Thyroid Cancer and Fibroblasts
Abstract
:Simple Summary
Abstract
1. Introduction
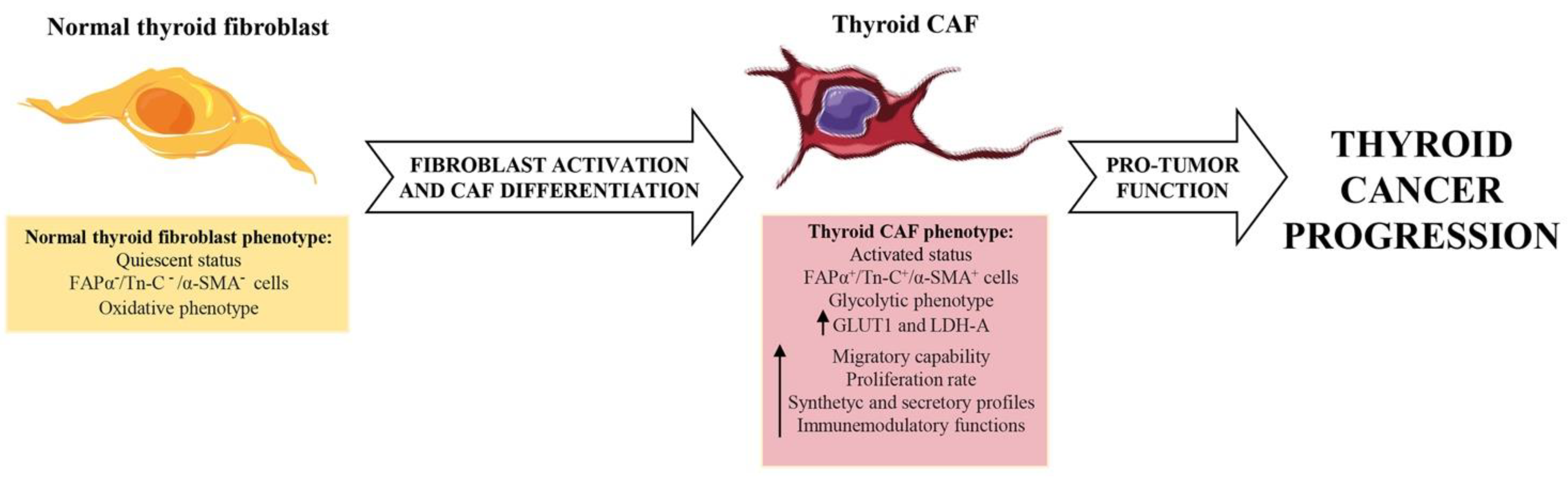
2. Cancer Associated Fibroblasts of Thyroid Desmoplastic Tumor Microenvironment
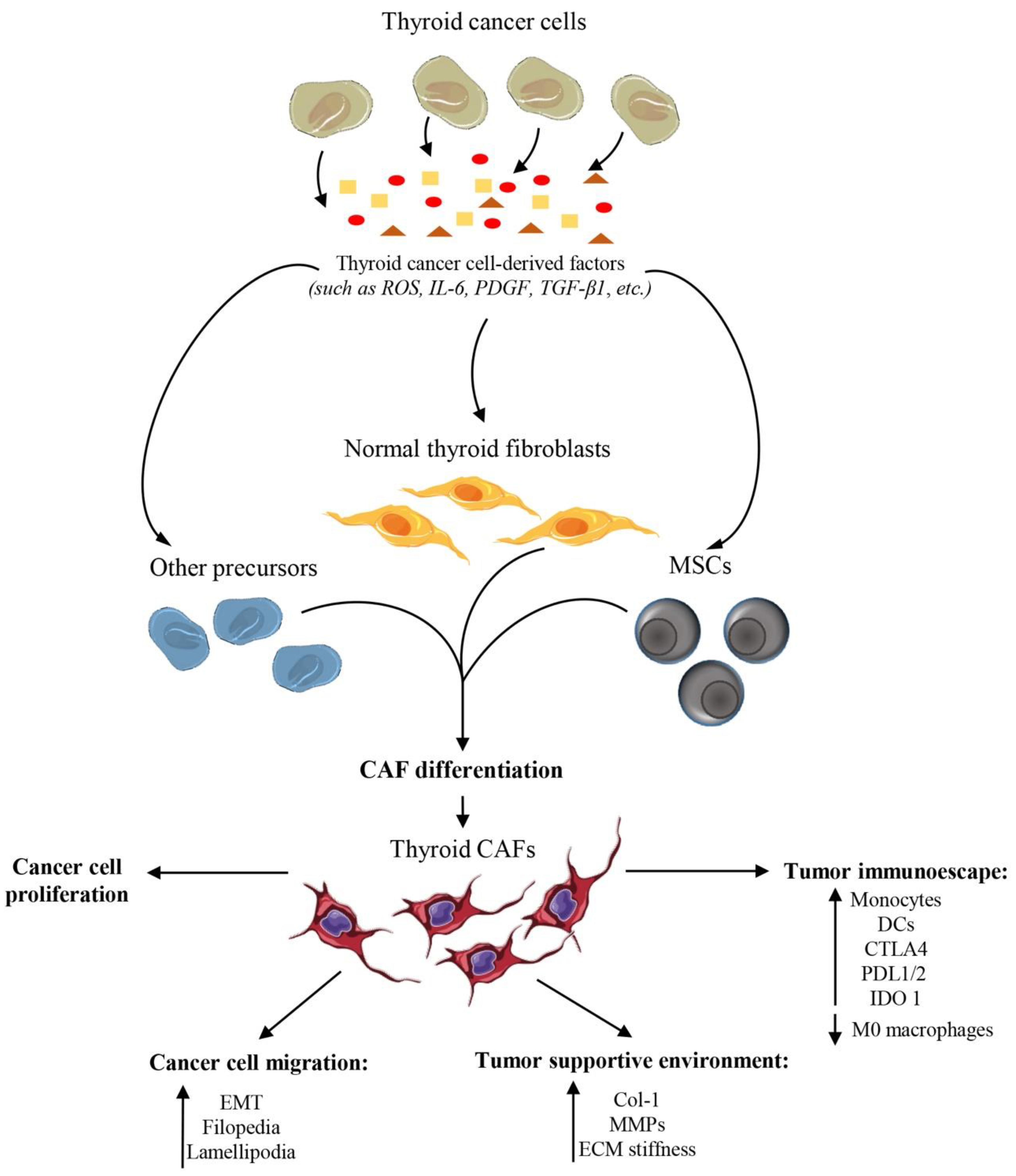
3. Fibroblasts in Thyroid Cancers: Origin and Features
4. Exploring the Role of Fibroblasts in Thyroid Cancers
5. Therapeutic Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Allen, E.; Fingeret, A. Anatomy, Head and Neck, Thyroid. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Nilsson, M.; Fagman, H. Development of the thyroid gland. Development 2017, 144, 2123–2140. [Google Scholar] [CrossRef]
- Khan, A.; Nosé, V. Pathology of Thyroid Gland. In Endocrine Pathology: Differential Diagnosis and Molecular Advances; Lloyd, R.V., Ed.; Springer: New York, NY, USA, 2010; pp. 181–235. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Sosa, J.A. The changing incidence of thyroid cancer. Nat. Rev. Endocrinol. 2016, 12, 646–653. [Google Scholar] [CrossRef]
- Xing, M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef]
- Wei, W.; Hardin, H.; Luo, Q.Y. Targeting autophagy in thyroid cancers. Endocr.-Relat. Cancer 2019, 26, R181–R194. [Google Scholar] [CrossRef]
- Prete, A.; Borges de Souza, P.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Front. Endocrinol. 2020, 11, 102. [Google Scholar] [CrossRef]
- Song, H.J.; Xue, Y.L.; Xu, Y.H.; Qiu, Z.L.; Luo, Q.Y. Rare metastases of differentiated thyroid carcinoma: Pictorial review. Endocr.-Relat. Cancer 2011, 18, R165–R174. [Google Scholar] [CrossRef]
- Avagliano, A.; Fiume, G.; Ruocco, M.R.; Martucci, N.; Vecchio, E.; Insabato, L.; Russo, D.; Accurso, A.; Masone, S.; Montagnani, S.; et al. Influence of Fibroblasts on Mammary Gland Development, Breast Cancer Microenvironment Remodeling, and Cancer Cell Dissemination. Cancers 2020, 12, 1697. [Google Scholar] [CrossRef]
- Avagliano, A.; Ruocco, M.R.; Nasso, R.; Aliotta, F.; Sanita, G.; Iaccarino, A.; Bellevicine, C.; Cali, G.; Fiume, G.; Masone, S.; et al. Development of a Stromal Microenvironment Experimental Model Containing Proto-Myofibroblast Like Cells and Analysis of Its Crosstalk with Melanoma Cells: A New Tool to Potentiate and Stabilize Tumor Suppressor Phenotype of Dermal Myofibroblasts. Cells 2019, 8, 1435. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Arcucci, A.; Ruocco, M.R.; Granato, G.; Sacco, A.M.; Montagnani, S. Cancer: An Oxidative Crosstalk between Solid Tumor Cells and Cancer Associated Fibroblasts. BioMed Res. Int. 2016, 2016, 4502846. [Google Scholar] [CrossRef]
