Simple Summary
Pazopanib treatment in advanced solitary fibrous tumour patients, assessed in the prospective GEIS-32 phase II clinical trial, has shown longer progression-free survival and overall survival versus chemotherapy treatment in control patients. In recent years, the interest in the prognostic and predictive value of different peripheral inflammatory indexes, such as neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and red cell distribution width, has been increased in sarcomas, showing significant results in different soft tissue sarcomas. However, they have not been previously analysed in solitary fibrous tumour (SFT) patients. These indexes were retrospectively analysed in the typical- and malignant-SFT cohorts treated with pazopanib of the GEIS-32 trial to evaluate their predictive or prognostic value.
Abstract
Pazopanib was assessed prospectively in the GEIS-32 phase II study (NCT02066285) on advanced solitary fibrous tumour (SFT), resulting in a longer progression-free survival (PFS) and overall survival (OS) compared with historical controls treated with chemotherapy. A retrospective analysis of peripheral inflammatory indexes in patients enrolled into GEIS-32 was performed to evaluate their prognostic and predictive value. Patients received pazopanib 800 mg/day as the first antiangiogenic line. The impacts of baseline neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and red cell distribution width (RDW) on PFS, OS, and Choi response were evaluated by univariate and multivariate analysis. Metastasis-free interval (MFI), mitotic count, and ECOG were also included as potential prognostic factors. Sixty-seven SFT patients, enrolled in this study, showed a median age of 63 years and a female/male distribution of 57/43. The median follow-up from treatment initiation was 16.8 months. High baseline NLR, PLR, and standardised RDW were significantly associated with worse PFS and OS. NLR, RDW, MFI, and mitotic count were independent variables for PFS, while RDW and ECOG were independent for OS. Further, NLR and mitotic count were independent factors for Choi response. High baseline NLR and RDW values were independent prognostic biomarkers for worse outcome in advanced SFT patients treated with pazopanib.
1. Introduction
Solitary fibrous tumour (SFT) is a rare and ubiquitous fibroblastic mesenchymal neoplasm with a specific histological architecture, which harbours an intrachromosomal NAB2/STAT6 fusion gene and shows nuclear immunoreactivity for STAT6 [1]. Typical and malignant SFT subtypes were differentiated in the WHO 2013 classification of soft tissue tumours, taking into account the number of mitoses per 10 high-power fields, the presence of necrosis, and nuclear pleomorphism. However, despite the differences in these parameters, up to 45% of cases in both subtypes can develop metastasis. Thus, the last WHO 2020 classification of soft tissue and bone tumours advises the use of risk stratification models to determine prognosis in SFT, based on age, tumour size, and number of mitoses or even with the presence of necrosis [2]. Dedifferentiated SFT is a more rare and aggressive subtype, which shows a fast transition to a high-grade sarcoma. The most frequent localisations of SFTs are abdominal and thoracic cavities, followed by limbs and intracranial locations. Surgical resection is the preferred treatment for localised disease (>90% of cases), showing a 10-year overall survival (OS), which ranges from 54% to 89%. However, locally advanced or metastatic disease is treated with conventional chemotherapy, although it has limited efficacy [3,4]. Pazopanib is an antiangiogenic drug that has shown longer progression-free survival (PFS) and OS in advanced SFTs compared with chemotherapy in historical controls [5,6]. Pazopanib was approved in second lines of advanced soft tissue sarcomas (STS) based on a significant improvement in PFS compared with placebo [7]. However, the identification of prognostic biomarkers of pazopanib response seems necessary to improve the clinical effectiveness and cost-effectiveness of this drug.
Pazopanib is a tyrosine kinase inhibitor (TKI) targeting VEGFRs, PDGFR α/β, FGFR, and KIT, which are differentially expressed in SFT, although not showing any prognostic significance in primary tumours [8]. Pazopanib inhibits angiogenesis and the proliferation of tumour cells and also modifies different components of the tumour microenvironment [9]. Resistance to pazopanib has been associated with a specific immunological profile, as was observed in metastatic renal cell carcinoma, with increased levels of IL6, IL8, VEGF, and numbers of granulocytic myeloid-derived suppressor cells [10]. Moreover, an increased neutrophil/lymphocyte ratio (NLR) after pazopanib treatment has been independently associated with significantly shorter PFS and OS in some STS types different from SFT [11]. Additionally, high NLR values have been also associated with adverse survival in many solid tumours [12,13,14] and metastasis at diagnosis in STS [15]. Other peripheral inflammatory indexes, such as high platelet/lymphocyte ratio (PLR), have been associated with worse survival in STS [16] and other solid tumours [17] or high red cell distribution width (RDW), which has been associated with worse OS in solid tumours, including osteosarcomas [17,18,19,20,21].
