Risk of Diabetes Mellitus after Radiotherapy for Gastric Mucosa-Associated Lymphoid Tissue Lymphoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Treatment of GML
2.3. Dosimetric Evaluation
2.4. Outcome Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jaffe, E.S. The 2008 WHO classification of lymphomas: Implications for clinical practice and translational research. ASH Educ. Program Book 2009, 2009, 523–531. [Google Scholar] [CrossRef]
- Tsang, R.W.; Gospodarowicz, M.K.; Pintilie, M.; Wells, W.; Hodgson, D.C.; Sun, A.; Crump, M.; Patterson, B.J. Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J. Clin. Oncol. 2003, 21, 4157–4164. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, D.F.; Heydenrych, J.J.; Smit, B.; Zuurmond, T.; Louw, G.; Laker, L.; Els, D.; Weideman, A.; Wolfe-Coote, S.; Du Toit, L.B.; et al. The effect of ionizing radiation on the primate pancreas: An endocrine and morphologic study. J. Surg. Oncol. 1987, 34, 43–52. [Google Scholar] [CrossRef] [PubMed]
- De Vathaire, F.; El-Fayech, C.; Ben Ayed, F.F.; Haddy, N.; Guibout, C.; Winter, D.; Thomas-Teinturier, C.; Veres, C.; Jackson, A.; Pacquement, H.; et al. Radiation dose to the pancreas and risk of diabetes mellitus in childhood cancer survivors: A retrospective cohort study. Lancet Oncol. 2012, 13, 1002–1010. [Google Scholar] [CrossRef]
- Friedman, D.N.; Moskowitz, C.S.; Hilden, P.; Howell, R.M.; Weathers, R.E.; Smith, S.A.; Wolden, S.L.; Tonorezos, E.S.; Mostoufi-Moab, S.; Chow, E.J.; et al. Radiation Dose and Volume to the Pancreas and Subsequent Risk of Diabetes Mellitus: A Report from the Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2020, 112, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.Y.; Lim, D.H.; Oh, D.; Nam, H.; Kim, J.J.; Lee, J.H.; Min, B.-H.; Lee, H. Increased Risk of Diabetes after Definitive Radiotherapy in Patients with Indolent Gastroduodenal Lymphoma. Cancer Res. Treat. 2022, 54, 294–300. [Google Scholar] [CrossRef]
- Pinnix, C.C.; Gunther, J.R.; Milgrom, S.A.; Cruz-Chamorro, R.J.; Medeiros, L.J.; Khoury, J.D.; Amini, B.; Neelapu, S.; Lee, H.J.; Westin, J.; et al. Outcomes after Reduced-Dose Intensity Modulated Radiation Therapy for Gastric Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 447–455. [Google Scholar] [CrossRef]
- Yahalom, J.; Illidge, T.; Specht, L.; Hoppe, R.T.; Li, Y.-X.; Tsang, R.; Wirth, A. Modern radiation therapy for extranodal lymphomas: Field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 11–31. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253. [Google Scholar]
- Lee, D.-C.; Park, I.; Jun, T.-W.; Nam, B.-H.; Cho, S.-I.; Blair, S.-N.; Kim, Y.-S. Physical activity and body mass index and their associations with the development of type 2 diabetes in korean men. Am. J. Epidemiol. 2012, 176, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.-H.; Ku, H.; Park, K.S. Prevalence and socioeconomic burden of diabetes mellitus in South Korean adults: A population-based study using administrative data. BMC Public Health 2021, 21, 548. [Google Scholar] [CrossRef]
- Teinturier, C.; Tournade, M.-F.; Caillat-Zucman, S.; Boitard, C.; Amoura, Z.; Bougneres, P.F.; Timsit, J. Diabetes mellitus after abdominal radiation therapy. Lancet 1995, 346, 633–634. [Google Scholar] [CrossRef]