- Ciavardelli, D.; Bellomo, M.; Consalvo, A.; Crescimanno, C.; Vella, V. Metabolic Alterations of Thyroid Cancer as Potential Therapeutic Targets. BioMed Res. Int. 2017, 2017, 2545031. [Google Scholar] [CrossRef]
- MacDonald, L.; Jenkins, J.; Purvis, G.; Lee, J.; Franco, A.T. The Thyroid Tumor Microenvironment: Potential Targets for Therapeutic Intervention and Prognostication. Horm. Cancer 2020, 11, 205–217. [Google Scholar] [CrossRef]
- De Wever, O.; Mareel, M. Role of tissue stroma in cancer cell invasion. J. Pathol. 2003, 200, 429–447. [Google Scholar] [CrossRef]
- Dvorak, H.F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef]
- Liotta, L.A.; Kohn, E.C. The microenvironment of the tumour-host interface. Nature 2001, 411, 375–379. [Google Scholar] [CrossRef]
- Koperek, O.; Akin, E.; Asari, R.; Niederle, B.; Neuhold, N. Expression of hypoxia-inducible factor 1 alpha in papillary thyroid carcinoma is associated with desmoplastic stromal reaction and lymph node metastasis. Virchows Arch. Int. J. Pathol. 2013, 463, 795–802. [Google Scholar] [CrossRef]
- Koperek, O.; Asari, R.; Niederle, B.; Kaserer, K. Desmoplastic stromal reaction in papillary thyroid microcarcinoma. Histopathology 2011, 58, 919–924. [Google Scholar] [CrossRef]
- Koperek, O.; Scheuba, C.; Puri, C.; Birner, P.; Haslinger, C.; Rettig, W.; Niederle, B.; Kaserer, K.; Garin Chesa, P. Molecular characterization of the desmoplastic tumor stroma in medullary thyroid carcinoma. Int. J. Oncol. 2007, 31, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Scheuba, C.; Kaserer, K.; Kaczirek, K.; Asari, R.; Niederle, B. Desmoplastic stromal reaction in medullary thyroid cancer-an intraoperative “marker” for lymph node metastases. World J. Surg. 2006, 30, 853–859. [Google Scholar] [CrossRef]
- Harach, H.R.; Franssila, K.O.; Wasenius, V.M. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer 1985, 56, 531–538. [Google Scholar] [CrossRef]
- Cho, J.G.; Byeon, H.K.; Oh, K.H.; Baek, S.K.; Kwon, S.Y.; Jung, K.Y.; Woo, J.S. Clinicopathological significance of cancer-associated fibroblasts in papillary thyroid carcinoma: A predictive marker of cervical lymph node metastasis. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.Y.; Jung, W.H.; Koo, J.S. Expression of cancer-associated fibroblast-related proteins in thyroid papillary carcinoma. Tumour Biol. 2016, 37, 8197–8207. [Google Scholar] [CrossRef]
