2015, 10, e0120358. [Google Scholar] [CrossRef]
- Hiroshima, Y.; Zhao, M.; Zhang, Y.; Maawy, A.; Hassanein, M.K.; Uehara, F.; Miwa, S.; Yano, S.; Momiyama, M.; Suetsugu, A.; et al. Comparison of efficacy of Salmonella typhimurium A1-R and chemotherapy on stem-like and non-stem human pancreatic cancer cells. Cell Cycle 2013, 12, 2774–2780. [Google Scholar] [CrossRef]
- Gu, J.; Li, Y.; Zeng, J.; Wang, B.; Ji, K.; Tang, Y.; Sun, Q. Knockdown of HIF-1α by siRNA-expressing plasmid delivered by attenuated Salmonella enhances the antitumor effects of cisplatin on prostate cancer. Sci. Rep. 2017, 7, 7546. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, X.; Lai, Y.; Zhou, Y.; Cao, W.; Hua, Z.C. Salmonella VNP20009-mediated RNA interference of ABCB5 moderated chemoresistance of melanoma stem cell and suppressed tumor growth more potently. Oncotarget 2016, 7, 14940–14950. [Google Scholar] [CrossRef]
- Binder, D.C.; Arina, A.; Wen, F.; Tu, T.; Zhao, M.; Hoffman, R.M.; Wainwright, D.A.; Schreiber, H. Tumor relapse prevented by combining adoptive T cell therapy with Salmonella typhimurium. Oncoimmunology 2016, 5, e1130207. [Google Scholar] [CrossRef]
- Igarashi, K.; Kawaguchi, K.; Zhao, M.; Kiyuna, T.; Miyake, K.; Miyake, M.; Nelson, S.D.; Dry, S.M.; Li, Y.; Yamamoto, N.; et al. Exquisite Tumor Targeting by Salmonella A1-R in Combination with Caffeine and Valproic Acid Regresses an Adult Pleomorphic Rhabdomyosarcoma Patient-Derived Orthotopic Xenograft Mouse Model. Transl. Oncol. 2020, 13, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Linley, A.J.; Ahmad, M.; Rees, R.C. Tumour-associated antigens: Considerations for their use in tumour immunotherapy. Int. J. Hematol. 2011, 93, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Dougan, G.; John, V.; Palmer, S.; Mastroeni, P. Immunity to salmonellosis. Immunol. Rev. 2011, 240, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Duan, X.; Liu, Y.; Zhu, J.; Zhang, K.; Zhang, Y.; Xia, T.; Fei, Y.; Ye, J. T cell immunity induced by a bivalent Salmonella-based CEACAM6 and 4-1BBL vaccines in a rat colorectal cancer model. Oncol. Lett. 2017, 13, 3753–3759. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, S.N.; Phan, T.X.; Nam, T.K.; Nguyen, V.H.; Kim, H.S.; Bom, H.S.; Choy, H.E.; Hong, Y.; Min, J.J. Inhibition of tumor growth and metastasis by a combination of Escherichia coli-mediated cytolytic therapy and radiotherapy. Mol. Ther. 2010, 18, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Rooseboom, M.; Commandeur, J.N.; Vermeulen, N.P. Enzyme-catalyzed activation of anticancer prodrugs. Pharmacol. Rev. 2004, 56, 53–102. [Google Scholar] [CrossRef]
- Barak, Y.; Thorne, S.H.; Ackerley, D.F.; Lynch, S.V.; Contag, C.H.; Matin, A. New enzyme for reductive cancer chemotherapy, YieF, and its improvement by directed evolution. Mol. Cancer Ther. 2006, 5, 97–103. [Google Scholar] [CrossRef]
- Dresselaers, T.; Theys, J.; Nuyts, S.; Wouters, B.; de Bruijn, E.; Anné, J.; Lambin, P.; Van Hecke, P.; Landuyt, W. Non-invasive 19F MR spectroscopy of 5-fluorocytosine to 5-fluorouracil conversion by recombinant Salmonella in tumours. Br. J. Cancer 2003, 89, 1796–1801. [Google Scholar] [CrossRef][Green Version]
- Cunningham, C.; Nemunaitis, J. A phase I trial of genetically modified Salmonella typhimurium expressing cytosine deaminase (TAPET-CD, VNP20029) administered by intratumoral injection in combination with 5-fluorocytosine for patients with advanced or metastatic cancer. Protocol no: CL-017. Version: April 9, 2001. Hum. Gene Ther. 2001, 12, 1594–1596. [Google Scholar]
- Weiss, S.; Chakraborty, T. Transfer of eukaryotic expression plasmids to mammalian host cells by bacterial carriers. Curr. Opin. Biotechnol. 2001, 12, 467–472. [Google Scholar] [CrossRef]
