Factors Predicting 30-Day Grade IIIa–V Clavien–Dindo Classification Complications and Delayed Chemotherapy Initiation after Cytoreductive Surgery for Advanced-Stage Ovarian Cancer: A Prospective Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Variables and Definitions
2.3. Study Outcomes
2.4. Statistical Analysis
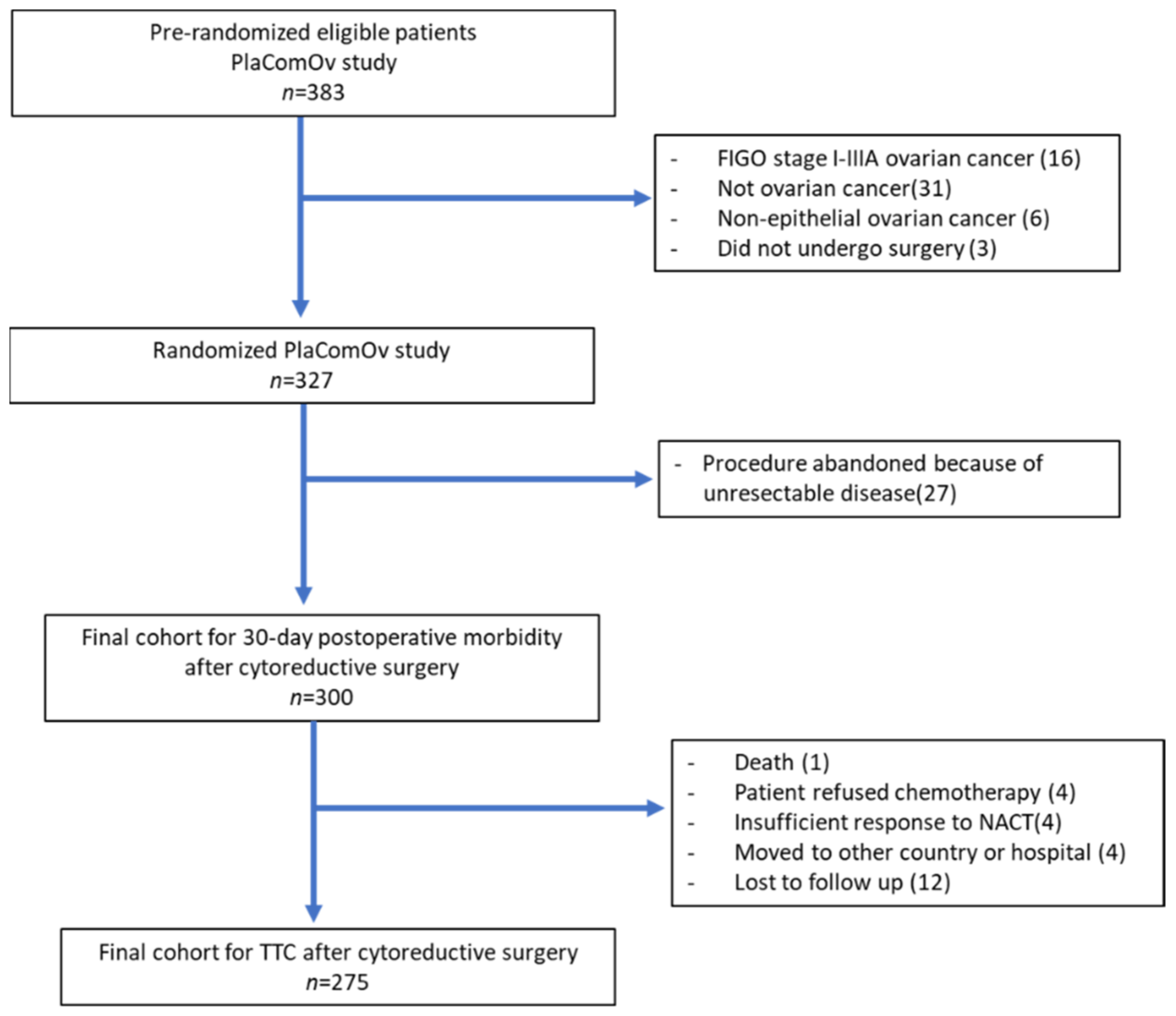
3. Results
3.1. Patient Characteristics and Surgical Outcome
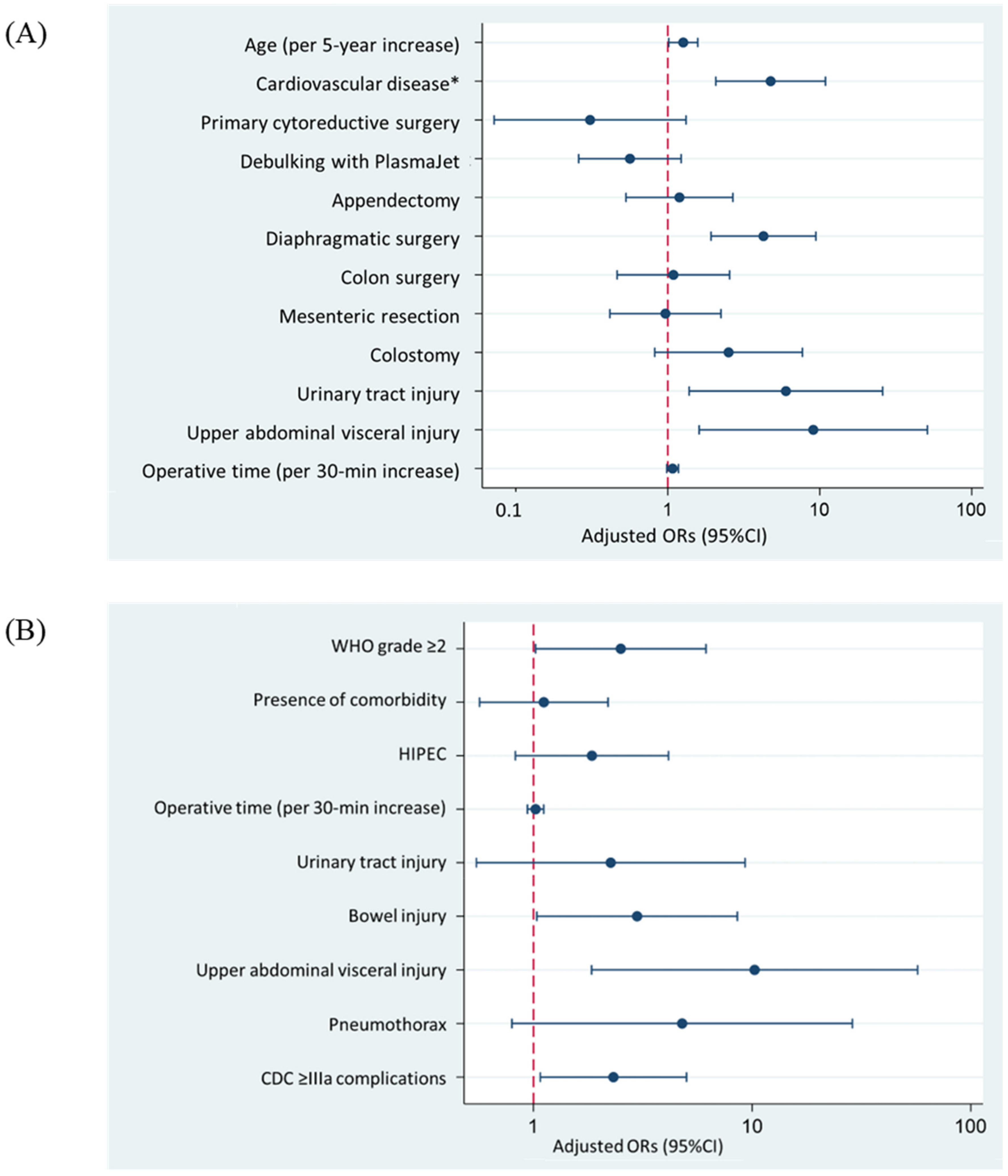
3.2. Regression Analysis of Factors Related to Clavien–Dindo Classification Grade ≥ IIIa
3.3. Regression Analysis of Factors Related to Time to Adjuvant Chemotherapy >42 Days
3.4. Interval Cytoreductive Surgery
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEOC | Advanced-stage epithelial ovarian cancer |
| BMI | Body mass index |
| CA125 | Cancer antigen 125 |
| CDC | Clavien–Dindo classification |
| CRS | Cytoreductive surgery |
| ERAS | Enhanced recovery after surgery |
| FIGO | International Federation of Gynecology and Obstetrics |
| ICU | Intensive care unit |
| ICS | Interval cytoreductive surgery |
| IQR | Interquartile range |
| HIPEC | Hyperthermic intraperitoneal chemotherapy |
| NACT | Neoadjuvant chemotherapy |
| OS | Overall survival |
| PCS | Primary cytoreductive surgery |
| PFS | Progression free survival |
| SD | Standard deviation |
| TTC | Time to chemotherapy |
| WHO | World Health Organization |
References
- Mahmood, R.D.; Morgan, R.D.; Edmondson, R.J.; Clamp, A.R.; Jayson, G.C. First-Line Management of Advanced High-Grade Serous Ovarian Cancer. Curr. Oncol. Rep. 2020, 22, 64. [Google Scholar] [CrossRef] [PubMed]
- Tangjitgamol, S.; Manusirivithaya, S.; Laopaiboon, M.; Lumbiganon, P.; Bryant, A. Interval debulking surgery for advanced epithelial ovarian cancer. Cochrane Database Syst. Rev. 2016, 2016, CD006014. [Google Scholar] [CrossRef] [PubMed]
- Coleridge, S.L.; Bryant, A.; Kehoe, S.; Morrison, J. Neoadjuvant chemotherapy before surgery versus surgery followed by chemotherapy for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst. Rev. 2021, 7, CD005343. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Elattar, A.; Bryant, A.; Winter-Roach, B.A.; Hatem, M.; Naik, R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst. Rev. 2011, 2011, CD007565. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Becerra, A.Z.; Justiniano, C.F.; Aquina, C.T.; Fleming, F.J.; Boscoe, F.P.; Schymura, M.J.; Sinno, A.K.; Chaoul, J.; Morrow, G.R.; et al. Complications and Survivorship Trends After Primary Debulking Surgery for Ovarian Cancer. J. Surg. Res. 2020, 246, 34–41. [Google Scholar] [CrossRef]
- Tewari, K.S.; Java, J.J.; Eskander, R.N.; Monk, B.J.; Burger, R.A. Early initiation of chemotherapy following complete resection of advanced ovarian cancer associated with improved survival: NRG Oncology/Gynecologic Oncology Group study. Ann. Oncol. 2016, 27, 114–121. [Google Scholar] [CrossRef]
