Abscopal Response in Metastatic Melanoma: Real-World Data of a Retrospective, Multicenter Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Population
2.2. Outcome
2.3. Statistical Analysis
3. Results
3.1. Population Characteristics
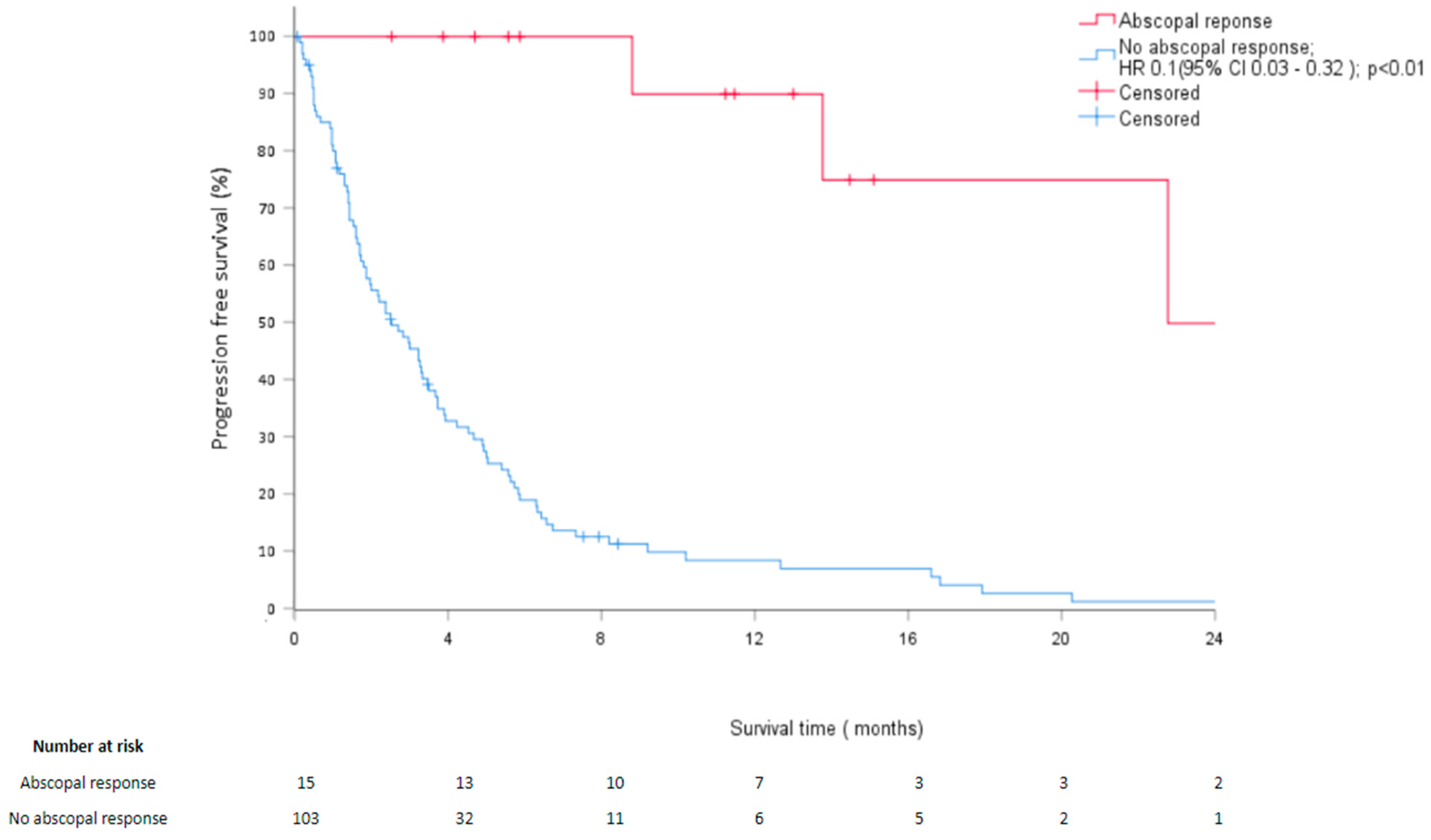
3.2. Survival Analysis
3.3. Abscopal Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AJCC 8th | American Joint Committee on Cancer 8th edition AJCC 8th |
| AUC | Area Under the curve |
| AR | Abscopal Response |
| CR | complete response |
| CRP | C-Reactive Protein |
| CTCAE v5 | Common Terminology Criteria for Adverse Events version 5 |
| CTLA-4 | cytotoxic T lymphocyte antigen |
| EBRT | external beam radiation therapy |
| 18F-FDG-PET/CT | 18F-Fluorodeoxyglucose positron emission tomography with CT |
| HR | hazard ratio |
| ICI | immune checkpoint inhibitors |
| LC | local control of the irradiated lesion |
| MM | Metastatic melanoma |
| OS | Overall survival |
| PD-1 | programmed cell death 1 |
| PD-L1 | programmed cell death-ligand 1 |
| PFS | progression-free survival |
| PTV | planning target volume |
| PR | partial response |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| RT | Radiotherapy |
| SBRT | Stereotactic body radiation therapy |
References
- Formenti, S.C.; Demaria, S. Radiation therapy to convert the tumor into an in situ vaccine. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 879–880. [Google Scholar] [CrossRef]
- Demaria, S.; Bhardwaj, N.; McBride, W.H.; Formenti, S.C. Combining radiotherapy and immunotherapy: A revived partnership. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 655–666. [Google Scholar] [CrossRef]
- Formenti, S.C.; Demaria, S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. J. Natl. Cancer Inst. 2013, 105, 256–265. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. Available online: https://www.nejm.org/doi/10.1056/NEJMoa1910836 (accessed on 24 August 2020). [CrossRef]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Mole, R.H. Whole body irradiation; radiobiology or medicine? Br. J. Radiol. 1953, 26, 234–241. [Google Scholar] [CrossRef]
- Abuodeh, Y.; Venkat, P.; Kim, S. Systematic review of case reports on the abscopal effect. Curr. Probl. Cancer 2016, 40, 25–37. [Google Scholar] [CrossRef]
- Dagoglu, N.; Karaman, S.; Caglar, H.B.; Oral, E.N. Abscopal Effect of Radiotherapy in the Immunotherapy Era: Systematic Review of Reported Cases. Cureus 2019, 11, e4103. [Google Scholar] [CrossRef]
- Hatten, S.J.; Lehrer, E.J.; Liao, J.; Sha, C.M.; Trifiletti, D.M.; Siva, S.; McBride, S.M.; Palma, D.; Holder, S.L.; Zaorsky, N.G. A Patient-Level Data Meta-analysis of the Abscopal Effect. Adv. Radiat. Oncol. 2022, 7, 100909. [Google Scholar] [CrossRef]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Update on Tumor Neoantigens and Their Utility: Why It Is Good to Be Different—PubMed [Internet]. Available online: https://pubmed.ncbi.nlm.nih.gov/29751996/ (accessed on 24 October 2020).