- Pilgrim, S.; Stritzker, J.; Schoen, C.; Kolb-Mäurer, A.; Geginat, G.; Loessner, M.; Gentschev, I.; Goebel, W. Bactofection of mammalian cells by Listeria monocytogenes: Improvement and mechanism of DNA delivery. Gene Ther. 2003, 10, 2036–2045. [Google Scholar] [CrossRef][Green Version]
- Darji, A.; Guzmán, C.A.; Gerstel, B.; Wachholz, P.; Timmis, K.N.; Wehland, J.; Chakraborty, T.; Weiss, S. Oral somatic transgene vaccination using attenuated S. typhimurium. Cell 1997, 91, 765–775. [Google Scholar] [CrossRef]
- Stegantseva, M.V.; Shinkevich, V.A.; Tumar, E.M.; Meleshko, A.N. Multi-antigen DNA vaccine delivered by polyethylenimine and Salmonella enterica in neuroblastoma mouse model. Cancer Immunol. Immunother. 2020, 69, 2613–2622. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wu, C.L.; Shiau, A.L. Systemic administration of attenuated Salmonella choleraesuis carrying thrombospondin-1 gene leads to tumor-specific transgene expression, delayed tumor growth and prolonged survival in the murine melanoma model. Cancer Gene Ther. 2005, 12, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wu, C.L.; Shiau, A.L. Endostatin gene therapy delivered by Salmonella choleraesuis in murine tumor models. J. Gene Med. 2004, 6, 1382–1393. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Adiseshaiah, P.P.; Crist, R.M.; Hook, S.S.; McNeil, S.E. Nanomedicine strategies to overcome the pathophysiological barriers of pancreatic cancer. Nat. Rev. Clin. Oncol. 2016, 13, 750–765. [Google Scholar] [CrossRef]
- Chen, Y.; Du, M.; Yu, J.; Rao, L.; Chen, X.; Chen, Z. Nanobiohybrids: A synergistic integration of bacteria and nanomaterials in cancer therapy. BIO Integr. 2020, 1, 25–36. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Han, J.-W.; Choi, Y.; Cho, S.; Zheng, S.; Ko, S.Y.; Park, J.-O.; Park, S. Active Tumor-Therapeutic Liposomal Bacteriobot Combining a Drug (Paclitaxel)-Encapsulated Liposome with Targeting Bacteria (Salmonella typhimurium). Sens. Actuators B Chem. 2015, 224, 217–224. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, M.; Fang, C.; Cheng, C.; Zhao, M.; Fang, W.; Chu, P.K.; Ping, Y.; Tang, G. Engineering nanoparticle-coated bacteria as oral DNA vaccines for cancer immunotherapy. Nano Lett. 2015, 15, 2732–2739. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-J.; Chang, W.-W.; Lin, S.-T.; Chen, M.-C.; Lee, C.-H. Salmonella Overcomes Drug Resistance in Tumor through P-glycoprotein Downregulation. Int. J. Med. Sci. 2018, 15, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Luna, M.A.; Díaz de León-Ortega, R.; Hernández-Cueto, D.D.; Gaxiola-Centeno, R.; Castro-Luna, R.; Martínez-Cristóbal, L.; Huerta-Yépez, S.; Luria-Pérez, R. Bactofection of sequences encoding a Bax protein peptide chemosensitizes prostate cancer tumor cells. Boletín Médico Del Hosp. Infant. De México 2016, 73, 388–396. [Google Scholar] [CrossRef] [PubMed]
- King, I.; Bermudes, D.; Lin, S.; Belcourt, M.; Pike, J.; Troy, K.; Le, T.; Ittensohn, M.; Mao, J.; Lang, W.; et al. Tumor-targeted Salmonella expressing cytosine deaminase as an anticancer agent. Hum. Gene Ther. 2002, 13, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Nemunaitis, J.; Cunningham, C.; Senzer, N.; Kuhn, J.; Cramm, J.; Litz, C.; Cavagnolo, R.; Cahill, A.; Clairmont, C.; Sznol, M. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther. 2003, 10, 737–744. [Google Scholar] [CrossRef]
- ClinicalTrials.gov [Internet], Guangzhou Sinogen Pharmaceutical Co., Ltd. 2021 Sep 8. Identifier NCT05038150, IStudy of SGN1 in Patients With Advanced Solid Tumor. Available online: https://www.clinicaltrials.gov/ct2/show/NCT05038150 (accessed on 29 March 2022).