- Meacham, L.R.; Sklar, C.A.; Li, S.; Liu, Q.; Gimpel, N.; Yasui, Y.; Whitton, J.A.; Stovall, M.; Robison, L.L.; Oeffinger, K.C. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: A report for the childhood cancer survivor study. Arch. Intern. Med. 2009, 169, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Van Nimwegen, F.A.; Schaapveld, M.; Janus, C.P.; Krol, A.D.; Raemaekers, J.M.; Kremer, L.C.; Stovall, M.; Aleman, B.M.; van Leeuwen, F.E. Risk of diabetes mellitus in long-term survivors of Hodgkin lymphoma. J. Clin. Oncol. 2014, 32, 3257–3263. [Google Scholar] [CrossRef]
- Groot, H.J.; Gietema, J.A.; Aleman, B.M.P.; Incrocci, L.; de Wit, R.; Witjes, J.A.; Groenewegen, G.; de Brouwer, P.; Meijer, O.W.M.; Hulshof, M.C.C.M.; et al. Risk of diabetes after para-aortic radiation for testicular cancer. Br. J. Cancer 2018, 119, 901–907. [Google Scholar] [CrossRef]
- Atkins, K.M.; Rawal, B.; Chaunzwa, T.L.; Lamba, N.; Bitterman, D.S.; Williams, C.L.; Kozono, D.E.; Baldini, E.H.; Chen, A.B.; Nguyen, P.L.; et al. Cardiac Radiation Dose, Cardiac Disease, and Mortality in Patients with Lung Cancer. J. Am. Coll. Cardiol. 2019, 73, 2976–2987. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Wirth, A.; Gospodarowicz, M.; Aleman, B.M.P.; Bressel, M.; Ng, A.; Chao, M.; Hoppe, R.T.; Thieblemont, C.; Tsang, R.; Moser, L.; et al. Long-term outcome for gastric marginal zone lymphoma treated with radiotherapy: A retrospective, multi-centre, International Extranodal Lymphoma Study Group study. Ann. Oncol. 2013, 24, 1344–1351. [Google Scholar] [CrossRef]
- Goda, J.S.; Gospodarowicz, M.; Pintilie, M.; Wells, W.; Hodgson, D.C.; Sun, A.; Crump, M.; Tsang, R.W. Long-term outcome in localized extranodal mucosa-associated lymphoid tissue lymphomas treated with radiotherapy. Cancer 2010, 116, 3815–3824. [Google Scholar] [CrossRef]
- Lowry, L.; Smith, P.; Qian, W.; Falk, S.; Benstead, K.; Illidge, T.; Linch, D.; Robinson, M.; Jack, A.; Hoskin, P. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: A randomized phase III trial. Radiother. Oncol. 2011, 100, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.J.; Kirkwood, A.A.; Popova, B.; Smith, P.; Robinson, M.; Gallop-Evans, E.; Coltart, S.; Illidge, T.; Madhavan, K.; Brammer, C.; et al. 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): A randomised phase 3 non-inferiority trial. Lancet Oncol. 2014, 15, 457–463. [Google Scholar] [CrossRef]
- Saifi, O.; Lester, S.C.; Rule, W.; Stish, B.J.; Stafford, S.; Pafundi, D.H.; Jiang, L.; Menke, D.; Moustafa, M.A.; Rosenthal, A.; et al. Comparable Efficacy of Reduced Dose Radiation Therapy for the Treatment of Early Stage Gastric Extranodal Marginal Zone Lymphoma of Mucosa-Associated Lymphoid Tissue. Adv. Radiat. Oncol. 2021, 6, 100714. [Google Scholar] [CrossRef] [PubMed]
- Rehn, S.; Reinartz, G.; Oertel, M.; Hering, D.; Pott, C.; Greve, B.; Eich, H. Phase II Trial to assess the Efficacy of Low Radiation Dose of 20 Gy for the Treatment of Marginal Zone Lymphoma or Follicular Lymphoma Stage I-II localized in the Stomach or the Duodenum. Strahlenther. Onkol. 2021, 197, S170–S171. [Google Scholar]
- US National Library of Medicine. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03680586?cond=lymphoma+gastric&draw=2&rank=8 (accessed on 23 March 2021).