- Lee, Y.J.; Chung, Y.S.; Lee, J.Y.; Nam, E.J.; Kim, S.W.; Kim, S.; Kim, Y.T. Impact of the time interval from completion of neoadjuvant chemotherapy to initiation of postoperative adjuvant chemotherapy on the survival of patients with advanced ovarian cancer. Gynecol. Oncol. 2018, 148, 62–67. [Google Scholar] [CrossRef]
- Hofstetter, G.; Concin, N.; Braicu, I.; Chekerov, R.; Sehouli, J.; Cadron, I.; Van Gorp, T.; Trillsch, F.; Mahner, S.; Ulmer, H.; et al. The time interval from surgery to start of chemotherapy significantly impacts prognosis in patients with advanced serous ovarian carcinoma—Analysis of patient data in the prospective OVCAD study. Gynecol. Oncol. 2013, 131, 15–20. [Google Scholar] [CrossRef]
- Lin, H.; Chen, W.H.; Wu, C.H.; Ou, Y.C.; Chen, Y.J.; Chen, Y.Y.; Lin, Y.H.; Fu, H.C. Impact of the Time Interval Between Primary Debulking Surgery and Start of Adjuvant Chemotherapy in Advanced Epithelial Ovarian Cancer. Cancer Manag. Res. 2021, 13, 5413–5422. [Google Scholar] [CrossRef]
- Somashekhar, S.P.; Ramya, Y.; Ashwin, K.R.; Shabber, S.Z.; Ahuja, V.K.; Amit, R.; Rohit, K.C. Evaluation of delay in time to adjuvant chemotherapy after HIPEC and its impact on oncological outcome in advanced epithelial ovarian cancer. Pleura Peritoneum 2020, 5, 2020-0103. [Google Scholar] [CrossRef]
- Timmermans, M.; van der Aa, M.A.; Lalisang, R.I.; Witteveen, P.O.; Van de Vijver, K.K.; Kruitwagen, R.F.; Sonke, G.S. Interval between debulking surgery and adjuvant chemotherapy is associated with overall survival in patients with advanced ovarian cancer. Gynecol. Oncol. 2018, 150, 446–450. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, T.; Wu, Q.; Jiao, Y.; Gong, T.; Ma, X.; Li, D. Relationship between initiation time of adjuvant chemotherapy and survival in ovarian cancer patients: A dose-response meta-analysis of cohort studies. Sci. Rep. 2017, 7, 9461. [Google Scholar] [CrossRef]
- Baldewpersad Tewarie, N.M.S.; van Driel, W.J.; van Ham, M.; Wouters, M.W.; Kruitwagen, R. Postoperative outcomes of primary and interval cytoreductive surgery for advanced ovarian cancer registered in the Dutch Gynecological Oncology Audit (DGOA). Gynecol. Oncol. 2021, 162, 331–338. [Google Scholar] [CrossRef]
- De Groot, J.J.; Maessen, J.M.; Slangen, B.F.; Winkens, B.; Dirksen, C.D.; van der Weijden, T. A stepped strategy that aims at the nationwide implementation of the Enhanced Recovery After Surgery programme in major gynaecological surgery: Study protocol of a cluster randomised controlled trial. Implement. Sci. IS 2015, 10, 106. [Google Scholar] [CrossRef][Green Version]