- ClinicalTrials.gov [Internet], Guangzhou Sinogen Pharmaceutical Co., Ltd. 2021 Nov 2021. Identifier NCT05103345, Study of SGN1 Administered Via Intratumoral Injection in Patients With Advanced Solid Tumor. Available online: https://www.clinicaltrials.gov/ct2/show/NCT05103345 (accessed on 5 April 2022).
- Schmitz-Winnenthal, F.H.; Hohmann, N.; Schmidt, T.; Podola, L.; Friedrich, T.; Lubenau, H.; Springer, M.; Wieckowski, S.; Breiner, K.M.; Mikus, G.; et al. A phase 1 trial extension to assess immunologic efficacy and safety of prime-boost vaccination with VXM01, an oral T cell vaccine against VEGFR2, in patients with advanced pancreatic cancer. Oncoimmunology 2018, 7, e1303584. [Google Scholar] [CrossRef]
- ClinicalTrials.gov [Internet]. Salspera LLC. 2019 Oct 19. Identifier NCT04589234, Saltikva for Metastatic Pancreatic Cancer. Available online: https://www.clinicaltrials.gov/ct2/show/study/NCT04589234 (accessed on 28 March 2022).
- Gniadek, T.J.; Augustin, L.; Schottel, J.; Leonard, A.; Saltzman, D.; Greeno, E.; Batist, G. A Phase I, Dose Escalation, Single Dose Trial of Oral Attenuated Salmonella typhimurium Containing Human IL-2 in Patients with Metastatic Gastrointestinal Cancers. J. Immunother. 2020, 43, 217–221. [Google Scholar] [CrossRef]
- ClinicalTrials.gov [Internet]. Masonic Cancer Center, University of Minnesota. 2010 Apr 7. Identifier NCT01099631, IL-2 Expressing, Attenuated Salmonella typhimurium in Unresectable Hepatic Spread. Available online: https://clinicaltrials.gov/ct2/show/NCT01099631 (accessed on 8 October 2021).
- ClinicalTrials.gov [Internet]. Pharmaceuticals, V. National Cancer Institute (NCI). 2004 Feb 25. Identifier NCT00004216, VNP20009 in Treating Patients With Advanced or Metastatic Solid Tumors That Have Not Responded to Previous Therapy. Available online: https://clinicaltrials.gov/ct2/show/NCT00004216 (accessed on 8 October 2021).
- ClinicalTrials.gov [Internet]. National Cancer Institute (NCI): National Institutes of Health Clinical Center (CC). 2000 Mar 20. Identifier NCT00004988, Treatment of Patients with Cancer with Genetically Modified Salmonella typhimurium Bacteria. Available online: https://clinicaltrials.gov/ct2/show/NCT00004988 (accessed on 8 October 2021).
- ClinicalTrials.gov [Internet]. Pharmaceuticals, V. National Cancer Institute (NCI). 2003 Sep 3. Identifier NCT00006254, VNP20009 in Treating Patients with Advanced Solid Tumors. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00006254 (accessed on 8 October 2021).
- ClinicalTrials.gov [Internet]. Jichlinski, P. University of Lausanne Hospitals. 2018 Feb 5. Identifier NCT03421236, Intravesical Ty21a for the Treatment of Patients with Non-muscle-invasive Bladder Cancer (NMIBC). Available online: https://www.clinicaltrials.gov/ct2/show/NCT03421236 (accessed on 8 October 2021).