This study aims to evaluate retrospectively the prognostic value of NLR, PLR, and RDW peripheral inflammatory indexes in a cohort of advanced SFT patients, treated within a prospective phase 2 clinical trial with pazopanib [5,6]. This study also tries to correlate changes among these peripheral inflammatory indexes and gene expression profiles in the tumour microenvironment.
2. Materials and Methods
2.1. Patients
Patients enrolled in two SFT cohorts (typical or malignant/dedifferentiated SFT), within a single-arm phase 2 trial (ClinicalTrials.gov, accessed on 15 July 2022, NCT02066285, EudraCT number 2013-005456-15; GEIS-32), were analysed retrospectively. Each cohort was composed of patients aged over 18 years who were diagnosed with unresectable or metastatic SFT (32 typical, 33 malignant, and 2 dedifferentiated SFT) in any location, and they showed progression in the previous 6 months (by RECIST and Choi criteria) and an ECOG performance status of 0–2. Each patient received an oral dose of 800 mg of pazopanib daily, only interrupted in case of disease progression (according to Choi criteria) or intolerance.
2.2. Peripheral Inflammatory Indexes
Baseline values of neutrophils (109/L), platelets (109/L), and lymphocytes (109/L) were obtained from complete blood count tests (some hours before first dose or, in some cases, within 72 h before the first dose) to determine NLR and PLR before treatment with pazopanib. RDW values at baseline were also obtained from complete blood tests and normalised by the highest value detected in its hospital. The optimal cut-off point to categorise patients between high and low NLR (3.78), PLR (242), and RDW (1.03) was calculated using the maxstat package for PFS and OS, choosing the value of OS for all indexes. PLR and NLR ratios were not available in 1 and 2 malignant SFT patients, respectively. RDW ratio was not available in 6 typical and 4 malignant SFT patients.
2.3. Immuno-Oncology Assay
The gene expression of 549 human RNA transcripts involved in the innate and adaptive immune response to cancer was quantified with the immuno-oncology assay (HTG Molecular Diagnostics, Tucson, AZ, USA) in 49 formalin-fixed paraffin-embedded tumour samples (22 typical, 25 malignant, and 2 dedifferentiated SFT tumour samples). Sample preparation, assay performance, and data analysis were performed as we previously described [5,6].
2.4. Statistical Analysis
Time-to-event variables, PFS and OS, were estimated by Kaplan–Meier survival analysis from the treatment onset. PFS was assessed by median time and measured from treatment start date until progression or death. OS was assessed by median time and measured from treatment start date until death.
Univariate analysis for comparing variables of interest (age, sex, tumour size at diagnosis (mm), presence of necrosis, metastasis-free interval (MFI), Eastern Cooperative Oncology Group (ECOG) performance status, number of mitoses per 10 hpf, neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and red cell distribution width (RDW)) was performed by log-rank test. Multivariate analysis was carried out according to the Cox proportional hazard regression model. Two side p-values lower than 0.05 were considered significant.
The regulated genes associated with each peripheral inflammatory index were determined by comparing high versus low NLR, PLR, or RDW indexes using DESeq2. Transcriptomic data were correlated with the NLR, PLR, and RDW peripheral inflammatory indexes using a generalised linear model (GLM) in R (t-test).
3. Results
3.1. Patients
Peripheral inflammatory indexes were analysed in 67 SFT adult patients enrolled in the two cohorts of the GEIS-32 trial. The first cohort included 32 patients diagnosed as typical SFT, and the second cohort included 35 patients diagnosed as malignant/dedifferentiated SFT, of which only 2 were dedifferentiated. The median age was 63 years (range: 24–87), with 57% being female. Among them, 82% had metastatic disease before pazopanib initiation (Table 1).

Table 1.
Clinicopathologic characteristics of accrued patients.
At a median follow-up of 16.8 months, the overall response rate (ORR) according to Choi criteria by central review was 53.7%. The median PFS was 7.4 months (95% CI 3.7–11.1), and the median OS was 49.8 months (95% CI 14.1–85.4).
3.2. High NLR, PLR, and RDW Are Associated with Worse Survival
Patients with pretreatment NLR values higher than 3.78 showed worse median PFS (4.5 (95% CI 1.9–7.0) vs. 10.8 months (95% CI 8.7–12.9), p = 0.010) and worse median OS (11.7 months (95% CI 3.5–19.8) vs. NR, p < 0.001) (Figure 1a and Table 2) compared with patients with lower NLR. Patients with pretreatment PLR values higher than 242 showed worse median PFS (4.5 (95% CI 2.0–7.0) vs. 10.1 months (95% CI 6.3–13.9), p = 0.005) and worse median OS (10.7 (95% CI 5.2–16.2) vs. 49.8 months (95% CI 14.6–85.0), p < 0.001) (Figure 1b and Table 2) compared with patients with lower PLR. Patients with pretreatment RDW values higher than 1.03 showed worse median PFS (4.0 (95% CI 0.9–7.0) vs. 9.8 months (7.4–12.3), p = 0.001), worse median OS (10.7 (95% CI 3.8–17.5) vs. 49.8 months (95% CI 9.4–90.2), p < 0.001) (Figure 1c and Table 2), and worse Choi response (3 (23%) vs. 25 patients (59%), p < 0.029) compared with patients with lower RDW.