- Nelson, G.; Bakkum-Gamez, J.; Kalogera, E.; Glaser, G.; Altman, A.; Meyer, L.A.; Taylor, J.S.; Iniesta, M.; Lasala, J.; Mena, G.; et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations—2019 update. Int. J. Gynecol. Cancer 2019, 29, 651. [Google Scholar] [CrossRef]
- Noh, J.J.; Kim, M.-S.; Lee, Y.-Y. The implementation of enhanced recovery after surgery protocols in ovarian malignancy surgery. Gland Surg. 2021, 10, 1182–1194. [Google Scholar] [CrossRef]
- Tankou, J.I.; Foley, O.; Falzone, M.; Kalyanaraman, R.; Elias, K.M. Enhanced recovery after surgery protocols improve time to return to intended oncology treatment following interval cytoreductive surgery for advanced gynecologic cancers. Int. J. Gynecol. Cancer 2021, 31, 1145. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Paudel, P.; Rajbhandari, B.; Pradhan, G.B.; Shrestha, S.; Bhattachan, C.L. An analysis of surgical complications; a tool to improve surgical outcome. Nepal Med. Coll. J. NMCJ 2014, 16, 115–118. [Google Scholar]
- Boer, G.M.N.; Hofhuis, W.; Reesink-Peters, N.; Willemsen, S.; Boere, I.A.; Schoots, I.G.; Piek, J.M.J.; Hofman, L.N.; Beltman, J.J.; van Driel, W.J.; et al. Adjuvant Use of PlasmaJet Device During Cytoreductive Surgery for Advanced-Stage Ovarian Cancer: Results of the PlaComOv-study, a Randomized Controlled Trial in The Netherlands. Ann. Surg. Oncol. 2022, 29, 4833–4843. [Google Scholar] [CrossRef] [PubMed]
- Eggink, F.A.; Mom, C.H.; Kruitwagen, R.F.; Reyners, A.K.; Van Driel, W.J.; Massuger, L.F.; Niemeijer, G.C.; Van der Zee, A.G.; Van der Aa, M.A.; Nijman, H.W. Improved outcomes due to changes in organization of care for patients with ovarian cancer in the Netherlands. Gynecol. Oncol. 2016, 141, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.; Gentry-Maharaj, A.; Nordin, A.; Burnell, M.; Liston, R.; Manchanda, R.; Das, N.; Desai, R.; Gornall, R.; Beardmore-Gray, A.; et al. Predictors of complications in gynaecological oncological surgery: A prospective multicentre study (UKGOSOC—UK gynaecological oncology surgical outcomes and complications). Br. J. Cancer 2015, 112, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.G.R.; Dos Reis, R.; Cintra, G.F.; De Assunção Sousa, M.M.; Vieira, M.D.A.; Andrade, C.E.M.D.C. Predictive Factors for Surgical Morbidities and Adjuvant Chemotherapy Delay for Advanced Ovarian Cancer Patients Treated by Primary Debulking Surgery or Interval Debulking Surgery. Int. J. Gynecol. Cancer 2018, 28, 1520–1528. [Google Scholar] [CrossRef]
- Van Smeden, M.; Moons, K.G.; de Groot, J.A.; Collins, G.S.; Altman, D.G.; Eijkemans, M.J.; Reitsma, J.B. Sample size for binary logistic prediction models: Beyond events per variable criteria. Stat. Methods Med. Res. 2019, 28, 2455–2474. [Google Scholar] [CrossRef]