- Tarabichi, M.; Antoniou, A.; Le Pennec, S.; Gacquer, D.; de Saint Aubain, N.; Craciun, L.; Cielen, T.; Laios, I.; Larsimont, D.; Andry, G.; et al. Distinctive Desmoplastic 3D Morphology Associated With BRAFV600E in Papillary Thyroid Cancers. J. Clin. Endocrinol. Metab. 2018, 103, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, K.E.; Myong, J.P.; Park, J.H.; Jeon, Y.K.; Min, H.S.; Park, S.Y.; Jung, K.C.; Koo do, H.; Youn, Y.K. BRAF V600E mutation is associated with tumor aggressiveness in papillary thyroid cancer. World J. Surg. 2012, 36, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Lupi, C.; Giannini, R.; Ugolini, C.; Proietti, A.; Berti, P.; Minuto, M.; Materazzi, G.; Elisei, R.; Santoro, M.; Miccoli, P.; et al. Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2007, 92, 4085–4090. [Google Scholar] [CrossRef]
- Finkelstein, A.; Levy, G.H.; Hui, P.; Prasad, A.; Virk, R.; Chhieng, D.C.; Carling, T.; Roman, S.A.; Sosa, J.A.; Udelsman, R.; et al. Papillary thyroid carcinomas with and without BRAF? V600E mutations are morphologically distinct. Histopathology 2012, 60, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wei, X.; Pan, Y.; Xu, J.; Si, Y.; Min, Z.; Yu, B. A new risk factor indicator for papillary thyroid cancer based on immune infiltration. Cell Death Dis. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jolly, L.A.; Novitskiy, S.; Owens, P.; Massoll, N.; Cheng, N.; Fang, W.; Moses, H.L.; Franco, A.T. Fibroblast-Mediated Collagen Remodeling Within the Tumor Microenvironment Facilitates Progression of Thyroid Cancers Driven by BrafV600E and Pten Loss. Cancer Res. 2016, 76, 1804–1813. [Google Scholar] [CrossRef] [Green Version]
- Minna, E.; Brich, S.; Todoerti, K.; Pilotti, S.; Collini, P.; Bonaldi, E.; Romeo, P.; Cecco, L.; Dugo, M.; Perrone, F.; et al. Cancer Associated Fibroblasts and Senescent Thyroid Cells in the Invasive Front of Thyroid Carcinoma. Cancers 2020, 12, 112. [Google Scholar] [CrossRef]
- Wen, S.; Qu, N.; Ma, B.; Wang, X.; Luo, Y.; Xu, W.; Jiang, H.; Zhang, Y.; Wang, Y.; Ji, Q. Cancer-Associated Fibroblasts Positively Correlate with Dedifferentiation and Aggressiveness of Thyroid Cancer. OncoTargets Ther. 2021, 14, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Avagliano, A.; Granato, G.; Ruocco, M.R.; Romano, V.; Belviso, I.; Carfora, A.; Montagnani, S.; Arcucci, A. Metabolic Reprogramming of Cancer Associated Fibroblasts: The Slavery of Stromal Fibroblasts. BioMed Res. Int. 2018, 2018, 6075403. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Eyden, B.P. Interfollicular fibroblasts in the human thyroid gland: Recognition of a CD34 positive stromal cell network communicated by gap junctions and terminated by autonomic nerve endings. J. Submicrosc. Cytol. Pathol. 1997, 29, 461–476. [Google Scholar] [PubMed]