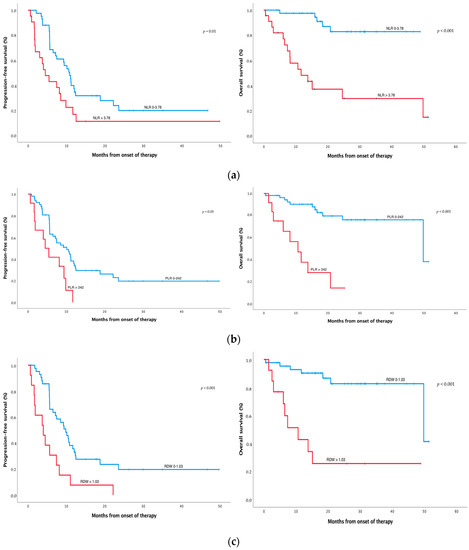
Figure 1.
Survival analysis of SFT treated with pazopanib. Progression-free survival (PFS) and overall survival (OS), according to Choi criteria, of SFT patients with high NLR (>3.78) vs. low NLR (a), high PLR (>242) vs. low PLR (b), and high RDW (>1.03) vs. low (c). NLR, PLR, and RDW were measured before pazopanib treatment. The optimal cut-off point to categorise patients between high and low levels of each inflammatory index was calculated by the maxstat package. Significance between groups was defined at p-values < 0.05.

Table 2.
Univariate analysis of clinicopathological factors and inflammatory indexes according to progression-free survival (PFS), overall survival (OS), and Choi response.
Moreover, other clinicopathological factors showed prognostic value for either PFS or OS. In this case, the presence of necrosis (p = 0.002), metastasis-free interval (MFI) ≤ 8 months (p = 0.01), and number of mitoses higher than 3 per 10 hpf (p < 0.001) were significantly associated with worse PFS, while tumour size at diagnosis higher than 85 mm (p < 0.001) and ECOG status higher than 1 (p = 0.001) were significantly associated with worse OS. Neither age nor sex was a prognostic factor for PFS or OS (Table 2). Moreover, MFI ≤ 8 months (p < 0.001) was significantly associated with a worse Choi response.
In the multivariate analysis of inflammatory indexes and clinicopathological factors (Table 3), NLR higher than 3.78 was an independent factor for worse PFS (p = 0.008) and Choi response (p = 0.009), while RDW higher than 1.03 was an independent factor for worse PFS (p = 0.012) and OS (p = 0.001). Moreover, the number of mitoses higher than 3 per hpf was also independently associated with worse PFS (p < 0.001) and Choi response (p = 0.028), while MFI ≤ 8 months (p = 0.042) and ECOG status higher than 1 (p = 0.005) were independently associated with worse PFS (p = 0.042) or worse OS (p = 0.005), respectively.

Table 3.
Multivariate analysis of clinicopathological factors according to progression-free survival (PFS), overall survival (OS), and Choi response.
3.3. Gene Expression Profiling According to High NLR and RDW
Gene expression profile was analysed in 51 tumour samples and correlated with inflammatory indexes. Tumour samples from patients with NLR values higher than 3.78 showed 12 genes differentially expressed (TYK2, RIPK2, TRAF2, IKBKB, DUSP6, PTGS2, CCND3, RUNX1, HSPA1A, ELK1, TFRC, and NFATC4, p-value < 0.05 and FDR < 0.05) versus patients with low NLR (Table 4). All of them were upregulated in patients with high NLR, with PTGS2 and RUNX1 being the genes with higher levels of overexpression (Log2FC = 1.31 and 1.47, respectively). PTGS2 was the only gene differentially expressed (Log2FC = 1.88, p-value < 0.001, FDR = 0.011) in tumour samples from patients with high RDW (>1.03) versus low RDW. No significantly regulated genes were identified for high PLR versus low PLR.

Table 4.
Genes significantly expressed in patients with high NLR versus low NLR.