- Keung, E.Z.; Gershenwald, J.E. The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: Implications for melanoma treatment and care. Expert Rev. Anticancer Ther. 2018, 18, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Kluger, H.M.; Tawbi, H.A.; Ascierto, M.L.; Bowden, M.; Callahan, M.K.; Cha, E.; Chen, H.X.; Drake, C.G.; Feltquate, D.M.; Ferris, R.L.; et al. Defining tumor resistance to PD-1 pathway blockade: Recommendations from the first meeting of the SITC Immuno-therapy Resistance Taskforce. J. Immunother. Cancer 2020, 8, e000398. [Google Scholar] [CrossRef]
- Vermeire, S.; Van Assche, G.; Rutgeerts, P. Laboratory markers in IBD: Useful, magic, or unnecessary toys? Gut 2006, 55, 426–431. [Google Scholar] [CrossRef]
- Mulkey, F.; Theoret, M.R.; Keegan, P.; Pazdur, R.; Sridhara, R. Comparison of iRECIST versus RECIST V.1.1 in patients treated with an anti-PD-1 or PD-L1 antibody: Pooled FDA analysis. J. Immunother. Cancer 2020, 8, e000146. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef]
- National Cancer Institute (US). Cancer Therapy Evaluation Program. In Common Terminology Criteria for Adverse Events (CTCAE); Cancer Therapy Evaluation Program: Rockville, MD, USA, 2017; p. 155. [Google Scholar]
- D’Andrea, M.A.; Reddy, G.K. Systemic Antitumor Effects and Abscopal Responses in Melanoma Patients Receiving Radiation Therapy. Oncology 2020, 98, 202–215. [Google Scholar] [CrossRef]
- Golden, E.B.; Chhabra, A.; Chachoua, A.; Adams, S.; Donach, M.; Fenton-Kerimian, M.; Friedman, K.; Ponzo, F.; Babb, J.S.; Goldberg, J.; et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: A proof-of-principle trial. Lancet Oncol. 2015, 16, 795–803. [Google Scholar] [CrossRef]
- Ugurel, S.; Röhmel, J.; Ascierto, P.A.; Flaherty, K.T.; Grob, J.J.; Hauschild, A.; Larkin, J.; Long, G.V.; Lorigan, P.; McArthur, G.A.; et al. Survival of patients with advanced metastatic melanoma: The impact of novel therapies-update 2017. Eur. J. Cancer 2017, 83, 247–257. [Google Scholar] [CrossRef]
- Borcoman, E.; Kanjanapan, Y.; Champiat, S.; Kato, S.; Servois, V.; Kurzrock, R.; Goel, S.; Bedard, P.; Le Tourneau, C. Novel patterns of response under immunotherapy. Ann. Oncol. 2019, 30, 385–396. [Google Scholar] [CrossRef]
- Chicas-Sett, R.; Morales-Orue, I.; Rodriguez-Abreu, D.; Lara-Jimenez, P. Combining radiotherapy and ipilimumab induces clinically relevant radiation-induced abscopal effects in metastatic melanoma patients: A systematic review. Clin. Transl. Radiat. Oncol. 2017, 9, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Montalvo-Ortiz, W.; Yu, L.; Krasco, A.; Ebstein, S.; Cortez, C.; Lowy, I.; Murphy, A.J.; Sleeman, M.A.; Skokos, D. Sequence of αPD-1 relative to local tumor irradiation determines the induction of abscopal antitumor immune responses. Sci. Immunol. 2021, 6, eabg0117. [Google Scholar] [CrossRef]