- Sennett, R.; Rendl, M. Developmental biology. A scar is born: Origins of fibrotic skin tissue. Science 2015, 348, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Celetta, G.; Tomasek, J.J.; Gabbiani, G.; Chaponnier, C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol. Biol. Cell 2001, 12, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Ryder, M.; Gild, M.; Hohl, T.M.; Pamer, E.; Knauf, J.; Ghossein, R.; Joyce, J.A.; Fagin, J.A. Genetic and pharmacological targeting of CSF-1/CSF-1R inhibits tumor-associated macrophages and impairs BRAF-induced thyroid cancer progression. PLoS ONE 2013, 8, e54302. [Google Scholar] [CrossRef]
- Fozzatti, L.; Alamino, V.A.; Park, S.; Giusiano, L.; Volpini, X.; Zhao, L.; Stempin, C.C.; Donadio, A.C.; Cheng, S.Y.; Pellizas, C.G. Interplay of fibroblasts with anaplastic tumor cells promotes follicular thyroid cancer progression. Sci. Rep. 2019, 9, 8028. [Google Scholar] [CrossRef]
- Fozzatti, L.; Cheng, S.Y. Tumor Cells and Cancer-Associated Fibroblasts: A Synergistic Crosstalk to Promote Thyroid Cancer. Endocrinol. Metab. 2020, 35, 673–680. [Google Scholar] [CrossRef]
- Avagliano, A.; Arcucci, A. Insights into melanoma fibroblast populations and therapeutic strategy perspectives: Friends or foes? Curr. Med. Chem. 2022. [Google Scholar] [CrossRef]
- Ruocco, M.R.; Avagliano, A.; Granato, G.; Imparato, V.; Masone, S.; Masullo, M.; Nasso, R.; Montagnani, S.; Arcucci, A. Involvement of Breast Cancer-Associated Fibroblasts in Tumor Development, Therapy Resistance and Evaluation of Potential Therapeutic Strategies. Curr. Med. Chem. 2018, 25, 3414–3434. [Google Scholar] [CrossRef]
- Parascandolo, A.; Rappa, F.; Cappello, F.; Kim, J.; Cantu, D.A.; Chen, H.; Mazzoccoli, G.; Hematti, P.; Castellone, M.D.; Salvatore, M.; et al. Extracellular Superoxide Dismutase Expression in Papillary Thyroid Cancer Mesenchymal Stem/Stromal Cells Modulates Cancer Cell Growth and Migration. Sci. Rep. 2017, 7, 41416. [Google Scholar] [CrossRef] [PubMed]
- Avagliano, A.; Fiume, G.; Pelagalli, A.; Sanita, G.; Ruocco, M.R.; Montagnani, S.; Arcucci, A. Metabolic Plasticity of Melanoma Cells and Their Crosstalk With Tumor Microenvironment. Front. Oncol. 2020, 10, 722. [Google Scholar] [CrossRef] [PubMed]
- Romano, V.; Belviso, I.; Venuta, A.; Ruocco, M.R.; Masone, S.; Aliotta, F.; Fiume, G.; Montagnani, S.; Avagliano, A.; Arcucci, A. Influence of Tumor Microenvironment and Fibroblast Population Plasticity on Melanoma Growth, Therapy Resistance and Immunoescape. Int. J. Mol. Sci. 2021, 22, 5283. [Google Scholar] [CrossRef] [PubMed]