4. Discussion
In this study, high NLR, PLR, and RDW peripheral inflammatory indexes showed a significant association with worse PFS and OS in patients diagnosed with advanced SFT and treated with pazopanib. Among the peripheral inflammatory indexes, high RDW was identified as an independent significant prognostic biomarker of worse outcome for PFS and OS, while high NLR was an independent prognostic factor just for worse OS. Importantly, these peripheral inflammatory indexes were evaluated in a series of advanced SFT patients enrolled in a prospective clinical trial that explored pazopanib as systemic treatment. Therefore, patients were strictly controlled in follow-up assessments. This phase II trial, GEIS-32 [5,6], was the first ever trial conducted in SFT and was activated at 16 European hospitals. The study included two different cohorts (formerly named typical and malignant SFT) and reported an outcome in PFS, OS, and ORR according to Choi criteria, clearly better than historical control achievement with chemotherapy. Similar to our results, high pretreatment and/or increased NLR values have been previously reported as predictive factors for a shorter PFS and OS in other types of STS treated with pazopanib, such as undifferentiated pleomorphic sarcoma and leiomyosarcoma [11,12]. Likewise, the prognostic role of NLR before treatment has been confirmed in different solid tumours [13], including STS [15]. On the other hand, high RDW has also been previously reported as a predictive factor in different tumours [21], including some types of sarcomas, such as osteosarcomas [20], but SFT patients were not included in these studies. In our study, other clinicopathological factors retrospectively explored, such as the presence of necrosis, MFI ≤ 8 months, and number of mitoses > 3/10 hpf or tumour size at diagnosis > 85 mm and ECOG > 1, were associated with statistically significant worse PFS or OS, respectively, in the univariate analysis. Moreover, MFI ≤ 8 months was associated with significantly worse Choi response. According to our results, NLR and RDW indexes may be used as prognostic biomarkers in advanced SFT patients treated with pazopanib.
Systemic inflammation, detectable through increased levels of C-reactive protein, cytokines, leukocytes, and their subtypes and hypoalbuminemia, was detected in our study with increased NLR and RDW values, which can be induced by the inflammatory response of the tumour microenvironment [22]. High NLR is a consequence of neutrophilia, which inhibits cytolytic activity of immune cells, such as lymphocytes, activated T cells, and natural killer cells. This fact might entail the reduction of T cell lymphocyte proportion in the tumour microenvironment. Additionally, high NLR has been associated with elevated infiltration of tumour-associated macrophages in the tumour microenvironment and elevated circulating cytokines (IL-1ra, IL-6, IL-7, IL-8, IL-9, IL-12, IFN-γ, IP10, MCP-1, MIP-1β, and PDGF-BB). Sometimes that may indicate M2 polarisation of tumour-associated macrophages [13]. On the other hand, high RDW reflects a high heterogeneity in the size of circulating erythrocytes, which indicates impaired erythropoiesis caused by inflammation among other stimuli (renal insufficiency or malnutrition). High RDW can be related to plasma inflammatory biomarkers, such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and IL-6 levels [21]. Various cytokines affect erythropoiesis (IL-6, IFN-γ, IL-1β, and TNF-α) via erythropoietin (EPO) production, inhibition of erythroid progenitors (IL-1α and IL-1β), and reduction in iron release (IL6). Together, this indicates a deleterious prognostic impact of inflamed circulating factors that might also reflect an inflamed tumour microenvironment in SFT.
The latent inflammation in the tumour microenvironment may promote tumour proliferation, survival of malignant cells, angiogenesis, and metastasis; subverts adaptive immune response, and hinders the action of chemotherapeutic agents [23,24,25,26]. In addition to tumour cells, neutrophils, macrophages, and lymphocytes infiltrated into the tumour microenvironment can contribute to amplifying inflammatory signalling through cytokines and chemokines. Neutrophils and macrophages secrete tumour-growth-promoting factors, including VEGF, HGF, IL-6, IL-8, MMPs, and elastases [12,27]. Our analysis of gene expression profiles in the tumour microenvironment identified genes implicated in inflammation or immune response, such as PTGS2 and TYK2. High PTGS2 (COX2) expression, a key enzyme that catalyses the conversion of arachidonic acid to prostaglandin, was detected in SFT in a significantly positive correlation with patients expressing high NLR and RDW levels. COX2 is the inducible isoform of COX, activated by growth factors, inflammatory stimuli, or carcinogenic factors. Although it is usually undetectable in most normal tissues, it has been previously described as a poor prognosis factor in breast [28], lung [29], pancreas [30], colorectal [31], and ovarian [32] cancer, as well as in osteosarcoma [33,34,35,36]. COX2 is released by cancer-associated fibroblasts (CAFs), type 2 macrophages (M2), and cancer cells to the tumour microenvironment (TME), where it induces cancer stem cell (CSC)-like activity, proliferation, angiogenesis, inflammation, invasion, and metastasis [37]. COX2 inhibitors sensitise cancer cells to radio-/chemotherapy and could reduce the risk of metastasis [38], so it could be a target for advanced SFT. TYK2 is a nonreceptor tyrosine kinase that mediates cytokine signalling and is significantly correlated with high NLR index. TYK2 is part of the Janus kinase (JAK) family and heterodimerises with JAK1 and 2. Once activated, JAKs recruit and phosphorylate signal transducers and activators of transcription (STAT). TYK2 specifically transduces the activation of STAT1, 2, and 5 and transmits signalling of type I and II interferons (IFNs) [39]. Other STATs can also transduce type I IFN signalling in some conditions and cell types; somewhat that should be tested in SFT with STAT6, where it is constitutively activated by the NAB2–STAT6 fusion transcript. Notably, type I IFNs stimulate the transcription of over 1000 genes involved in inflammation and immune functions [40]. Not surprisingly, high peripheral inflammatory indexes indicating high levels of circulatory cytokines are detected in SFT, and these indexes showed a significant correlation with TYK2 expression. Besides, in cancer cells, TYK2 activation can lead to increase cell survival, cell growth, invasion, and resistance to chemotherapy. Both TYK2 and STATs are considered promising targets in cancer [41,42] and should be tested in SFT. All the remaining genes in Table 4 have shown some implication in inflammation or immune response whose thorough discussion is beyond the scope of this paper. However, runt-related transcription factor 1, RUNX1, which expressed the highest logarithmic fold change in the correlation with high NLR signature, will be also commented on. Bioinformatic data revealed that RUNX1 is overexpressed in cancer, and its overexpression was also linked to a worse prognosis. Importantly, RUNX1 expression was positively correlated with cancer-associated fibroblasts (CAFs) in more than 30 different cancers [43]. There is well-known evidence that several cytokines or chemokines, mainly orchestrated by transforming growth factor beta (TGFb), are involved in the conversion of fibroblasts or myofibroblasts into CAFs [44]. Some of these form a feedback loop between cancer cells and CAFs, with the latter being a relevant tumour-promoting component. Thus, RUNX1 inhibition might be also a reasonable target in SFT.
Interpretation might be affected by the low number of the patients enrolled in this prospective clinical trial, despite being a considerable number for a rare entity. NLR may be altered by different pathologies, such as hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease, chronic kidney disease, heart failure, thyroid dysfunction, cerebrovascular disease, and peripheral arterial disease. RDW may also be altered by inadequate production of erythropoietin, observed in cases of undernutrition and impaired renal function. It is very improbable that these pathologies affected our results, taking into account the inclusion criteria of the trial.
5. Conclusions
In summary, high NLR and RDW are prognostic biomarkers of worse outcome in advanced SFT patients treated with pazopanib. The underlying tumour and microenvironment context that correlates with these peripheral inflammatory biomarkers seems to be governed, in some way, by tumour-promoting inflammation and resistance for immune response and for chemotherapy actions. The inhibition of COX2, TYK2, and RUNX1 will be tested soon by our lab team in SFT.
Author Contributions
S.H.-R. and J.C.-G. contributed equally to this work. Conceptualisation, J.M.-B.; formal analysis, A.G.; writing—original draft preparation, S.H.-R., J.C.-G., D.S.M. and J.M.-B.; writing—review and editing, S.H.-R., J.C.-G., D.S.M., S.S., A.L.-P., A.R., A.I., A.G., G.G., N.H., J.-A.L.-G., X.G.d.M., J.M.T., E.P., A.S.G., D.B., A.L.C., P.G.C., J.-Y.B., J.C.J. and J.M.-B.; supervision, J.M.-B. All authors have read and agreed to the published version of the manuscript.
Funding
S.H.-R. and J.C.-G. report institutional research grants from PharmaMar and Karyopharm. D.S.M. reports institutional research grants from PharmaMar, Eisai, Immix Biopharma, and Novartis outside the submitted work and travel support from PharmaMar, Eisai, Celgene, Bayer, and Pfizer. N.H. reports grants, personal fees, and nonfinancial support from PharmaMar; research grants from Eisai, Immix Biopharma, and Novartis outside the submitted work; and re-search funding for clinical studies (institutional) from PharmaMar, Eli Lilly and Company, AROG, Bayer, Eisai, Lixte, Karyopharm, Deciphera, GSK, Novartis, Blueprint, Nektar, Forma, Amgen, and Daiichi-Sankyo. J.M.B. reports research grants from PharmaMar, Eisai, Immix Biopharma and Novartis outside the submitted work; honoraria for advisory board participation and expert testimony from PharmaMar; honoraria for advisory board participation from Eli Lilly and Company, Bayer, and Eisai; and research funding for clinical studies (institutional) from PharmaMar, Eli Lilly and Company, AROG, Bayer, Eisai, Lixte, Karyopharm, Deciphera, GSK, Novartis, Blueprint, Nektar, Forma, Amgen, and Daiichi-Sankyo. X.G. reports research grant of AstraZeneca outside the submitted work; honoraria for advisory role and/or invited speaker from Pfizer, BMS, Ipsen, Astellas Pharma, Roche, PharmaMar, Eisai, and EUSA Pharma. J.C.J. reports speaker honoraria from Glaxo, AstraZeneca, Roche, Novartis, PharmaMar, Eisai, Lilly, Pfizer, Seagen, Glaxo, Gilead, and Daiichi-Sankyo; consultant/advisory role from AstraZeneca, Roche, Novartis, PharmaMar, Eisai, Lilly, Pfizer, Seagen, Glaxo, Gilead, and Daiichi-Sankyo. D.B. declares proctor/consultancy role for Boston and speaker fees from PharmaMar. A.L.C. declares bureau fees from PharmaMar, Deciphera, and Bayer. A.R. reports research funding (institutional) from PharmaMar, Eisai, and Roche Farma outside the submitted work; honoraria for advisory board participation from PharmaMar, Clovis, GSK, AstraZeneca, and MSD; and honoraria for speaking from PharmaMar, Clovis, GSK, AstraZeneca, and MSD.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Research Ethic Committee of the Illes Balears (protocol code GEIS-32, nº eudraCT 2013-005456-15, approved the 15th April 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available in a private area of the sponsor’s website (www.grupogeis.org, accessed on 15 July 2022) and will be available beginning 3 months and ending 5 years after publication of the initial study results. Data requests should be sent to secretaria@grupogeis.org.