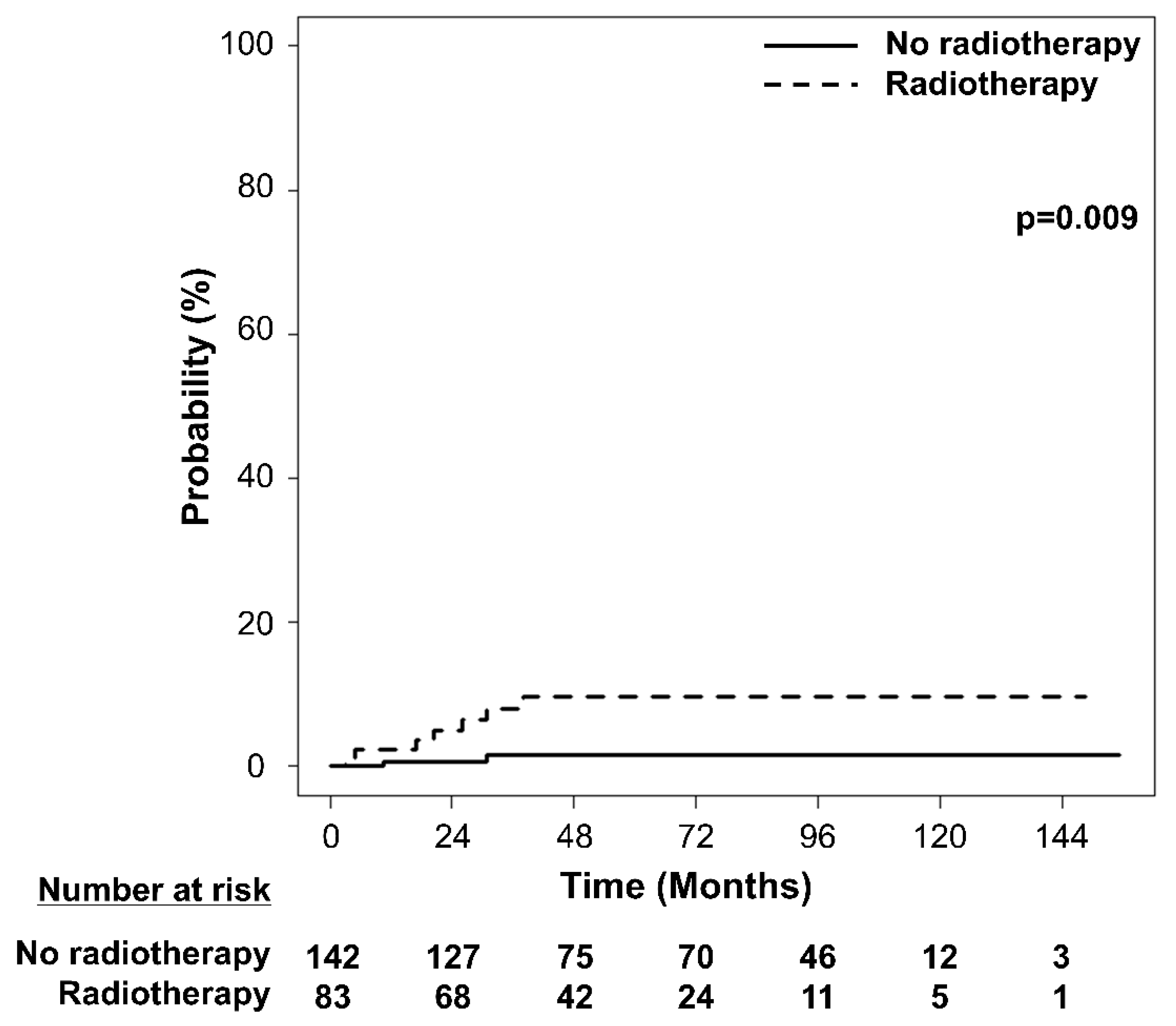
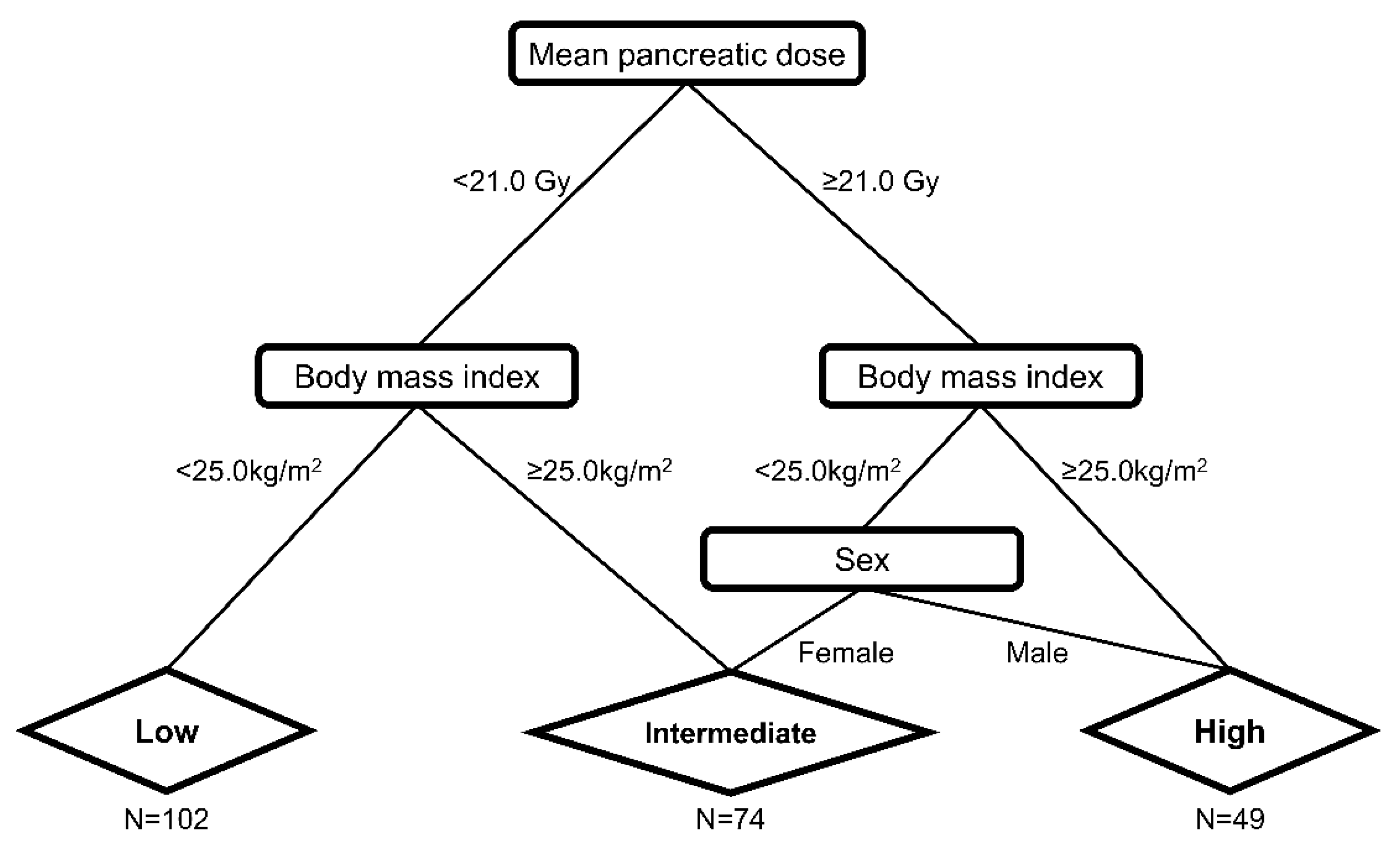
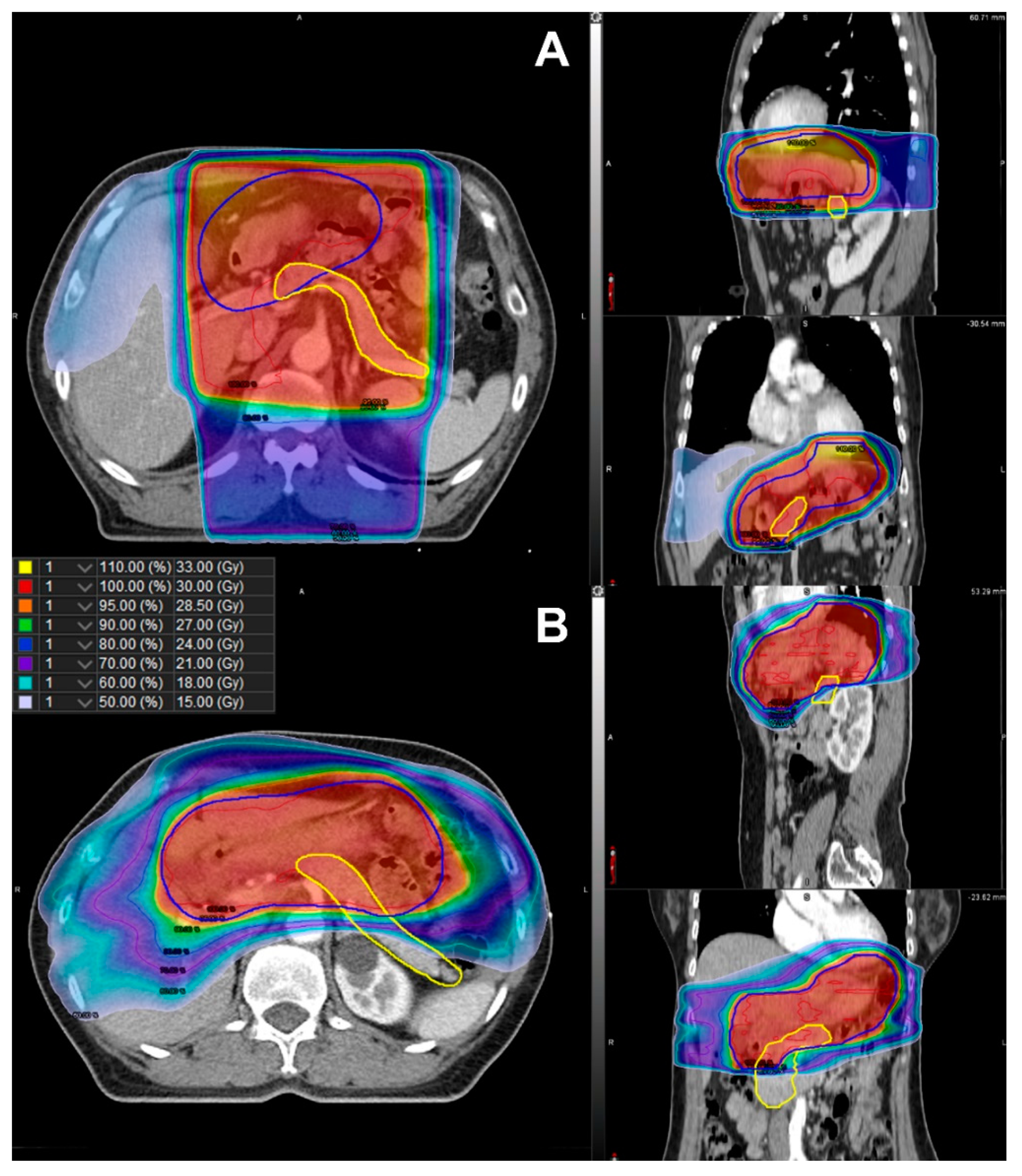
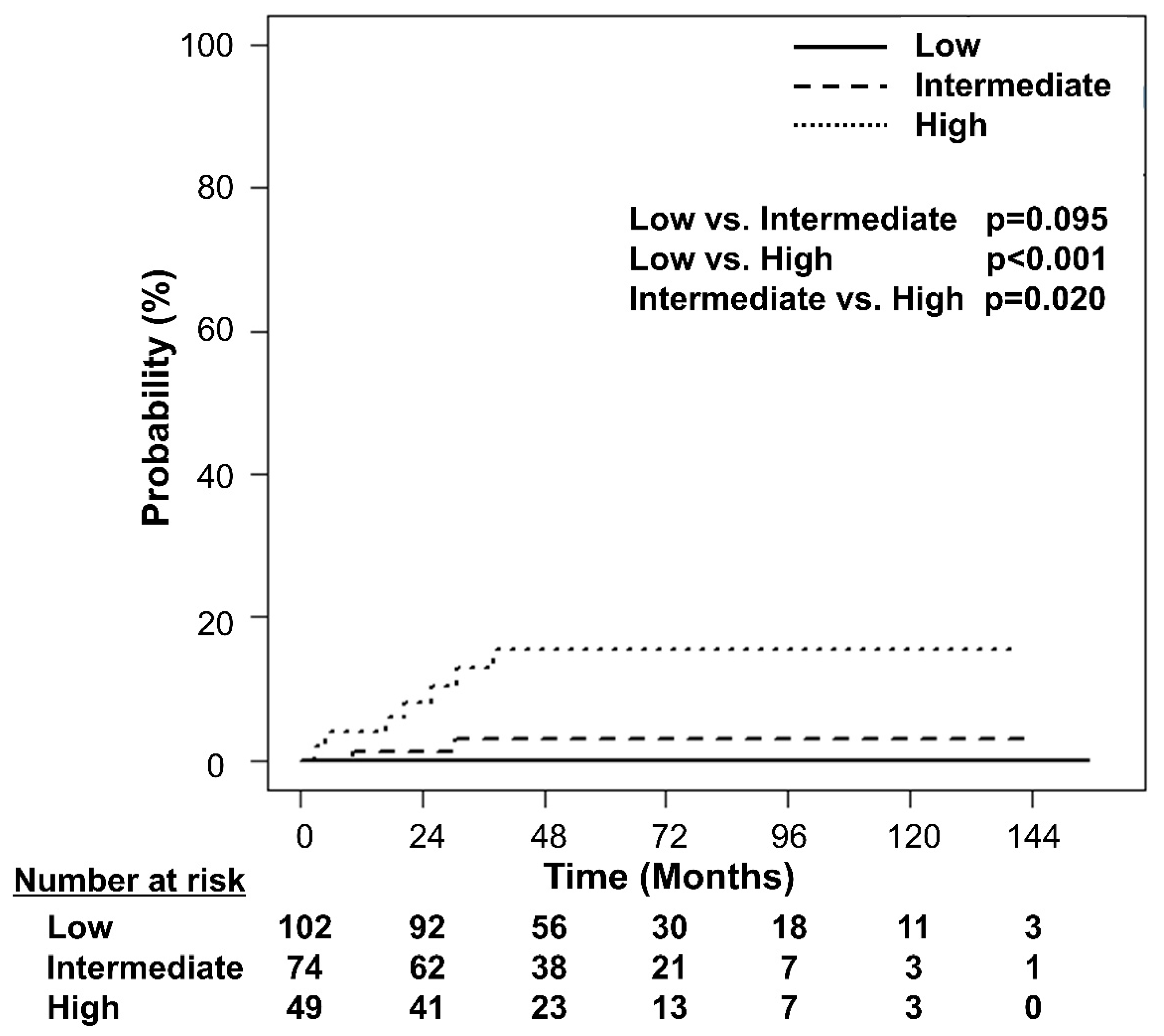
| Characteristics | Total | RT | No RT | ||||
|---|---|---|---|---|---|---|---|
| N = 225 | % | N = 83 | % | N = 142 | % | p-Value | |
| Age, years (median [range]) | 55 (31–86) | 57 (34–86) | 55 (31–77) | 0.219 | |||
| Sex | 0.050 | ||||||
| Female | 138 | 61.3 | 44 | 53.0 | 94 | 66.2 | |
| Male | 87 | 38.7 | 39 | 47.0 | 48 | 33.8 | |
| Body mass index | 0.310 | ||||||
| <25.0 kg/m2 | 159 | 70.7 | 62 | 74.7 | 97 | 68.3 | |
| ≥25.0 kg/m2 | 66 | 29.3 | 21 | 25.3 | 45 | 31.7 | |
| Hypertension | 0.180 | ||||||
| No | 203 | 90.2 | 72 | 86.7 | 131 | 92.3 | |
| Yes | 22 | 9.8 | 11 | 13.3 | 11 | 7.7 | |