- Lippert, T.P.; Greenberg, R.A. The abscopal effect: A sense of DNA damage is in the air. J. Clin. Investig. 2021, 131, 148274. [Google Scholar] [CrossRef] [PubMed]
- Sundahl, N.; Seremet, T.; Van Dorpe, J.; Neyns, B.; Ferdinande, L.; Meireson, A.; Brochez, L.; Kruse, V.; Ost, P. Phase 2 Trial of Nivolumab Combined with Stereotactic Body Radiation Therapy in Patients with Metastatic or Locally Advanced Inoperable Melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Souto, J.C.; Vila, L.; Brú, A. Polymorphonuclear neutrophils and cancer: Intense and sustained neutrophilia as a treatment against solid tumors. Med. Res. Rev. 2011, 31, 311–363. [Google Scholar] [CrossRef]
- Takeshima, T.; Pop, L.M.; Laine, A.; Iyengar, P.; Vitetta, E.S.; Hannan, R. Key role for neutrophils in radiation-induced antitumor immune responses: Potentiation with G-CSF. Proc. Natl. Acad. Sci. USA 2016, 113, 11300–11305. [Google Scholar] [CrossRef] [PubMed]
- Schernberg, A.; Blanchard, P.; Chargari, C.; Deutsch, E. Neutrophils, a candidate biomarker and target for radiation therapy? Acta Oncol. 2017, 56, 1522–1530. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef]
- Demaria, S.; Guha, C.; Schoenfeld, J.; Morris, Z.; Monjazeb, A.; Sikora, A.; Crittenden, M.; Shiao, S.; Khleif, S.; Gupta, S.; et al. Radiation dose and fraction in immunotherapy: One-size regimen does not fit all settings, so how does one choose? J. Immunother. Cancer 2021, 9, e002038. [Google Scholar] [CrossRef]
- Yamazaki, T.; Kirchmair, A.; Sato, A.; Buqué, A.; Rybstein, M.; Petroni, G.; Bloy, N.; Finotello, F.; Stafford, L.; Manzano, E.N.; et al. Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat. Immunol. 2020, 21, 1160–1171. [Google Scholar] [CrossRef]
- Formenti, S.C.; Rudqvist, N.P.; Golden, E.; Cooper, B.; Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Friedman, K.; Ferrari de Andrade, L.; Wucherpfennig, K.W.; et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 2018, 24, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; DeMaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Onderdonk, B.E.; Bhave, S.R.; Karrison, T.; Lemons, J.M.; Chang, P.; Zha, Y.; Carll, T.; Krausz, T.; Huang, L.; et al. Improved Survival Associated with Local Tumor Response Following Multisite Radiotherapy and Pembrolizumab: Secondary Analysis of a Phase I Trial. Clin. Cancer Res. 2020, 26, 6437–6444. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz-Merino, L.; Illescas-Vacas, A.; Grueso-López, A.; Barco-Sánchez, A.; Míguez-Sánchez, C. Radiation for Awakening the Dormant Immune System, a Promising Challenge to be Explored. Front. Immunol. 2014, 5, 102. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3953712/ (accessed on 14 September 2020). [CrossRef]