Acknowledgments
D.S.M. is the recipient of a Sara Borrell postdoctoral fellowship funded by the National Institute of Health Carlos III (ISCIII) (CD20/00155). The authors would also like to thank the SELNET project. SELNET has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement no. 825806. The authors would like to thank the Instituto de Salud Carlos III (ISCIII)–Fondo Europeo de Desarrollo Regional (FEDER), project reference PI18/01728. APSATUR scholarship for research on solitary fibrous tumour. Patricio Ledesma for data management.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thway, K.; Ng, W.; Noujaim, J.; Jones, R.L.; Fisher, C. The Current Status of Solitary Fibrous Tumor: Diagnostic Features, Variants, and Genetics. Int. J. Surg. Pathol. 2016, 24, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Martin-Broto, J.; Mondaza-Hernandez, J.L.; Moura, D.S.; Hindi, N. A Comprehensive Review on Solitary Fibrous Tumor: New Insights for New Horizons. Cancers 2021, 13, 2913. [Google Scholar] [CrossRef] [PubMed]
- DeVito, N.; Henderson, E.; Han, G.; Reed, D.; Bui, M.M.; Lavey, R.; Robinson, L.; Zager, J.S.; Gonzalez, R.J.; Sondak, V.K.; et al. Clinical Characteristics and Outcomes for Solitary Fibrous Tumor (SFT): A Single Center Experience. PLoS ONE 2015, 10, e0140362. [Google Scholar] [CrossRef]
- Demicco, E.G.; Park, M.S.; Araujo, D.M.; Fox, P.S.; Bassett, R.L.; Pollock, R.E.; Lazar, A.J.; Wang, W.-L. Solitary Fibrous Tumor: A Clinicopathological Study of 110 Cases and Proposed Risk Assessment Model. Mod. Pathol. 2012, 25, 1298–1306. [Google Scholar] [CrossRef]
- Martin-Broto, J.; Stacchiotti, S.; Lopez-Pousa, A.; Redondo, A.; Bernabeu, D.; de Alava, E.; Casali, P.G.; Italiano, A.; Gutierrez, A.; Moura, D.S.; et al. Pazopanib for Treatment of Advanced Malignant and Dedifferentiated Solitary Fibrous Tumour: A Multicentre, Single-Arm, Phase 2 Trial. Lancet Oncol. 2019, 20, 134–144. [Google Scholar] [CrossRef]
- Martin-Broto, J.; Cruz, J.; Penel, N.; Le Cesne, A.; Hindi, N.; Luna, P.; Moura, D.S.; Bernabeu, D.; de Alava, E.; Lopez-Guerrero, J.A.; et al. Pazopanib for Treatment of Typical Solitary Fibrous Tumours: A Multicentre, Single-Arm, Phase 2 Trial. Lancet Oncol. 2020, 21, 456–466. [Google Scholar] [CrossRef]
- Van der Graaf, W.T.A.; Blay, J.-Y.; Chawla, S.P.; Kim, D.-W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for Metastatic Soft-Tissue Sarcoma (PALETTE): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Demicco, E.G.; Wani, K.; Fox, P.S.; Bassett, R.L.; Young, E.D.; Lev, D.; Aldape, K.D.; Lazar, A.J.; Wang, W.-L. Histologic Variability in Solitary Fibrous Tumors Reflects Angiogenic and Growth Factor Signaling Pathway Alterations. Hum. Pathol. 2015, 46, 1015–1026. [Google Scholar] [CrossRef]
- Kwilas, A.R.; Donahue, R.N.; Tsang, K.Y.; Hodge, J.W. Immune Consequences of Tyrosine Kinase Inhibitors That Synergize with Cancer Immunotherapy. Cancer Cell Microenviron. 2015, 2, e677. [Google Scholar] [CrossRef]
- Pal, S.K.; Hossain, D.M.S.; Zhang, Q.; Frankel, P.H.; Jones, J.O.; Carmichael, C.; Ruel, C.; Lau, C.; Kortylewski, M. Pazopanib as Third Line Therapy for Metastatic Renal Cell Carcinoma: Clinical Efficacy and Temporal Analysis of Cytokine Profile. J. Urol. 2015, 193, 1114–1121. [Google Scholar] [CrossRef]