- Chi, D.S.; Zivanovic, O.; Levinson, K.L.; Kolev, V.; Huh, J.; Dottino, J.; Gardner, G.J.; Leitao, M.M., Jr.; Levine, D.A.; Sonoda, Y.; et al. The incidence of major complications after the performance of extensive upper abdominal surgical procedures during primary cytoreduction of advanced ovarian, tubal, and peritoneal carcinomas. Gynecol. Oncol. 2010, 119, 38–42. [Google Scholar] [CrossRef]
- Bolliger, M.; Kroehnert, J.A.; Molineus, F.; Kandioler, D.; Schindl, M.; Riss, P. Experiences with the standardized classification of surgical complications (Clavien-Dindo) in general surgery patients. Eur. Surg. 2018, 50, 256–261. [Google Scholar] [CrossRef]
- Benedetti Panici, P.; Di Donato, V.; Fischetti, M.; Casorelli, A.; Perniola, G.; Musella, A.; Marchetti, C.; Palaia, I.; Berloco, P.; Muzii, L. Predictors of postoperative morbidity after cytoreduction for advanced ovarian cancer: Analysis and management of complications in upper abdominal surgery. Gynecol. Oncol. 2015, 137, 406–411. [Google Scholar] [CrossRef]
- Inci, M.G.; Rasch, J.; Woopen, H.; Mueller, K.; Richter, R.; Sehouli, J. ECOG and BMI as preoperative risk factors for severe postoperative complications in ovarian cancer patients: Results of a prospective study (RISC-GYN-trial). Arch. Gynecol. Obstet. 2021, 304, 1323–1333. [Google Scholar] [CrossRef]
- Cham, S.; Chen, L.; St. Clair, C.M.; Hou, J.Y.; Tergas, A.I.; Melamed, A.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L.; Wright, J.D. Development and validation of a risk-calculator for adverse perioperative outcomes for women with ovarian cancer. Am. J. Obstet. Gynecol. 2019, 220, 571.E1–571.E8. [Google Scholar] [CrossRef]
- Rausei, S.; Uccella, S.; D’Alessandro, V.; Gisone, B.; Frattini, F.; Lianos, G.; Rovera, F.; Boni, L.; Dionigi, G.; Ghezzi, F. Aggressive surgery for advanced ovarian cancer performed by a multidisciplinary team: A retrospective analysis on a large series of patients. Surg. Open Sci. 2019, 1, 43–47. [Google Scholar] [CrossRef]
- Yalcin, Y.; Tatar, B.; Erdemoglu, E.; Erdemoglu, E. The parameters to estimate postoperative severe complications classified through Clavien-Dindo after upper abdominal surgery in patients with primary and recurrent ovarian cancer. Ginekol. Pol. 2019, 90, 557–564. [Google Scholar] [CrossRef]
- Gerestein, C.G.; Nieuwenhuyzen-de Boer, G.M.; Eijkemans, M.J.; Kooi, G.S.; Burger, C.W. Prediction of 30-day morbidity after primary cytoreductive surgery for advanced stage ovarian cancer. Eur. J. Cancer 2010, 46, 102–109. [Google Scholar] [CrossRef]
- Gunakan, E.; Tohma, Y.A.; Tunc, M.; Akilli, H.; Sahin, H.; Ayhan, A. Factors associated with surgical morbidity of primary debulking in epithelial ovarian cancer. Obstet. Gynecol. Sci. 2020, 63, 64–71. [Google Scholar] [CrossRef]