- ClinicalTrials.gov [Internet]. Meleshko, A. Belarusian Research Center for Pediatric Oncology, Hematology and Immunology. 2018 Ago 8. Identifier NCT04049864, DNA Vaccination Against Neuroblastoma. Available online: https://clinicaltrials.gov/ct2/show/NCT04049864 (accessed on 8 October 2021).
- ClinicalTrials.gov [Internet]. Lulla, P. Baylor College of Medicine (USA). 2018 Dic 3. Identifier NCT03762291, Multiple Myeloma Trial of Orally Administered Salmonella Based Survivin Vaccine (MAPSS). Available online: https://clinicaltrials.gov/ct2/show/NCT03762291 (accessed on 8 October 2021).
- Felgner, S.; Kocijancic, D.; Frahm, M.; Weiss, S. Bacteria in Cancer Therapy: Renaissance of an Old Concept. Int. J. Microbiol. 2016, 2016, 8451728. [Google Scholar] [CrossRef]
- Zhou, S.; Gravekamp, C.; Bermudes, D.; Liu, K. Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer 2018, 18, 727–743. [Google Scholar] [CrossRef]
- Mignon, C.; Sodoyer, R.; Werle, B. Antibiotic-free selection in biotherapeutics: Now and forever. Pathogens 2015, 4, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Forbes, N.S.; Coffin, R.S.; Deng, L.; Evgin, L.; Fiering, S.; Giacalone, M.; Gravekamp, C.; Gulley, J.L.; Gunn, H.; Hoffman, R.M.; et al. White paper on microbial anti-cancer therapy and prevention. J. Immunother. Cancer 2018, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.T.; Lee, J.W.; Cameron, D.E.; Bashor, C.J.; Collins, J.J. ‘Deadman’ and ‘Passcode’ microbial kill switches for bacterial containment. Nat. Chem. Biol. 2016, 12, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, M.R.; Isabella, V.M.; Li, N.; Kurtz, C.B. Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat. Commun. 2020, 11, 1738. [Google Scholar] [CrossRef]
- Martinez-Morales, F.; Borges, A.C.; Martinez, A.; Shanmugam, K.T.; Ingram, L.O. Chromosomal integration of heterologous DNA in Escherichia coli with precise removal of markers and replicons used during construction. J. Bacteriol. 1999, 181, 7143–7148. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, Y.; Liu, X.; Min, J.J.; Tan, W.; Zheng, J.H. Targeted cancer immunotherapy with genetically engineered oncolytic Salmonella typhimurium. Cancer Lett. 2020, 469, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Pálffy, R.; Gardlík, R.; Hodosy, J.; Behuliak, M.; Resko, P.; Radvánský, J.; Celec, P. Bacteria in gene therapy: Bactofection versus alternative gene therapy. Gene Ther. 2006, 13, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Felgner, S.; Kocijancic, D.; Frahm, M.; Heise, U.; Rohde, M.; Zimmermann, K.; Falk, C.; Erhardt, M.; Weiss, S. Engineered Salmonella enterica serovar Typhimurium overcomes limitations of anti-bacterial immunity in bacteria-mediated tumor therapy. Oncoimmunology 2018, 7, e1382791. [Google Scholar] [CrossRef]
- Lee, C.H.; Lin, Y.H.; Hsieh, J.L.; Chen, M.C.; Kuo, W.L. A polymer coating applied to Salmonella prevents the binding of Salmonella-specific antibodies. Int. J. Cancer 2013, 132, 717–725. [Google Scholar] [CrossRef]
- Food and Drug Administration. Recommendations for Microbial Vectors Used for Gene Therapy. Available online: https://www.fda.gov/media/94200/download (accessed on 5 May 2022).