- Romano, V.; Ruocco, M.R.; Carotenuto, P.; Barbato, A.; Venuta, A.; Acampora, V.; De Lella, S.; Vigliar, E.; Iaccarino, A.; Troncone, G.; et al. Generation and Characterization of a Tumor Stromal Microenvironment and Analysis of Its Interplay with Breast Cancer Cells: An In Vitro Model to Study Breast Cancer-Associated Fibroblast Inactivation. Int. J. Mol. Sci. 2022, 23, 6875. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Chen, G.; Li, X.; Wei, X.; Liu, C.; Derwahl, M. Effect of IL-6 on proliferation of human thyroid anaplastic cancer stem cells. Int. J. Clin. Exp. Pathol. 2019, 12, 3992–4001. [Google Scholar] [PubMed]
- Kobawala, T.P.; Trivedi, T.I.; Gajjar, K.K.; Patel, D.H.; Patel, G.H.; Ghosh, N.R. Significance of Interleukin-6 in Papillary Thyroid Carcinoma. J. Thyroid. Res. 2016, 2016, 6178921. [Google Scholar] [CrossRef]
- Wu, X.; Tao, P.; Zhou, Q.; Li, J.; Yu, Z.; Wang, X.; Li, J.; Li, C.; Yan, M.; Zhu, Z.; et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget 2017, 8, 20741–20750. [Google Scholar] [CrossRef]
- Jia, C.; Wang, G.; Wang, T.; Fu, B.; Zhang, Y.; Huang, L.; Deng, Y.; Chen, G.; Wu, X.; Chen, J.; et al. Cancer-associated Fibroblasts induce epithelial-mesenchymal transition via the Transglutaminase 2-dependent IL-6/IL6R/STAT3 axis in Hepatocellular Carcinoma. Int. J. Biol. Sci. 2020, 16, 2542–2558. [Google Scholar] [CrossRef]
- Kang, Y.E.; Kim, J.T.; Lim, M.A.; Oh, C.; Liu, L.; Jung, S.N.; Won, H.R.; Lee, K.; Chang, J.W.; Yi, H.S.; et al. Association between Circulating Fibroblast Growth Factor 21 and Aggressiveness in Thyroid Cancer. Cancers 2019, 11, 1154. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Li, D.; Jing, S. Notch and TGF-beta/Smad3 pathways are involved in the interaction between cancer cells and cancer-associated fibroblasts in papillary thyroid carcinoma. Tumour Biol. 2014, 35, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, O.; Mitsutake, N.; Nakayama, T.; Nagayama, Y. Fibroblast-mediated in vivo and in vitro growth promotion of tumorigenic rat thyroid carcinoma cells but not normal Fisher rat thyroid follicular cells. Thyroid 2009, 19, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Miana, R.D.C.; Della Vedova, A.B.; De Paul, A.L.; Remedi, M.M.; Guantay, M.L.; Gilardoni, M.B.; Pellizas, C.G.; Donadio, A.C. Thyroid tumor cells-fibroblasts crosstalk: Role of extracellular vesicles. Endocr. Connect. 2020, 9, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Laatikainen, L.E.; Castellone, M.D.; Hebrant, A.; Hoste, C.; Cantisani, M.C.; Laurila, J.P.; Salvatore, G.; Salerno, P.; Basolo, F.; Nasman, J.; et al. Extracellular superoxide dismutase is a thyroid differentiation marker down-regulated in cancer. Endocr.-Relat. Cancer 2010, 17, 785–796. [Google Scholar] [CrossRef]