- Zighelboim, I.; Kizer, N.; Taylor, N.P.; Case, A.S.; Gao, F.; Thaker, P.H.; Rader, J.S.; Massad, L.S.; Mutch, D.G.; Powell, M.A. "Surgical Apgar Score" predicts postoperative complications after cytoreduction for advanced ovarian cancer. Gynecol. Oncol. 2010, 116, 370–373. [Google Scholar] [CrossRef]
- Kengsakul, M.; Nieuwenhuyzen-de Boer, G.M.; Udomkarnjananun, S.; Kerr, S.J.; Niehot, C.D.; van Beekhuizen, H.J. Factors predicting postoperative morbidity after cytoreductive surgery for ovarian cancer: A systematic review and meta-analysis. J. Gynecol. Oncol. 2022, 33, e53. [Google Scholar] [CrossRef]
- Llueca, A.; Serra, A.; Maiocchi, K.; Delgado, K.; Jativa, R.; Gomez, L.; Escrig, J. Predictive model for major complications after extensive abdominal surgery in primary advanced ovarian cancer. Int. J. Women’s Health 2019, 11, 161–167. [Google Scholar] [CrossRef]
- Kumar, A.; Janco, J.M.; Mariani, A.; Bakkum-Gamez, J.N.; Langstraat, C.L.; Weaver, A.L.; McGree, M.E.; Cliby, W.A. Risk-prediction model of severe postoperative complications after primary debulking surgery for advanced ovarian cancer. Gynecol. Oncol. 2016, 140, 15–21. [Google Scholar] [CrossRef]
- Sánchez-Iglesias, J.L.; Carbonell-Socias, M.; Pérez-Benavente, M.A.; Monreal Clua, S.; Manrique-Muñoz, S.; García Gorriz, M.; Burgos-Peláez, R.; Segurola Gurrutxaga, H.; Pamies Serrano, M.; Pilar Gutiérrez-Barceló, M.D.; et al. PROFAST: A randomised trial implementing enhanced recovery after surgery for highcomplexity advanced ovarian cancer surgery. Eur. J. Cancer 2020, 136, 149–158. [Google Scholar] [CrossRef]
- Liu, X.D.; Liu, Y.; Gong, T.T.; Guo, J.Y.; Wang, Y.N.; Wang, L.; Wu, Q.J.; Jiao, Y.S. Prognostic Influence of the Time Interval between Surgery and Chemotherapy in Epithelial Ovarian Cancer. J. Cancer 2018, 9, 4172–4178. [Google Scholar] [CrossRef]
- Rocher, G.; Gaillard, T.; Uzan, C.; Collinet, P.; Bolze, P.A.; Ballester, M.; Bendifallah, S.; Ouldamer, L.; Touboul, C.; Huchon, C.; et al. Does Time-to-Chemotherapy after Primary Complete Macroscopic Cytoreductive Surgery Influence Prognosis for Patients with Epithelial Ovarian Cancer? A Study of the FRANCOGYN Group. J. Clin. Med. 2021, 10, 1058. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Guetzko, M.; Resnick, K. Preoperative predictors of delay in initiation of adjuvant chemotherapy in patients undergoing primary debulking surgery for ovarian cancer. Gynecol. Oncol. 