| Species | Strain | Mutation | Treated Malignancy | Reference |
|---|---|---|---|---|
| Mutations in genes of metabolic pathways | ||||
| Salmonella Typhimurium | SL3235 | aroA | Plasmacytoma, Non-Hodgkin lymphoma | [6,11,12,13,14] |
| Salmonella Typhimurium | SL7207 | hisG46, DEL407 aroA544::Tn10 (Tcs) | Lung cancer | [15,16] |
| Salmonella Typhimurium | YB1 | asd | Hepatocellular carcinoma | [17] |
| Salmonella Typhimurium | LVR01 | aroC | B cell lymphoma | [18,19] |
| Salmonella Typhimurium | BRD509 | aroA and aroD | Murine melanoma | [20] |
| Salmonella Typhimurium | A1-R | Leu,Arg | Prostate cancer, Spinal glioma, Pancreatic cancer, and Fibrosarcoma | [21,22,23,24,25,26,27] |
| Salmonella Typhimurium | VNP20009 | purI,msbB | Metastatic melanoma | [28,29] |
| Salmonella Typhi | CVD915 | guaBA | Breast adenocarcinoma, T cell lymphoma | [30,31] |
| Mutations in genes associated with virulence | ||||
| Salmonella Typhimurium | VNP20009 | purI,msbB | Metastatic melanoma | [28,29] |
| Salmonella Typhimurium | RE88 | aroA and dam | Breast Carcinoma | [32] |
| Salmonella Typhimurium | SB824 | sptP::Kan | Fibrosarcoma | [33] |
| Salmonella Typhimurium | ST8 | aroA::Tn10, gmd::Plac-T7RNAP,htrA::PpepT-asd-PsodA, infA::Ptet-tetR. | Colon cancer | [34] |
| Salmonella Typhimurium | 14028 | rfaL, rfaG, rfaH, rfaD, rfaP and msbB | Colon cancer | [35] |
| Salmonella Typhimurium | S634 | pagP, pagL and lpxR | Colon carcinoma | [36,37] |
| Salmonella Typhimurium | LH340 | PhoP/PhoQ | Prostate cancer | [39] |
| Salmonella Typhimurium | ppGpp | relA::cat,spoT::kan | Colon adenocarcinoma | [40,41,42] |
| Gene mutations associated with tumor selectivity | ||||
| Salmonella Typhimurium | SA186 | znuABC | Breast Adenocarcinoma | [43] |
| Salmonella Typhimurium | X4550 | Cya-1, Crp-1 | Adenocarcinoma | [44] |
| Mutations in multiple genes | ||||
| Salmonella Typhimurium | YS7211 | Pur, Ilv, Arg and Pur, Ilv, Ura. | Melanoma | [45] |
| Salmonella Typhimurium | SL1344 | cheY, fliGHI, invG, phoP, sseD, ssrB, aroA, and purA | Colon Carcinoma | [46] |
| Salmonella Typhi | TY21A | Chemical attenuation, UDP-glucose-4-epimerase | Murine bladder cancer | [47] |
| Species | Mutation | Heterologous Molecule | Type of Tumor in Murine Model | Generated AntiTumor Response | References |
|---|---|---|---|---|---|
| Tumor-associated antigens/Tumor-specific antigens | |||||
| Salmonella Typhimurium | aroA | PSA | Prostate cancer | Cytotoxic CD8+ T cells | [130] |
| Salmonella Typhimurium | aroA | VEGFR-2 | Melanoma | Cytotoxic CD8+ T cells | [131] |
| Salmonella Typhimurium | aroA | C-RaF | Lung adenocarcinoma | Cytotoxic CD8+ T cells | [16] |
| Salmonella Typhimurium | aroA | Peptide 217-225 of protein P60 | Fibrosarcoma | Effector CD8+ T cells | [33,132] |
| Salmonella Typhimurium | phoP, phoQ | NY-ESO1 | Fibrosarcoma | Specific CD4+ and CD8+ T cells | [133] |
| Salmonella Typhimurium | aroA | E7 (HPV16E7) | Cervical cancer | INFγ and TNFα | [134] |
| Salmonella Typhimurium | aroA | Melan-A | Melanoma | Th1 and CTL response | [135,136] |
| Salmonella Typhimurium | purD, htrA | SVN | Colon cancer and lymphoma | Induction of CD8+ Treg cells | [137] |
| Cytotoxic molecules | |||||
| Salmonella Typhimurium | waaN, purl, aroA | HlyE | Breast cancer | Increased LDH | [138] |
| Salmonella Typhimurium | ppGpp | ClyA | Colon cancer and hepatocellular carcinoma | Decrease in tumor size | [139,140] |