- Kobayashi, H.; Okuma, T.; Oka, H.; Hirai, T.; Ohki, T.; Ikegami, M.; Sawada, R.; Shinoda, Y.; Akiyama, T.; Sato, K.; et al. Neutrophil-to-Lymphocyte Ratio after Pazopanib Treatment Predicts Response in Patients with Advanced Soft-Tissue Sarcoma. Int. J. Clin. Oncol. 2018, 23, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Mirili, C.; Paydaş, S.; Guney, I.B.; Ogul, A.; Gokcay, S.; Buyuksimsek, M.; Yetisir, A.E.; Karaalioglu, B.; Tohumcuoglu, M.; Seydaoglu, G. Assessment of Potential Predictive Value of Peripheral Blood Inflammatory Indexes in 26 Cases with Soft Tissue Sarcoma Treated by Pazopanib: A Retrospective Study. Cancer Manag. Res. 2019, 11, 3445–3453. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.-K.; Liu, Z.-L.; Shen, D.; Lin, Q.-F.; Su, J.; Mao, W.-D. Prognostic Performance of Pre-Treatment NLR and PLR in Patients Suffering from Osteosarcoma. World J. Surg. Oncol. 2016, 14, 127. [Google Scholar] [CrossRef]
- Chan, J.Y.; Zhang, Z.; Chew, W.; Tan, G.F.; Lim, C.L.; Zhou, L.; Goh, W.L.; Poon, E.; Somasundaram, N.; Selvarajan, S.; et al. Biological Significance and Prognostic Relevance of Peripheral Blood Neutrophil-to-Lymphocyte Ratio in Soft Tissue Sarcoma. Sci. Rep. 2018, 8, 11959. [Google Scholar] [CrossRef]
- Que, Y.; Qiu, H.; Li, Y.; Chen, Y.; Xiao, W.; Zhou, Z.; Zhang, X. Preoperative Platelet-Lymphocyte Ratio Is Superior to Neutrophil-Lymphocyte Ratio as a Prognostic Factor for Soft-Tissue Sarcoma. BMC Cancer 2015, 15, 648. [Google Scholar] [CrossRef]
- Templeton, A.J.; Ace, O.; McNamara, M.G.; Al-Mubarak, M.; Vera-Badillo, F.E.; Hermanns, T.; Seruga, B.; Ocaña, A.; Tannock, I.F.; Amir, E. Prognostic Role of Platelet to Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1204–1212. [Google Scholar] [CrossRef]
- Montagnana, M.; Danese, E. Red Cell Distribution Width and Cancer. Ann. Transl. Med. 2016, 4, 399. [Google Scholar] [CrossRef]
- Hu, L.; Li, M.; Ding, Y.; Pu, L.; Liu, J.; Xie, J.; Cabanero, M.; Li, J.; Xiang, R.; Xiong, S. Prognostic Value of RDW in Cancers: A Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 16027–16035. [Google Scholar] [CrossRef]
- Zheng, J.; Yuan, X.; Guo, W. Relationship between Red Cell Distribution Width and Prognosis of Patients with Osteosarcoma. Biosci. Rep. 2019, 39, BSR20192590. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.-F.; Song, S.-Y.; Guo, H.; Wang, T.-J.; Liu, N.; Yan, C.-X. Prognostic Role of Pretreatment Red Blood Cell Distribution Width in Patients with Cancer: A Meta-Analysis of 49 Studies. J. Cancer 2019, 10, 4305–4317. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-Related Inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, C.S.D.; McMillan, D.C. Role of Systemic Inflammatory Response in Predicting Survival in Patients with Primary Operable Cancer. Future Oncol. 2010, 6, 149–163. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Olsson, A.K.; Cedervall, J. The Pro-Inflammatory Role of Platelets in Cancer. Platelets 2018, 29, 569–573. [Google Scholar] [CrossRef]
- Kusumanto, Y.H.; Dam, W.A.; Hospers, G.A.P.; Meijer, C.; Mulder, N.H. Platelets and Granulocytes, in Particular the Neutrophils, Form Important Compartments for Circulating Vascular Endothelial Growth Factor. Angiogenesis 2003, 6, 283–287. [Google Scholar] [CrossRef]
- Xu, F.; Li, M.; Zhang, C.; Cui, J.; Liu, J.; Li, J.; Jiang, H. Clinicopathological and Prognostic Significance of COX-2 Immunohistochemical Expression in Breast Cancer: A Meta-Analysis. Oncotarget 2017, 8, 6003–6012. [Google Scholar] [CrossRef]
- Laga, A.C.; Zander, D.S.; Cagle, P.T. Prognostic Significance of Cyclooxygenase 2 Expression in 259 Cases of Non-Small Cell Lung Cancer. Arch. Pathol. Lab. Med. 2005, 129, 1113–1117. [Google Scholar] [CrossRef]