- Grassberger, C.; Ellsworth, S.G.; Wilks, M.Q.; Keane, F.K.; Loeffler, J.S. Assessing the interactions between radiotherapy and antitumour immunity. Nat. Rev. Clin. Oncol. 2019, 16, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Yovino, S.; Kleinberg, L.; Grossman, S.A.; Narayanan, M.; Ford, E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: Modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Investig. 2013, 31, 140–144. [Google Scholar] [CrossRef]
- Bockel, S.; Durand, B.; Deutsch, E. Combining radiation therapy and cancer immune therapies: From preclinical findings to clinical applications. Cancer Radiother. 2018, 22, 567–580. [Google Scholar] [CrossRef]
- Merrick, A.; Errington, F.; Milward, K.; O’Donnell, D.; Harrington, K.; Bateman, A.; Pandha, H.; Vile, R.; Morrison, E.; Selby, P.; et al. Immunosuppressive effects of radiation on human dendritic cells: Reduced IL-12 production on activation and impairment of naïve T-cell priming. Br. J. Cancer 2005, 92, 1450–1458. [Google Scholar] [CrossRef]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef]
- Woloshin, S.; Schwartz, L.M. Distribution of C-reactive protein values in the United States. N. Engl. J. Med. 2005, 352, 1611–1613. [Google Scholar] [CrossRef]
- Kushner, I.; Rzewnicki, D.; Samols, D. What does minor elevation of C-reactive protein signify? Am. J. Med. 2006, 119, 166.e17–166.e28. [Google Scholar] [CrossRef]
- McKelvey, K.J.; Hudson, A.L.; Back, M.; Eade, T.; Diakos, C.I. Radiation, inflammation and the immune response in cancer. Mamm. Genome 2018, 29, 843–865. [Google Scholar] [CrossRef]
- Brooks, E.D.; Chang, J.Y. Time to abandon single-site irradiation for inducing abscopal effects. Nat. Rev. Clin. Oncol. 2019, 16, 123–135. [Google Scholar] [CrossRef]
- Formenti, S.C. Optimizing Dose Per Fraction: A New Chapter in the Story of the Abscopal Effect? Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 677–679. [Google Scholar] [CrossRef]
- Altorki, N.K.; McGraw, T.E.; Borczuk, A.C.; Saxena, A.; Port, J.L.; Stiles, B.M.; Lee, B.E.; Sanfilippo, N.J.; Scheff, R.J.; Pua, B.B.; et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: A single-centre, randomised phase 2 trial. Lancet Oncol. 2021, 22, 824–835. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ferris, R.L.; Psyrri, A.; Haddad, R.I.; Tahara, M.; Bourhis, J.; Harrington, K.; Chang, P.M.H.; Lin, J.C.; Razaq, M.A.; et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: A randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021, 22, 450–462. [Google Scholar] [CrossRef]