2016, 143, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, C.; Planchamp, F.; Aytulu, T.; Chiva, L.; Cina, A.; Ergönül, Ö.; Fagotti, A.; Haidopoulos, D.; Hasenburg, A.; Hughes, C.; et al. European Society of Gynaecological Oncology guidelines for the peri-operative management of advanced ovarian cancer patients undergoing debulking surgery. Int. J. Gynecol. Cancer 2021, 31, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Feijoo, B.; Agusti-Garcia, N.; Sebio, R.; López-Hernández, A.; Sisó, M.; Glickman, A.; Carreras-Dieguez, N.; Fuste, P.; Marina, T.; Martínez-Egea, J.; et al. Feasibility of a Multimodal Prehabilitation Programme in Patients Undergoing Cytoreductive Surgery for Advanced Ovarian Cancer: A Pilot Study. Cancers 2022, 14, 1635. [Google Scholar] [CrossRef]
| Variable | Value (%) |
|---|---|
| Age, mean ± SD (year) | 65 ± 10.4 |
| BMI, mean ± SD (kg/m2) | 25 ± 4.7 |
| CA125 at diagnosis, median (Q1–Q3) (kU/L) | 807 (301–2077) |
| Daily smoker | 29 (9.7) |
| Daily alcohol drinking | 82 (27.4) |
| WHO performance status | |
| 0: able to all normal activity | 175 (58.3) |
| 1: able to carry out light work | 105 (35.0) |
| 2: capable of self-care but not any work | 12 (4.0) |
| 3: capable of limited self-care, confined to bed or chair >50% of waking hours | 8 (2.7) |
| 4: disabled | 0 (0) |
| Comorbidity | |
| Diabetes mellitus | 27 (9.0) |
| Hypertension | 79 (26.3) |
| Cardiovascular disease * | 54 (18.0) |
| Primary cytoreductive surgery | 45 (15.0) |
| Extensive surgery ** | 209 (69.7) |
| HIPEC procedure | 61 (20.3) |
| Debulking with PlasmaJet | 138 (46) |
| Surgical outcome | |
| Complete cytoreduction (no visible tumor) | 235 (78.3) |
| Optimal cytoreduction (residual tumor ≤1 cm) | 50 (16.7) |
| Suboptimal cytoreduction (residual tumor >1 cm) | 15 (5.0) |
| FIGO stage | |
| Stage IIIB | 21 (7.0) |
| Stage IIIC | 187 (62.3) |
| Stage IV | 92 (30.7) |
| Post-surgery admission | |
| Nursing ward | 174 (58.0) |
| Post anesthetic care unit | 53 (17.7) |
| Intensive care unit | 73 (24.3) |
| Histology | |
| Serous | 287 (95.7) |
| Mucinous | 2 (0.7) |
| Endometrioid | 4 (1.3) |
| Clear cell | 6 (2.0) |
| Mixed epithelial carcinoma | 1 (0.3) |
| Type | Clavien–Dindo Grade | N (%) |
|---|---|---|
| Surgical Complications | ||
| Intraabdominal bleeding | IIIb | 1 (0.3) |
| Re-exploration for peritonitis | IIIb | 4 (1.3) |
| Anastomosis leakage | IIIb | 2 (0.7) |
| Gastric leakage | IIIb | 1 (0.3) |
| Pancreatic leakage | IIIa IIIb | 1 (0.3) 1 (0.3) |
| Intraabdominal abscess | IIIa IIIb | 4 (1.3) 1 (0.3) |
| Intestinal perforation | IIIa | 1 (0.3) |
| Splenic hemorrhage | IIIa | 1 (0.3) |
| Pneumothorax | IIIa | 4 (1.3) |
| Wound dehiscence | IIIa | 1 (0.3) |
| Acute cholecystitis | IIIb | 1 (0.3) |
| Medical complications | ||
| Cardiovascular related complications * | IVa | 19 (6.3) |
| Respiratory insufficiency | IVa | 11 (3.7) |
| Massive pulmonary embolism | IVa | 1 (0.3) |
| Acute kidney injury | IVa | 1 (0.3) |
| Acute transaminitis | IVa | 1 (0.3) |
| 30-day mortality | V | 1 (0.3) |
| One patient can endure more than one complication | 57 complications/51 patients |