| Salmonella Typhimurium | aroA, purl | PNP | Breast cancer | Increase in apoptosis | [141] |
| Salmonella Typhimurium | purI, msbB | PNP | Melanoma | Infiltration by CD8+ cells | [142] |
| Salmonella Typhimurium | aroA | HSV-TK | Lymphoma | Sensitivity of tumor cells | [143] |
| Salmonella Typhimurium | pul, msbB and asd | CPG2 | Breast and colon cancer, melanoma | Cytotoxicity of tumor cells and inhibition of tumor growth | [144] |
| Salmonella Typhimurium | ppGpp | TGFα-PE38 | Colon and breast cancer | Delay in tumor growth | [145] |
| Immunomodulating molecules and apoptosis inducers | |||||
| Salmonella Typhimurium | Pur, msb | CCL21 | Breast Carcinoma | Inhibition of tumor growth | [62] |
| Salmonella Typhimurium | Cya-1, Crp-1 | IL-2 | Adenocarcinoma | Decreases metastases | [44,146] |
| Salmonella Typhimurium | aroA | IL-4, IL-18 | Melanoma | Increases IFN-γ levels | [147] |
| Salmonella Typhimurium | Pur, msb | IL-18 | Colon Carcinoma | Inhibits tumor growth | [148] |
| Salmonella Typhimurium | aroA | IL-24, Apoptina | Gastric cancer | Inhibits tumor growth | [149] |
| Salmonella Typhimurium | aroA | TRAIL, VP3 | Gastric cancer | Increases caspase-3 and 9 expression | [150] |
| Salmonella Typhimurium | aroA, aroD | IFN-γ | Melanoma | Inhibition of tumor growth | [151] |
| Salmonella Typhimurium | purI,msbB | LIGHT | Breast Carcinoma | Inhibition of tumor growth | [152] |
| Salmonella Typhimurium | relA::cat, spoT::kan | TGFα-PE38 | Breast and colon cancer | Inhibition of tumor growth | [145] |
| Salmonella Typhimurium | relA::cat,spoT::kan | L-asparaginasa | Colon Adenocarcinoma, pancreas, and breast cancer | Inhibition of tumor growth | [153] |
| Salmonella Typhimurium | Cya-1, Crp-1 | IL-2 | Osteosarcoma | Decrease in metastases | [154] |
| Salmonella Typhimurium | aroA, aroD | TNF-α | Melanoma | Induction of de apoptosis | [155] |
| Salmonella Typhimurium | msbB, purI | Laz | Glioblastoma | Induction of apoptosis | [156] |
| Salmonella Typhimurium | purI, msbB | FasL | Breast Carcinoma | Inhibition of tumor growth | [157] |
| Salmonella Typhimurium | msbB, purI | FADD | Melanoma | Induction of apoptosis | [158] |
| Nucleid acids (Bactofection) | |||||
| Salmonella Typhimurium | aroA, aroD | Flt3 | Melanoma | Inhibition of tumor growth | [159] |
| Salmonella Typhimurium | PhoP/PhoQ | Apoptina | Larynx Cancer | Decreased cytotoxicity and increased apoptosis | [160] |
| Salmonella Typhimurium | hisG46, DEL407 aroA544::Tn10 (Tcs) | Tirosina hidrolasa | Neuroblastoma | Protection against tumor challenges | [161] |
| Salmonella Typhimurium | hisG46, DEL407 aroA544::Tn10 (Tcs) | IL-15 | Neuroblastoma | Tumor remission | [162] |
| Salmonella Typhimurium | aroA | Hsp70-TAA | Melanoma | Activation of T cells, tumor elimination | [163] |
| Salmonella Typhimurium | Chemical attenuation, UDP-glucose-4 epimerase | RBM5 | Lung Adenocarcinoma | Improves apoptosis | [164] |
| Salmonella Typhimurium | msbB, purI | IDO ShRNA | Melanoma | Increase in ROS and cell death | [165] |
| Salmonella Typhimurium | msbB, purI | Sox2 shRNA | Lung Adenocarcinoma | I Inhibition of angiogenesis, increase in apoptosis | [166] |
| Salmonella Typhimurium | hisG46, DEL407 aroA544::Tn10 (Tcs) | MDR1 siRNA | Tongue Squamous cell carcinoma | Suppression of tumor proliferation | [167] |