- Saddawi-Konefka, R.; O’Farrell, A.; Faraji, F.; Clubb, L.; Allevato, M.; Jensen, S.M.; Yung, B.S.; Wang, Z.; Wu, V.H.; Anang, N.A.; et al. Lymphatic-Preserving Treatment Sequencing with Immune Checkpoint Inhibition Unleashes cDC1-Dependent Antitumor Immunity in HNSC. Nat. Commun. 2022, 13, 4298. [Google Scholar] [CrossRef]
| Baseline Characteristics | Overall |
|---|---|
| (n = 118) | |
| Age, median (range) years | 66.5 (23.5–100.1) |
| Sex, men (%) | 64 (54.2) |
| Performance status (%) | |
| 0 | 36 (30.6) |
| 1 | 47 (39.8) |
| 2 | 28 (23.7) |
| 3 | 7 (5.9) |
| AJCC 8th stage (%) | |
| M1A/IV | 29 (24.6) |
| M1B/IV | 5 (4.2) |
| M1C/IV | 68 (57.6) |
| M1D/IV | 16 (13.6) |
| Irradiated metastases (%) | |
| Abdomen (spleen, liver) | 13 (11.0) |
| Lymph node | 28 (23.7) |
| Skin | 23 (19.5) |
| Bone | 44 (37.3) |
| Lung | 4 (3.4) |
| Breast | 2 (1.7) |
| Muscle | 4 (3.4) |
| RT total dose, median (range) Gy | 30 (6.5–60) |
| RT dose per fraction, median (range) Gy | 4 (2–20) |
| SBRT (n = 15), median (range) Gy | 9 (8–20) |
| RT duration, median (range) days | 10 (1–63) |
| Multiple (≥2) irradiated metastases | 22(18.6) |
| Concomitant IT (%) | 80 (67.8) |
| Ipilimumab | 11 (13.7) |
| Nivolumab | 47 (58.8) |
| Nivolumab + Ipilimumab | 6 (7.5) |
| Pembrolizumab | 16 (20.0) |
| Time between the first injection of IT and RT, median (range) months. | 2.9 (0.5–47) |
| Immune-related events | 16 (13.5) |
| Resistance to immunotherapy (%) | |
| Primary | 34 (42.5) |
| Secondary | 46 (57.5) |
| Sex | Age | 1r or 2r to IT or none | Single (1) Or Multiple (≥2) Co RT plans | Site of RT | RT schedules | RT irE | Co-RT anti-infectious agents 0 no 1 yes | LT Before RT | Timing of irE | iRE category | IT | OS (month) | Time between the start of RT and AR detection (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | 84 | 2 | 1 | Splenic metastasis | 3*6Gy | Gastritis Helicobacter Pylori | 0 | concomitant | light | Pembrolizumab | 20 | 17 | |
| F | 77 | 0 | 2 | Skin metastases of the leg | 7*6Gy | Erysipelas on the same leg | 1 | concomitant | moderate | - | 12 | 99 | |
| M | 53 | 1 | 2 | Pulmonary nodules | 5*12Gy 3*6Gy | infectious pneumonia | 1 | before | moderate | Nivolumab | 16 | 22 | |
| F | 61 | 1 | 1 | Left axillary lymph node | 3*6Gy | Left Elbow adenitis. rise of CRP: 30 mg/L | 1 | 630 | concomitant | light | Nivolumab | 18 | 12 |
| F | 75 | 2 | 2 | Skin metastases of the right leg | 3*5.5Gy | erysipelas and urinary infection | 1 | concomitant | moderate | Nivolumab | 32 | 24 | |
| F | 56 | 1 | 2 | Right axillary lymph node + Skin metastases | 3*6Gy | Unknown biological inflammatory syndrome. Leucocytes: 18.35 G/L. Neutrophil count: 15.38 G/L CRP: 130 mg/L | 0 | 1080 | concomitant | severe | Nivolumab | 16 | 41 |