- Cammarota, F.; de Vita, G.; Salvatore, M.; Laukkanen, M.O. Ras oncogene-mediated progressive silencing of extracellular superoxide dismutase in tumorigenesis. BioMed Res. Int. 2015, 2015, 780409. [Google Scholar] [CrossRef]
- Laurila, J.P.; Castellone, M.D.; Curcio, A.; Laatikainen, L.E.; Haaparanta-Solin, M.; Gronroos, T.J.; Marjamaki, P.; Martikainen, S.; Santoro, M.; Laukkanen, M.O. Extracellular superoxide dismutase is a growth regulatory mediator of tissue injury recovery. Mol. Ther. 2009, 17, 448–454. [Google Scholar] [CrossRef]
- Castellone, M.D.; Langella, A.; Cantara, S.; Laurila, J.P.; Laatikainen, L.E.; Bellelli, R.; Pacini, F.; Salvatore, M.; Laukkanen, M.O. Extracellular superoxide dismutase induces mouse embryonic fibroblast proliferative burst, growth arrest, immortalization, and consequent in vivo tumorigenesis. Antioxid. Redox Signal. 2014, 21, 1460–1474. [Google Scholar] [CrossRef]
- Laukkanen, M.O.; Cammarota, F.; Esposito, T.; Salvatore, M.; Castellone, M.D. Extracellular superoxide dismutase regulates the expression of small gtpase regulatory proteins GEFs, GAPs, and GDI. PLoS ONE 2015, 10, e0121441. [Google Scholar] [CrossRef]
- Laukkanen, M.O. Extracellular Superoxide Dismutase: Growth Promoter or Tumor Suppressor? Oxidative Med. Cell. Longev. 2016, 2016, 3612589. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; He, Q. Ursolic acid inhibits proliferation, migration and invasion of human papillary thyroid carcinoma cells via CXCL12/CXCR4/CXCR7 axis through cancer-associated fibroblasts. Hum. Exp. Toxicol. 2022, 41, 9603271221111333. [Google Scholar] [CrossRef]
- Sun, R.; Luo, H.; Su, J.; Di, S.; Zhou, M.; Shi, B.; Sun, Y.; Du, G.; Zhang, H.; Jiang, H.; et al. Olaparib Suppresses MDSC Recruitment via SDF1alpha/CXCR4 Axis to Improve the Anti-tumor Efficacy of CAR-T Cells on Breast Cancer in Mice. Mol. Ther. 2021, 29, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, L.; Teng, X.; Zhang, H.; Guan, H. The involvement of CXCR7 in modulating the progression of papillary thyroid carcinoma. J. Surg. Res. 2014, 191, 379–388. [Google Scholar] [CrossRef]