| Salmonella Typhimurium | hisG46, DEL407 aroA544::Tn10 (Tcs) | MDR1 siRNA | Ovary Cancer | Slow tumor growth and sensitization to cisplatin | [168] |
| Salmonella Typhimurium | Chemical attenuation, UDP-glucose-4 epimerase | Survivan siRNA, GRIM-19 | Larynx Cancer | Increase in apoptosis, inhibition of tumor growth | [169] |
| Salmonella Typhimurium | aroA, LT2 Trp Met Erpsl flaA R- M+ | Bcl-2 shRNA | Melanoma | Delays tumor growth and prolongs survival | [170] |
| Salmonella Typhimurium | phoP, phoQ | Stat-3 shRNA | Larynx Cancer | Suppression of tumor growth | [171] |
| Salmonella Typhimurium | PhoP, PhoQ | Stat-3 SiRNA | Prostate cancer | Inhibition of tumor growth, decrease in metastases | [39] |
| Nanomolecules | |||||
| Salmonella Typhimurium | purA::Tn10 | Liposomes loaded with doxorubicin | Triple negative murine breast cancer | Induces tumor cell death | [172] |
| Salmonella Typhimurium | msbB, purI | PLGA | Murine breast cancer | Improves the therapeutic efficiency of chemotherapy drugs | [173] |
| Salmonella Typhimurium | msbB | Liposomes loaded with doxorubicin | Murine colon cancer | Improves the selectivity and release of Dorubicin | [174] |
| Salmonella Typhimurium | asd | Nanoparticles loaded with indocyanine green | Murine melanoma | Inhibition of tumor growth | [175] |
| Salmonella Typhi | Chemical attenuation, UDP-glucose-4 epimerase | Gold nanoparticles covered in folic acid | Murine colon cancer | Enhances delivery of gold nanoparticles to the tumor | [176] |
| Combination of Salmonella enterica and conventional antitumor treatments | |||||
| Salmonella Typhimurium | relA::cat,spoT::kan | Radiotherapy | Colon cancer | Inhibition of tumor growth | [177] |
| Salmonella Typhimurium | aroC | CHOP | NHL | Increase in infiltrating lymphocytes, expression of cytokines and chemokines in tumor | [178] |
| Salmonella Typhimurium | Leu,Arg | Trastuzumab | Cervical cancer | Decrease in tumor volume | [179] |
| Salmonella Typhimurium | Leu,Arg | Chemotherapy (5-FU, cisplatin, gentamicin) | Pancreatic Cancer | Decrease in tumor | [180] |
| Salmonella Typhimurium | Leu,Arg | Cisplastin | Melanoma | Suppression of tumor growth | [79] |
| Salmonella Typhimurium | Leu,Arg | Recombinant methioninase | Metastasic osteosarcoma | Inhibition of tumor growth | [72] |
| Salmonella Typhimurium | Chemical attenuation, UDP-glucose-4 epimerase | Cisplatin | Prostate cancer | Induction of apoptosis | [181] |
| Salmonella Typhimurium | purI,msbB and PhoP/PhoQ | Chemotherapy (paclitaxel and doxorubicin) | Melanoma | Delays tumor growth and improves survival | [182] |
| Salmonella Typhimurium | Leu,Arg | Adaptive T-cell therapy | Fibrosarcoma | Tumor regression | [183] |
| Salmonella Typhimurium | Leu,Arg | Caffeine and valproic acid | Pleomorphic rhabdomyosarcoma | Inhibition of tumor growth | [184] |
| Species | Mutation | Heterologous Molecule | Treated Malignancy | Dose and Administration | References |
|---|---|---|---|---|---|
| Salmonella Typhimurium | Purl, msbB | None | Phase I; Metastatic melanoma, metastatic renal carcinoma | Intravenous 1 × 106, 1 × 109 CFU, single escalating dosing | [29] |
| Salmonella Typhimurium | Purl, msbB | IL-2 | Phase I, Metastatic liver carcinoma | Oral, escalating dosing with 1 × 105, 1 × 1010 CFU per dose | [214] |
| Salmonella Typhimurium | Purl, msbB | Cytosine deaminase | Phase I; Head and neck carcinoma/esophageal adenocarcinoma | Intratumoral injection of 3 × 106, 1 × 107, 3 × 107 CFU/m2 at escalating dosing, for various cycles | [208] |