| F | 73 | 1 | 1 | Skin metastases of the left leg | 4*5Gy | Inflammation axillary node Thyroiditis | 1 | concomitant | light | Nivolumab | 12 | 44 | |
| F | 66 | 1 | 1 | Left axillary lymphnode | 4*5Gy | Subcutaneous inflammatory hyper metabolism | 0 | before | light | Nivolumab | 63 | 25 | |
| M | 65 | 2 | 2 | Retrocava lymphnode | 4*5Gy | Sigmoiditis | 0 | 950 | before | moderate | Nivolumab and Ipilimumab | 32 | 26 |
| M | 58 | 2 | 2 | Oral floor+bone metastasis | 10*3Gy | Oral infection | 1 | concomitant | moderate | Nivolumab and Ipilimumab | 31 | 16 | |
| W | 86 | 2 | 1 | Left inguinal adenopathy | 3*6Gy | bullous pemphigoid | 0 | 900 | concomitant | light | Pembrolizumab | 13 | 21 |
| M | 72 | 1 | 2 | Pulmonary nodules | 5*12Gy | Rhinopharyngitis +follicular acnea | 0 | 1300 | concomitant | moderate | Nivolumab | 23 | 28 |
| M | 67 | 2 | 1 | Skin metastases | 13*3Gy | - | - | - | Ipilimumab | 67 | 19 | ||
| M | 85 | 2 | 1 | Cervical lymph node | 15*3Gy | sarcoidosis | - | - | moderate | Nivolumab | 18 | 57 | |
| M | 88 | 2 | 1 | Cervical lymph node | 30*2Gy | - | - | - | Nivolumab | 28 | 45 |
| Patient Characteristics | No AR | AR | p |
|---|---|---|---|
| n = 103 | n = 15 | ||
| Age, median (range) years | 66.4 (23.3-100.1) | 66.8 (53.5- 87.8) | 0.09 |
| Sex, Women (%) | 46 (44.7) | 8 (53.3) | 0.72 |
| PS (%) | 0.45 | ||
| 0 | 32 (31.1) | 4 (26.7) | |
| 1 | 40 (38.8) | 7 (46.7) | |
| 2 | 26 (25.2) | 2 (13.3) | |
| 3 | 5 (4.9) | 2 (13.3) | |
| AJCC 8th stage (%) | 0.10 | ||
| M1A/IV | 24 (23.3) | 5 (33.4) | |
| M1B/IV | 3 (2.9) | 2 (13.3) | |
| M1C/IV | 60 (58.3) | 8 (53.3) | |
| M1D/IV | 16 (15.5) | 0 (0.0) | |
| Irradiated metastases (%) | 0.02 | ||
| Abdomen (spleen, liver) | 11 (10.7) | 2 (13.3) | |
| Lymph node | 22 (21.4) | 6 (40.0) | |
| Skin metastases | 19 (18.4) | 4 (26.7) | |
| Bone | 43 (41.8) | 1 (6.7) | |
| Lung | 2 (1.9) | 2 (13.3) | |
| Breast | 2 (1.9) | 0 (0.0) | |
| Muscle | 4 (3.9) | 0 (0.0) | |
| RT total dose, median(range) Gy | 30 (6.5-60) | 30(16.5-60) | 0.17 |
| RT dose per fraction, median (range) Gy | 4(2.4-20) | 4 (2-18) | 0.09 |
| 10 (1-63) | 11 (2-22) | 0.49 | |
| RT duration, median (range) days | |||
| Multiple (≥2) irradiated metastases (%) | 16 (15.5) | 6 (40.0) | 0.05 |
| Concomitant immunotherapy (%) | 66 (55.9) | 14 (93.3) | 0.02 |
| Resistance to immunotherapy | 0.96 | ||
| Primary | 28 (42.4) | 6 (42.9) | |
| Secondary | 38 (57.6) | 8 (57.1) | |
| RT immune-related events * | 3 (3.2) | 13 (86.7) | <0.01 |
| Local response for the RT-targets ** | <0.01 | ||
| Progressive | 15 (14.6) | 0 (0.0) | |
| Stable disease | 58 (56.3) | 2 (13.3) | |
| Tumor regression | 30 (29.1) | 13 (86.7) |