| Helicobacter pylori infection | <0.001 | ||||||
| No | 123 | 54.7 | 61 | 73.5 | 62 | 43.7 | |
| Yes | 102 | 45.3 | 22 | 26.5 | 80 | 56.3 | |
| Helicobacter pylori eradication | <0.001 | ||||||
| No | 22 | 9.8 | 22 | 26.5 | 0 | 0.0 | |
| Yes | 203 | 90.2 | 61 | 73.5 | 142 | 100.0 | |
| Pancreas volume, cc (median [range]) | 65.7 (28.5–125.8) | 64.9 (28.5–114.3) | 65.8 (28.9–125.8) | 0.409 | |||
| 3D-CRT | IMRT | ||
|---|---|---|---|
| Median (Range) | Median (Range) | p-Value | |
| Dmean (Gy) | 28.8 (16.6–35.9) | 26.1 (20.7–30.8) | 0.009 |
| D0.03cc (Gy) | 31.2 (24.3–40.4) | 31.4 (23.9–32.8) | 0.463 |
| Dmin (Gy) | 5.8 (0.0–34.9) | 5.6 (0.8–28.7) | 0.484 |
| V5 Gy (%) | 100.0 (73.2–100.0) | 100.0 (82.8–100.0) | 0.802 |
| V10 Gy (%) | 98.7 (62.9–100.0) | 98.5 (72.4–100.0) | 0.903 |