| Variables | Univariable Analysis Unadjusted OR (95%CI) | p-Value | Multivariable Analysis Adjusted OR (95%CI) | p-Value |
|---|---|---|---|---|
| Pre-operative factor | ||||
| Age (per 5-year increase) | 1.19 (1.01–1.40) | 0.043 | 1.26 (1.02–1.58) | 0.036 |
| BMI (per kg/m2 increase) | 0.98 (0.92–1.05) | 0.57 | ||
| WHO performance status ≥2 | 0.72 (0.27–1.96) | 0.52 | ||
| Daily smoker | 1.62 (0.66–4.06) | 0.29 | ||
| Diabetes mellitus | 1.52 (0.85–2.69) | 0.16 | ||
| Hypertension | 1.10 (0.75–1.63) | 0.62 | ||
| Cardiovascular disease * | 3.67 (1.87–7.19) | <0.001 | 4.75 (2.07–10.91) | <0.001 |
| Primary cytoreductive surgery | 0.40 (0.14–1.16) | 0.09 | 0.31 (0.07–1.32) | 0.11 |
| Intraoperative procedure | ||||
| Pelvic peritonectomy | 1.62 (0.86–3.03) | 0.14 | ||
| Bladder surgery | 1.43 (0.78–2.62) | 0.25 | ||
| Small bowel surgery | 1.22 (0.72–2.08) | 0.45 | ||
| Colon surgery | 1.85 (0.97–3.51) | 0.06 | 1.09 (0.46–2.55) | 0.85 |
| Appendectomy | 1.85 (0.98–3.51) | 0.06 | 1.19 (0.53–2.68) | 0.67 |
| Mesenteric resection | 1.92 (1.02–3.62) | 0.04 | 0.97 (0.42–2.24) | 0.93 |
| Partial hepatectomy | 1.06 (0.44–2.57) | 0.89 | ||
| Splenectomy | 1.51 (0.53–4.32) | 0.44 | ||
| Pelvic lymph node resection | 1.40 (0.60–3.29) | 0.43 | ||
| Paraaortic lymph node resection | 1.51 (0.53–4.32) | 0.44 | ||
| Diaphragmatic surgery | 4.01 (2.12–7.57) | <0.001 | 4.26 (1.93–9.43) | <0.001 |
| Colostomy | 2.82 (1.23–6.51) | 0.02 | 2.51 (0.82–7.69) | 0.11 |
| HIPEC procedure | 1.13 (0.54–2.37) | 0.74 | ||
| Debulking with PlasmaJet | 0.59 (0.32–1.09) | 0.09 | 0.56 (0.26–1.22) | 0.15 |
| Operative time (per 30-min increase) | 1.10 (1.02–1.85) | 0.012 | 1.08 (0.98–1.18) | 0.11 |
| Intraoperative injury | ||||
| Urinary tract injury | 3.71 (1.13–12.02) | 0.031 | 6.00 (1.38–25.93) | 0.017 |
| Bowel injury | 2.05 (0.76–5.58) | 0.16 | ||
| Upper-abdominal visceral injury ** | 5.24 (1.46–18.83) | 0.011 | 9.07 (1.61–51.08) | 0.012 |
| Pneumothorax | 3.01 (0.69–13.03) | 0.14 | ||
| Blood loss >1 L | 1.65 (0.89–3.09) | 0.11 | ||
| Postoperative factor | ||||
| Complete cytoreduction | 1.34 (0.62–2.93) | 0.46 | ||
| FIGO stage | ||||
| Stage IIIB (reference) | ||||
| Stage IIIC | 1.31 (0.38–4.48) | 0.66 | ||
| Stage IV | 1.19 (0.60–2.36) | 0.61 |
| Variables | Univariable Analysis Unadjusted OR (95% CI) | p-Value | Multivariable Analysis Adjusted OR (95% CI) | p-Value |
|---|---|---|---|---|
| Preoperative factor | ||||
| Age (per 5-year increase) | 1.02 (0.89–1.18) | 0.75 | ||
| BMI (per kg/m2 increase) | 1.00 (0.96–1.07) | 0.70 | ||
| WHO performance status ≥2 | 1.89 (0.89–4.01) | 0.09 | 2.51 (1.02–6.15) | 0.045 |
| Daily smoker | 1.66 (0.69–3.95) | 0.25 | ||
| Presence of comorbidity * | 1.67 (0.92–3.04) | 0.09 | 1.12 (0.57–2.19) | 0.75 |