| Demographics | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR | p Value | HR | p Value | |
| Age (per 1 year increase) | 0.99 [0.98; 1.01] | 0.41 | ||
| Sex, female male | Reference 1.55 [1.03; 2.33] | 0.03 | 1.89 [1.21; 2.93] | <0.01 |
| Clinical characteristics | ||||
| Performance status 0 | Reference | |||
| 1 | 1.09 [0.67; 1.78] | 0.72 | ||
| 2 | 1.82 [1.06; 3.13] | 0.03 | 2.09 [1.21; 3.63] | <0.01 |
| 3 | 1.19 [0.42; 3.40] | 0.74 | ||
| AJCC 8th stage M1A/IV | Reference | |||
| M1B/IV | 0.45 [0.13; 1.49] | 0.19 | ||
| M1C/IV | 0.88 [0.55; 1.42] | 0.62 | ||
| M1D/IV | 1.30 [0.67; 2.51] | 0.43 | ||
| Number of irradiated metastases One metastasis Multiple metastasis (≥2) | Reference 0.56 [0.32; 0.98] | 0.04 | 0.83 [0.47; 1.49] | 0.53 |
| RT dose | 0.98 [0.97; 1.01] | 0.09 | ||
| Immunotherapy None Concomitant with RT | Reference 0.51 [0.33; 0.78] | <0.01 | 0.64 [0.40; 1.02] | 0.06 |
| Abscopal response No AR AR | Reference 0.08 [0.02; 0.24] | <0.01 | 0.1 [0.03; 0.32] | <0.01 |
| Demographics | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR | p-Value | HR | p-Value | |
| Age (per 1 year increase) | 0.99 [0.98; 1.01] | 0.64 | ||
| Gender female male | Reference 1.31 [0.84; 2.05] | 0.23 | ||
| Clinical characteristics | ||||
| Performance status 0 | Reference | |||
| 1 | 1.41 [0.79; 2.51] | 0.24 | ||
| 2 | 3.05 [1.65; 5.63] | <0.01 | 2.73 [1.46; 5.09] | <0.01 |
| 3 | 4.20 [1.44; 12.27] | 0.01 | 4.70 [1.59; 13.94] | <0.01 |
| AJCC 8th stage M1A/IV | Reference | |||
| M1B/IV | 0.63 [0.14; 2.72] | 0.53 | ||
| M1C/IV | 1.57 [0.90; 2.75] | 0.11 | ||
| M1D/IV | 2.20 [1.04; 4.67] | 0.04 | 2.45 [0.164; 3.97] | 0.63 |
| Number of irradiated metastases One metastasis Multiple metastasis (≥2) | Reference 0.38 [0.18; 0.78] | 0.01 | 0.48 [0.23; 1.02] | 0.05 |
| RT dose | 0.98 [0.96; 0.99] | 0.04 | 0.98 [0.96; 1.01] | 0.11 |
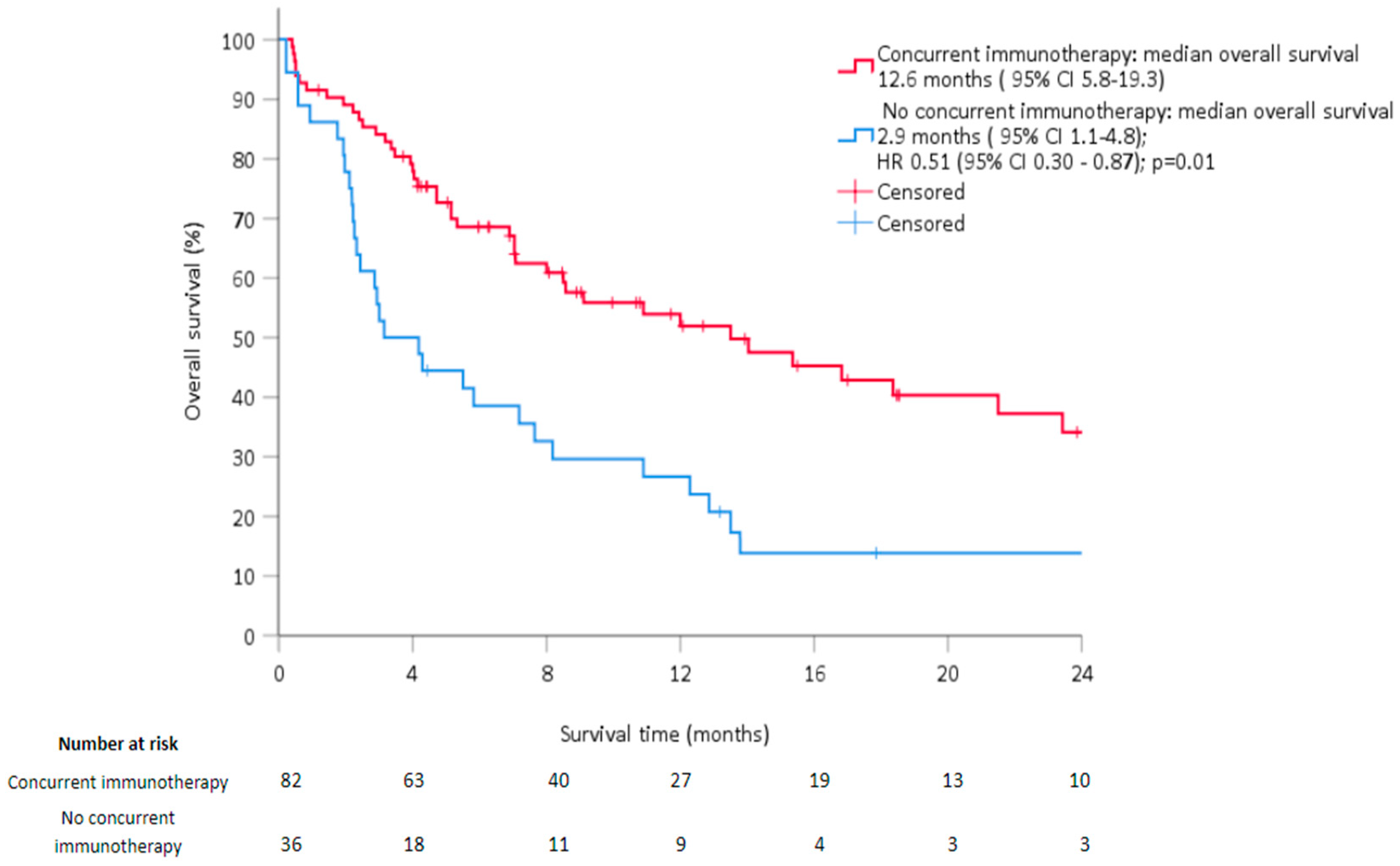
| Immunotherapy None Concomitant with RT | Reference 0.49 [0.31; 0.77] | <0.01 | 0.51 [0.30; 0.87] | 0.01 |
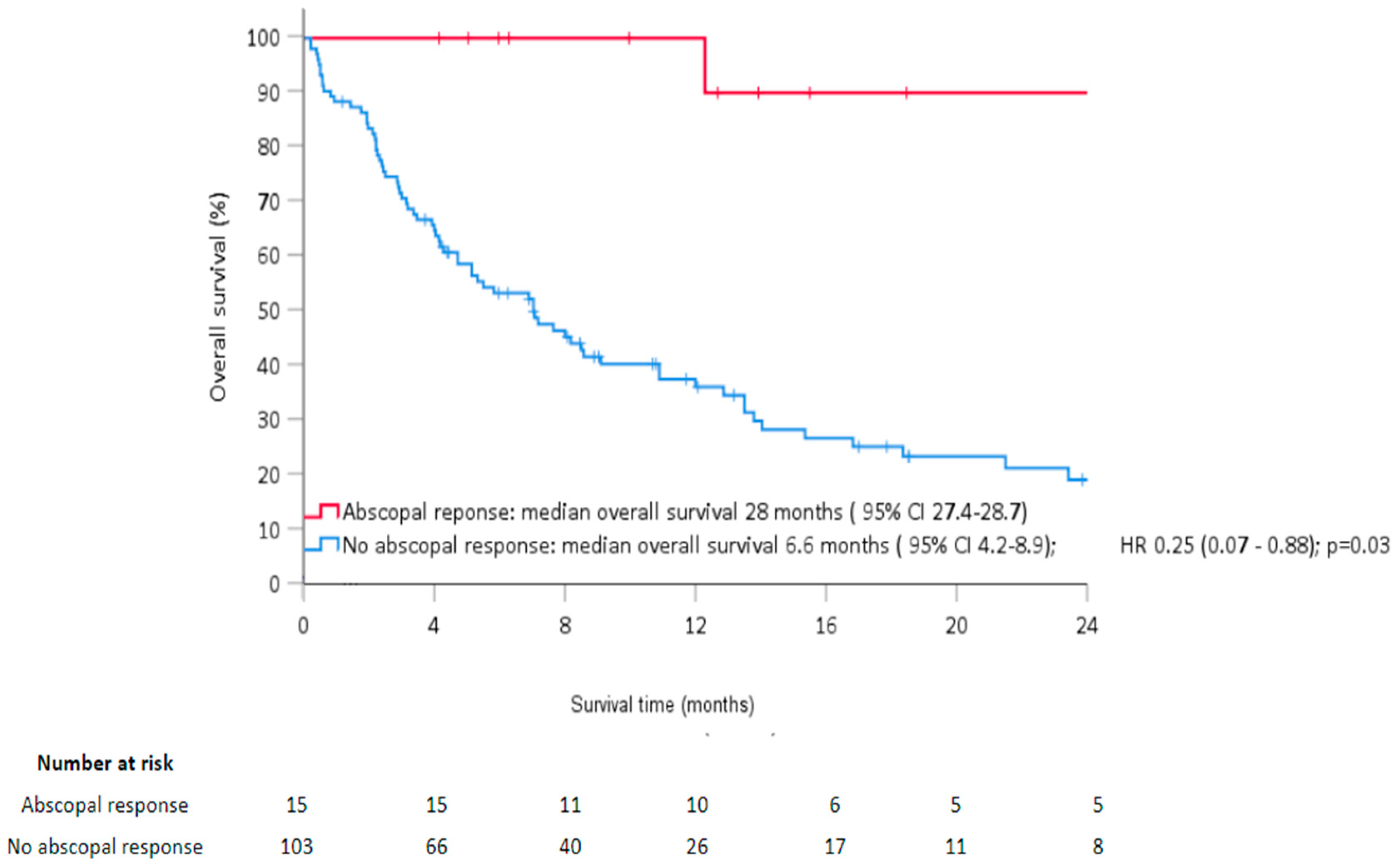
| Abscopal response | 0.19 [0.04; 0.46] | <0.01 | 0.25 [0.07; 0.88] | 0.03 |
| Demographics | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR | p-Value | HR | p-Value | |
| Age (per 1 year increase) | 1.03 [0.99; 1.06] | 0.09 | ||
| Gender Female Male | Reference 1.43 [0.52; 3.96] | 0.48 | ||
| Clinical characteristics | ||||
| Performance status 0 | Reference | |||
| 1 | 1.45 [0.42; 4.95] | 0.55 | ||
| 2 | 0.89 [0.16; 4.85] | 0.89 | ||
| 3 | 4.67 [0.85; 25.69] | 0.07 | ||
| AJCC 8th stage M1A/IV | Reference | |||
| M1B/IV | 2.80 [0.54; 14.47] | 0.22 | ||
| M1C/IV | 0.73 [0.24; 2.22] | 0.57 | ||
| M1D/IV | 0.00 [0.00; Inf] | 0.99 | ||
| Total RT dose | 1.02 [0.98; 1.06] | 0.27 | ||