| V15 Gy (%) | 96.3 (53.0–100.0) | 96.4 (65.8–100.0) | 0.497 |
| V20 Gy (%) | 94.1 (47.3–100.0) | 88.2 (62.3–100.0) | 0.090 |
| V25 Gy (%) | 90.9 (0.0–100.0) | 74.7 (0.0–100.0) | <0.001 |
| Univariate Analysis | ||
|---|---|---|
| HR (95% CI) | p-Value | |
| Dmean | 1.06 (1.01–1.11) | 0.015 |
| D0.03cc | 1.06 (1.01–1.10) | 0.016 |
| Dmin | 1.06 (1.01–1.11) | 0.023 |
| V5 Gy | 1.02 (1.00–1.04) | 0.021 |
| V10 Gy | 1.02 (1.00–1.03) | 0.020 |
| V15 Gy | 1.02 (1.00–1.03) | 0.018 |
| V20 Gy | 1.02 (1.00–1.03) | 0.016 |
| V25 Gy | 1.01 (1.00–1.03) | 0.049 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age * | 1.05 (1.00–1.10) | 0.058 | ||
| Sex | ||||
| Female | 1 (Reference) | 1 (Reference) | ||
| Male | 5.66 (1.20–26.76) | 0.029 | 4.85 (1.05–22.34) | 0.043 |