| Salmonella Typhimurium | Purl, msbB | None | Phase I; superficial solid tumors | Intratumoral injection of 3 escalating doses | [215] |
| Salmonella Typhimurium | Purl, msbB | None | Phase I; metastatic cancers | Intravenously with escalating doses every 35 days | [216] |
| Salmonella Typhimurium | Purl, msbB | None | Phase I, non-specific solid tumors | Intravenously with escalating dosing every 35 days | [217] |
| Salmonella Typhi | galE, rpoS, ilvD | VEGFR-2 | Pancreatic cancer | 106 to 1010 CFU Single dose | [126] |
| Salmonella Typhi | galE, rpoS, ilvD | None | Phase I: non-muscular bladder carcinoma | [218] | |
| Salmonella Typhimurium | --- | Neuroblastoma-associated antigen and protein of the potato virus X | Pilot study Recruitment period Neuroblastoma | 1010 CFU orally, at 1-week intervals, for 3–4 weeks | [219] |
| Salmonella Typhi | --- | Survivin | Pilot study Multiple myeloma | 2 escalating doses every 2 weeks 2.5 × 106–2.5 × 107 | [220] |
| Salmonella Typhimurium | Asd, cAMP y receptor de cAMP | IL-2 | Metastatic pancreatic cancer | 2.5 × 106 CFU every week for 6 weeks, orally | [212] |
| Salmonella Typhimurium | Purl, msbB | L-methioninase | Refractory solid tumors | 0.9–2.0 × 109 CFU, intravenously | [209] |
| Salmonella Typhimurium | Purl, msbB | Cytosine deaminase | Metastatic cancer | 2.5 × 106 CFU in mice. 1 × 1010 CFU in primates. Intravenous or intratumoral | [192] |
| Salmonella Typhimurium | Purl, msbB | L-methioninase | Head and neck advanced squamous cell carcinoma | 0.9–2.0 × 106 CFU Intratumoral | [210] |
| Salmonella Typhi | galE, rpoS, ilvD | VEGF | Pancreatic cancer | 106 or 107 CFU, orally | [211] |
| Salmonella Typhimurium | Asd, cAMP and cAMP receptor | IL-2 | Liver metastatic solid tumor | 1010 CFU, Orally | [213] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becerra-Báez, E.I.; Meza-Toledo, S.E.; Muñoz-López, P.; Flores-Martínez, L.F.; Fraga-Pérez, K.; Magaño-Bocanegra, K.J.; Juárez-Hernández, U.; Mateos-Chávez, A.A.; Luria-Pérez, R. Recombinant Attenuated Salmonella enterica as a Delivery System of Heterologous Molecules in Cancer Therapy. Cancers 2022, 14, 4224. https://doi.org/10.3390/cancers14174224
Becerra-Báez EI, Meza-Toledo SE, Muñoz-López P, Flores-Martínez LF, Fraga-Pérez K, Magaño-Bocanegra KJ, Juárez-Hernández U, Mateos-Chávez AA, Luria-Pérez R. Recombinant Attenuated Salmonella enterica as a Delivery System of Heterologous Molecules in Cancer Therapy. Cancers. 2022; 14(17):4224. https://doi.org/10.3390/cancers14174224
Chicago/Turabian StyleBecerra-Báez, Elayne Irene, Sergio Enrique Meza-Toledo, Paola Muñoz-López, Luis Fernando Flores-Martínez, Karla Fraga-Pérez, Kevin Jorge Magaño-Bocanegra, Uriel Juárez-Hernández, Armando Alfredo Mateos-Chávez, and Rosendo Luria-Pérez. 2022. "Recombinant Attenuated Salmonella enterica as a Delivery System of Heterologous Molecules in Cancer Therapy" Cancers 14, no. 17: 4224. https://doi.org/10.3390/cancers14174224
APA StyleBecerra-Báez, E. I., Meza-Toledo, S. E., Muñoz-López, P., Flores-Martínez, L. F., Fraga-Pérez, K., Magaño-Bocanegra, K. J., Juárez-Hernández, U., Mateos-Chávez, A. A., & Luria-Pérez, R. (2022). Recombinant Attenuated Salmonella enterica as a Delivery System of Heterologous Molecules in Cancer Therapy. Cancers, 14(17), 4224. https://doi.org/10.3390/cancers14174224