- Boufraqech, M.; Patel, D.; Nilubol, N.; Powers, A.; King, T.; Shell, J.; Lack, J.; Zhang, L.; Gara, S.K.; Gunda, V.; et al. Lysyl Oxidase Is a Key Player in BRAF/MAPK Pathway-Driven Thyroid Cancer Aggressiveness. Thyroid 2019, 29, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.L.; Wang, Z.; Cao, C.J.; Ke, Z.F.; Wang, F.; Wang, R.; Luo, C.Q.; Lu, X.; Wang, L.T. AEG-1 associates with metastasis in papillary thyroid cancer through upregulation of MMP2/9. Int. J. Oncol. 2017, 51, 812–822. [Google Scholar] [CrossRef]
- Shi, Y.; Su, C.; Hu, H.; Yan, H.; Li, W.; Chen, G.; Xu, D.; Du, X.; Zhang, P. Serum MMP-2 as a potential predictive marker for papillary thyroid carcinoma. PLoS ONE 2018, 13, e0198896. [Google Scholar] [CrossRef]
- Dadafarin, S.; Carnazza, M.; Islam, H.K.; Moscatello, A.; Tiwari, R.K.; Geliebter, J. Noncoding RNAs in Papillary Thyroid Cancer: Interaction with Cancer-Associated Fibroblasts (CAFs) in the Tumor Microenvironment (TME) and Regulators of Differentiation and Lymph Node Metastasis. Adv. Exp. Med. Biol. 2021, 1350, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef]
- Omi, Y.; Shibata, N.; Okamoto, T.; Obara, T.; Kobayashi, M. The role of CD147 in the invasiveness of follicular thyroid carcinoma cells. Thyroid 2012, 22, 383–394. [Google Scholar] [CrossRef]
- Huang, P.; Mao, L.F.; Zhang, Z.P.; Lv, W.W.; Feng, X.P.; Liao, H.J.; Dong, C.; Kaluba, B.; Tang, X.F.; Chang, S. Down-Regulated miR-125a-5p Promotes the Reprogramming of Glucose Metabolism and Cell Malignancy by Increasing Levels of CD147 in Thyroid Cancer. Thyroid 2018, 28, 613–623. [Google Scholar] [CrossRef]
- Tan, H.; Ye, K.; Wang, Z.; Tang, H. CD147 expression as a significant prognostic factor in differentiated thyroid carcinoma. Transl. Res. 2008, 152, 143–149. [Google Scholar] [CrossRef]
- Xin, X.; Zeng, X.; Gu, H.; Li, M.; Tan, H.; Jin, Z.; Hua, T.; Shi, R.; Wang, H. CD147/EMMPRIN overexpression and prognosis in cancer: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 32804. [Google Scholar] [CrossRef]
- Ohshio, Y.; Teramoto, K.; Hanaoka, J.; Tezuka, N.; Itoh, Y.; Asai, T.; Daigo, Y.; Ogasawara, K. Cancer-associated fibroblast-targeted strategy enhances antitumor immune responses in dendritic cell-based vaccine. Cancer Sci. 2015, 106, 134–142. [Google Scholar] [CrossRef]
- Yin, M.; Soikkeli, J.; Jahkola, T.; Virolainen, S.; Saksela, O.; Holtta, E. TGF-beta signaling, activated stromal fibroblasts, and cysteine cathepsins B and L drive the invasive growth of human melanoma cells. Am. J. Pathol. 2012, 181, 2202–2216. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Waldhauer, I.; Gonzalez-Nicolini, V.; Freimoser-Grundschober, A.; Nayak, T.K.; Fahrni, L.; Hosse, R.J.; Gerrits, D.; Geven, E.J.W.; Sam, J.; Lang, S.; et al. Simlukafusp alfa (FAP-IL2v) immunocytokine is a versatile combination partner for cancer immunotherapy. mAbs 2021, 13, 1913791. [Google Scholar] [CrossRef]