| Body mass index | ||||
| <25.0 kg/m2 | 1 (Reference) | 1 (Reference) | ||
| ≥25.0 kg/m2 | 4.96 (1.26–19.50) | 0.022 | 5.76 (1.56–21.17) | 0.008 |
| Hypertension | ||||
| No | 1 (Reference) | |||
| Yes | 2.74 (0.56–13.32) | 0.210 | ||
| Helicobacter pylori infection | ||||
| No | 1 (Reference) | |||
| Yes | 0.34 (0.07–1.63) | 0.180 | ||
| Pancreas volume * | 1.03 (0.99–1.07) | 0.220 | ||
| Dmean | ||||
| <21.0 Gy | 1 (Reference) | 1 (Reference) | ||
| ≥21.0 Gy | 6.86 (1.44–32.64) | 0.016 | 7.30 (1.65-32.30) | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Yoon, H.I.; Kim, J.; Cho, J.; Kim, K.H.; Suh, C.-O. Risk of Diabetes Mellitus after Radiotherapy for Gastric Mucosa-Associated Lymphoid Tissue Lymphoma. Cancers 2022, 14, 4110. https://doi.org/10.3390/cancers14174110
Lee J, Yoon HI, Kim J, Cho J, Kim KH, Suh C-O. Risk of Diabetes Mellitus after Radiotherapy for Gastric Mucosa-Associated Lymphoid Tissue Lymphoma. Cancers. 2022; 14(17):4110. https://doi.org/10.3390/cancers14174110
Chicago/Turabian StyleLee, Joongyo, Hong In Yoon, Jihun Kim, Jaeho Cho, Kyung Hwan Kim, and Chang-Ok Suh. 2022. "Risk of Diabetes Mellitus after Radiotherapy for Gastric Mucosa-Associated Lymphoid Tissue Lymphoma" Cancers 14, no. 17: 4110. https://doi.org/10.3390/cancers14174110
APA StyleLee, J., Yoon, H. I., Kim, J., Cho, J., Kim, K. H., & Suh, C.-O. (2022). Risk of Diabetes Mellitus after Radiotherapy for Gastric Mucosa-Associated Lymphoid Tissue Lymphoma. Cancers, 14(17), 4110. https://doi.org/10.3390/cancers14174110