- Juuti, A.; Louhimo, J.; Nordling, S.; Ristimäki, A.; Haglund, C. Cyclooxygenase-2 Expression Correlates with Poor Prognosis in Pancreatic Cancer. J. Clin. Pathol. 2006, 59, 382–386. [Google Scholar] [CrossRef] [Green Version]
- Kunzmann, A.T.; Murray, L.J.; Cardwell, C.R.; McShane, C.M.; McMenamin, U.C.; Cantwell, M.M. PTGS2 (Cyclooxygenase-2) Expression and Survival among Colorectal Cancer Patients: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Myung, S.-K.; Song, Y.-S. Prognostic Role of Cyclooxygenase-2 in Epithelial Ovarian Cancer: A Meta-Analysis of Observational Studies. Gynecol. Oncol. 2013, 129, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, H.; Zuo, J.; Gao, Z. Cyclooxygenase-2 Expression Correlates with Development, Progression, Metastasis, and Prognosis of Osteosarcoma: A Meta-Analysis and Trial Sequential Analysis. FEBS Open Bio 2019, 9, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, M.; Xiao, Z.; Wu, H.; Wu, Y. Quantitative Assessment of the Association of COX-2 (Cyclooxygenase-2) Immunoexpression with Prognosis in Human Osteosarcoma: A Meta-Analysis. PLoS ONE 2013, 8, e82907. [Google Scholar] [CrossRef]
- Jiao, G.; Ren, T.; Lu, Q.; Sun, Y.; Lou, Z.; Peng, X.; Liang, W.; Guo, W. Prognostic Significance of Cyclooxygenase-2 in Osteosarcoma: A Meta-Analysis. Tumor Biol. 2013, 34, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.I.; Hoots, W.K.; Koshkina, N.V.; Morales-Arias, J.A.; Arndt, C.A.; Inwards, C.Y.; Hawkins, D.S.; Munsell, M.F.; Kleinerman, E.S. COX-2 Expression Correlates with Survival in Patients with Osteosarcoma Lung Metastases. J. Pediatr. Hematol. Oncol. 2008, 30, 507–512. [Google Scholar] [CrossRef]
- Hashemi Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in Cancer: A Review. J. Cell. Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef]
- Tołoczko-Iwaniuk, N.; Dziemiańczyk-Pakieła, D.; Nowaszewska, B.K.; Celińska-Janowicz, K.; Miltyk, W. Celecoxib in Cancer Therapy and Prevention—Review. Curr. Drug Targets 2019, 20, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Borcherding, D.C.; He, K.; Amin, N.V.; Hirbe, A.C. TYK2 in Cancer Metastases: Genomic and Proteomic Discovery. Cancers 2021, 13, 4171. [Google Scholar] [CrossRef]
- Barrat, F.J.; Lu, T.T. Role of Type I Interferons and Innate Immunity in Systemic Sclerosis: Unbalanced Activities on Distinct Cell Types? Curr. Opin. Rheumatol. 2019, 31, 569–575. [Google Scholar] [CrossRef]
- Wrobleski, S.T.; Moslin, R.; Lin, S.; Zhang, Y.; Spergel, S.; Kempson, J.; Tokarski, J.S.; Strnad, J.; Zupa-Fernandez, A.; Cheng, L.; et al. Highly Selective Inhibition of Tyrosine Kinase 2 (TYK2) for the Treatment of Autoimmune Diseases: Discovery of the Allosteric Inhibitor BMS-986165. J. Med. Chem. 2019, 62, 8973–8995. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.L.A.; Hirpara, J.L.; Pervaiz, S.; Eu, J.-Q.; Sethi, G.; Goh, B.-C. Do STAT3 Inhibitors Have Potential in the Future for Cancer Therapy? Expert Opin. Investig. Drugs 2017, 26, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Tuo, Z.; Zhang, Y.; Wang, X.; Dai, S.; Liu, K.; Xia, D.; Wang, J.; Bi, L. RUNX1 Is a Promising Prognostic Biomarker and Related to Immune Infiltrates of Cancer-Associated Fibroblasts in Human Cancers. BMC Cancer 2022, 22, 523. [Google Scholar] [CrossRef]
- Kapusta, P.; Dulińska-Litewka, J.; Totoń-Żurańska, J.; Borys, A.; Konieczny, P.S.; Wołkow, P.P.; Seweryn, M.T. Dysregulation of Transcription Factor Activity During Formation of Cancer-Associated Fibroblasts. Int. J. Mol. Sci. 2020, 21, 8749. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).