| Primary cytoreductive surgery | 1.08 (0.51–2.30) | 0.84 | ||
| Intraoperative factor | ||||
| Extensive surgery ** | 0.87 (0.48–1.56) | 0.63 | ||
| Debulking with PlasmaJet | 0.77 (0.45–1.32) | 0.34 | ||
| HIPEC procedure | 2.05 (1.09–3.83) | 0.03 | 1.85 (0.83–4.15) | 0.13 |
| Operative time (per 30-min increase) | 1.06 (0.98–1.13) | 0.09 | 1.02 (0.94–1.11) | 0.52 |
| Intraoperative injury | ||||
| Urinary tract injury | 2.96 (0.83–10.53) | 0.09 | 2.26 (0.55–9.29) | 0.26 |
| Bowel injury | 3.47 (1.35–8.94) | 0.01 | 2.98 (1.04–8.56) | 0.043 |
| Upper abdominal visceral injury *** | 6.06 (1.47–24.91) | 0.01 | 10.26 (1.85–57.13) | 0.008 |
| Pneumothorax | 7.50 (1.42–39.56) | 0.02 | 4.79 (0.79–28.75) | 0.08 |
| Blood loss >1 L | 1.09 (0.624–1.910) | 0.78 | ||
| Postoperative factor | ||||
| Complete cytoreduction | 0.74 (0.390–1.425) | 0.38 | ||
| FIGO stage | ||||
| Stage IIIB (reference) | ||||
| Stage IIIC | 1.16 (0.34–4.01) | 0.82 | ||
| Stage IV | 1.36 (0.74–2.50) | 0.32 | ||
| Post-operative complications CDC ≥IIIa | 3.13 (1.63–6.02) | 0.001 | 2.32 (1.08–5.01) | 0.032 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kengsakul, M.; Nieuwenhuyzen-de Boer, G.M.; Udomkarnjananun, S.; Kerr, S.J.; van Doorn, H.C.; van Beekhuizen, H.J. Factors Predicting 30-Day Grade IIIa–V Clavien–Dindo Classification Complications and Delayed Chemotherapy Initiation after Cytoreductive Surgery for Advanced-Stage Ovarian Cancer: A Prospective Cohort Study. Cancers 2022, 14, 4181. https://doi.org/10.3390/cancers14174181
Kengsakul M, Nieuwenhuyzen-de Boer GM, Udomkarnjananun S, Kerr SJ, van Doorn HC, van Beekhuizen HJ. Factors Predicting 30-Day Grade IIIa–V Clavien–Dindo Classification Complications and Delayed Chemotherapy Initiation after Cytoreductive Surgery for Advanced-Stage Ovarian Cancer: A Prospective Cohort Study. Cancers. 2022; 14(17):4181. https://doi.org/10.3390/cancers14174181
Chicago/Turabian StyleKengsakul, Malika, Gatske M. Nieuwenhuyzen-de Boer, Suwasin Udomkarnjananun, Stephen J. Kerr, Helena C. van Doorn, and Heleen J. van Beekhuizen. 2022. "Factors Predicting 30-Day Grade IIIa–V Clavien–Dindo Classification Complications and Delayed Chemotherapy Initiation after Cytoreductive Surgery for Advanced-Stage Ovarian Cancer: A Prospective Cohort Study" Cancers 14, no. 17: 4181. https://doi.org/10.3390/cancers14174181
APA StyleKengsakul, M., Nieuwenhuyzen-de Boer, G. M., Udomkarnjananun, S., Kerr, S. J., van Doorn, H. C., & van Beekhuizen, H. J. (2022). Factors Predicting 30-Day Grade IIIa–V Clavien–Dindo Classification Complications and Delayed Chemotherapy Initiation after Cytoreductive Surgery for Advanced-Stage Ovarian Cancer: A Prospective Cohort Study. Cancers, 14(17), 4181. https://doi.org/10.3390/cancers14174181