- Hu, S.; Ma, J.; Su, C.; Chen, Y.; Shu, Y.; Qi, Z.; Zhang, B.; Shi, G.; Zhang, Y.; Zhang, Y.; et al. Engineered exosome-like nanovesicles suppress tumor growth by reprogramming tumor microenvironment and promoting tumor ferroptosis. Acta Biomater. 2021, 135, 567–581. [Google Scholar] [CrossRef]
- Nwani, N.G.; Deguiz, M.L.; Jimenez, B.; Vinokour, E.; Dubrovskyi, O.; Ugolkov, A.; Mazar, A.P.; Volpert, O.V. Melanoma Cells Block PEDF Production in Fibroblasts to Induce the Tumor-Promoting Phenotype of Cancer-Associated Fibroblasts. Cancer Res. 2016, 76, 2265–2276. [Google Scholar] [CrossRef]
- Shao, H.; Moller, M.; Cai, L.; Prokupets, R.; Yang, C.; Costa, C.; Yu, K.; Le, N.; Liu, Z.J. Converting melanoma-associated fibroblasts into a tumor-suppressive phenotype by increasing intracellular Notch1 pathway activity. PLoS ONE 2021, 16, e0248260. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, K.; Wickett, R.R.; Kadekaro, A.L.; Zhang, Y. Targeted deactivation of cancer-associated fibroblasts by beta-catenin ablation suppresses melanoma growth. Tumour Biol. 2016, 37, 14235–14248. [Google Scholar] [CrossRef]
- Biffi, G.; Tuveson, D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avagliano, A.; Fiume, G.; Bellevicine, C.; Troncone, G.; Venuta, A.; Acampora, V.; De Lella, S.; Ruocco, M.R.; Masone, S.; Velotti, N.; et al. Thyroid Cancer and Fibroblasts. Cancers 2022, 14, 4172. https://doi.org/10.3390/cancers14174172
Avagliano A, Fiume G, Bellevicine C, Troncone G, Venuta A, Acampora V, De Lella S, Ruocco MR, Masone S, Velotti N, et al. Thyroid Cancer and Fibroblasts. Cancers. 2022; 14(17):4172. https://doi.org/10.3390/cancers14174172
Chicago/Turabian StyleAvagliano, Angelica, Giuseppe Fiume, Claudio Bellevicine, Giancarlo Troncone, Alessandro Venuta, Vittoria Acampora, Sabrina De Lella, Maria Rosaria Ruocco, Stefania Masone, Nunzio Velotti, and et al. 2022. "Thyroid Cancer and Fibroblasts" Cancers 14, no. 17: 4172. https://doi.org/10.3390/cancers14174172
APA StyleAvagliano, A., Fiume, G., Bellevicine, C., Troncone, G., Venuta, A., Acampora, V., De Lella, S., Ruocco, M. R., Masone, S., Velotti, N., Carotenuto, P., Mallardo, M., Caiazza, C., Montagnani, S., & Arcucci, A. (2022). Thyroid Cancer and Fibroblasts. Cancers, 14(17), 4172. https://doi.org/10.3390/cancers14174172