| RT dose per fraction | 1.10 [0.98; 1.24] | 0.11 | ||
| RT duration | 0.96 [0.90; 1.04] | 0.34 | ||
| Number of irradiated metastases One metastasis Multiple metastasis (≥2) | * Reference 3.16 [1.12; 8.89] | 0.03 | 16.85 [2.16; 131.49] | <0.01 |
| Irradiated metastases | ||||
| Abdomen (spleen, liver) | 0.20 [0.01; 3.27] | 0.26 | ||
| Lymph node | 0.52 [0.06; 4.35] | 0.55 | ||
| Skin | 0.56 [0.06; 4.83] | 0.60 | ||
| Bone | 0.00 [0.00; Inf] | 0.99 | ||
| Lung | 1.56 [0.14; 17.29] | 0.72 | ||
| Breast | 0.00 [0.00; Inf] | 0.99 | ||
| Muscle | 0.00 [0.00; Inf] | 0.99 | ||
| Immunotherapy None Concomitant with RT | Reference 7.56 [0.99; 57.57] | 0.05 | ||
| Ipilimumab | Reference | |||
| Nivolumab | 2.05 [0.26; 16.21] | 0.49 | ||
| Nivolumab + ipilimumab | 3.79 [0.34; 41.86] | 0.28 | ||
| Pembrolizumab | 1.32 [0.12; 14.59] | 0.82 | ||
| Occurrence of immune-related events * | 64.83 [14.48; 290.18] | <0.01 | 403.45 [13.83; 11769.39] | <0.01 |
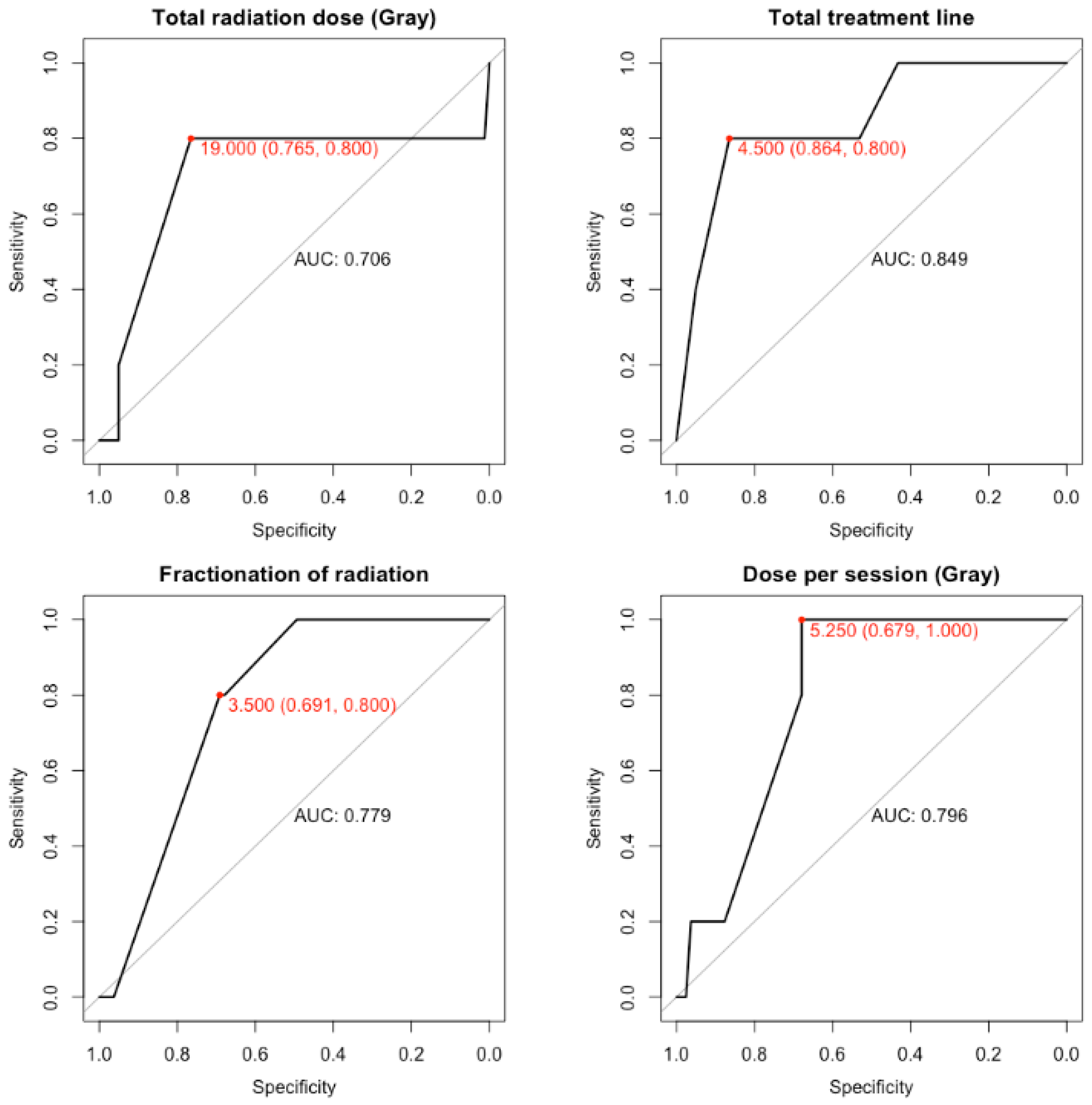
| Total Radiation Dose (Gray) | Dose per Session (Gray) | Fractionation of Radiation | Total Treatment Time, Including Breaks (Days) | |
|---|---|---|---|---|
| Optimal value | 19 | 5.25 | 3.5 | 4.5 |
| Specificity | 0.765 | 0.679 | 0.691 | 0.864 |
| Sensitivity | 0.8 | 1 | 0.8 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ollivier, L.; Orione, C.; Bore, P.; Misery, L.; Legoupil, D.; Leclere, J.-C.; Coste, A.; Girault, G.; Sicard-Cras, I.; Kacperek, C.; et al. Abscopal Response in Metastatic Melanoma: Real-World Data of a Retrospective, Multicenter Study. Cancers 2022, 14, 4213. https://doi.org/10.3390/cancers14174213
Ollivier L, Orione C, Bore P, Misery L, Legoupil D, Leclere J-C, Coste A, Girault G, Sicard-Cras I, Kacperek C, et al. Abscopal Response in Metastatic Melanoma: Real-World Data of a Retrospective, Multicenter Study. Cancers. 2022; 14(17):4213. https://doi.org/10.3390/cancers14174213
Chicago/Turabian StyleOllivier, Luc, Charles Orione, Paul Bore, Laurent Misery, Delphine Legoupil, Jean-Christophe Leclere, Anne Coste, Gilles Girault, Iona Sicard-Cras, Clemence Kacperek, and et al. 2022. "Abscopal Response in Metastatic Melanoma: Real-World Data of a Retrospective, Multicenter Study" Cancers 14, no. 17: 4213. https://doi.org/10.3390/cancers14174213
APA StyleOllivier, L., Orione, C., Bore, P., Misery, L., Legoupil, D., Leclere, J.-C., Coste, A., Girault, G., Sicard-Cras, I., Kacperek, C., Lucia, F., Stefan, D., Thillays, F., Rio, E., Lesueur, P., Berthou, C., Heymann, D., Champiat, S., Supiot, S., ... Kao, W. (2022). Abscopal Response in Metastatic Melanoma: Real-World Data of a Retrospective, Multicenter Study. Cancers, 14(17), 4213. https://doi.org/10.3390/cancers14174213









